Abstract
The effectiveness of inexpensive agro-industrial wastes such as sugarcane molasses, paper mill effluent, and dairy effluent for the production of polyhydroxyalkanoates (PHAs) using Bacillus subtilis RS1 was assessed in this study. Laboratory-scale fermentations were carried out with both raw and pre-treated agro-industrial effluents, and maximum PHAs yield was observed in pre-treated effluents. Experimental variables such as pH, inoculum dose, and incubation time were further optimized to maximize PHAs yield, and the optimal conditions were identified as incubation time 48 h; pH 7; and inoculum dose 10% v/v. PHAs yield in pre-treated sugarcane molasses increased to 70.5% under optimal conditions. Fourier transform infrared spectroscopy and gas chromatography–mass spectrometric analysis revealed that the extracted polymer was composed of penta- and hexadecanoic acid methyl esters, a copolymer of PHAs. Results indicated that the pre-treated sugarcane molasses could be used as an inexpensive substrate for the production of PHAs.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Environmental problems associated with petroleum-based plastics and rapid depletion of natural resources have motivated biologists to develop new biopolymers. Biopolymers such as polynucleotides, polyamides, polysaccharides, polyoxoesters, polythioesters, polyanhydrides, polyisoprenoids, and polyphenols have been reported as potential substitutes for synthetic plastics. Of these, polyhydroxyalkanoates (PHAs)—a group of polyoxoesters—have received intensive attention because of their biodegradability, biocompatibility, thermoplastic properties, chemical diversity, and their ability to be manufactured from renewable carbon sources (Muhammadi et al. 2015). PHAs are a family of naturally occurring polyesters synthesized by various microorganisms under unbalanced growth conditions as intracellular hydrophobic inclusions of carbon and energy storage compounds in the cytoplasm or by an electron sink mechanism for reducing redundant power under conditions of limiting nutritional elements such as N, P, S, O or Mg in the presence of excess carbon source.
The increasing availability of raw renewable materials and increasing demand for biodegradable polymers in biomedical, packaging, and food applications along with favourable green procurement policies are expected to benefit the market growth of PHAs (Kourmentza et al. 2017). According to a recent report published in 2017, the global PHAs market is expected to reach US$ 93.5 million by 2021. Several companies have initiated and industrialized the production of PHAs, but their high production cost and thus higher prices compared to conventional polymers remain a major limitation factor. Production cost ultimately depends on the substrate used as the carbon source and the extraction methodology. Hence, many efforts have been devoted to reducing the production cost of PHAs using inexpensive raw materials and efficient bacterial strains.
In recent years, there has been an increasing trend towards more efficient utilization of agro-industrial residues for the production of several fermentation-related products (Pandian et al. 2010). Carbohydrates, fats, proteins, minerals, and vitamins present in agro-industrial effluents act as nutrients for microbial growth and enhance the production of fermented products. Numerous technologies have been developed to produce PHAs from low-cost carbon substrates like agro-industrial wastes and their by-products such as palm oil mill effluent, cheese whey, sugarcane molasses, cardboard industry effluent, date syrup, wheat bran, tapioca hydrolysate, methanol and starch, food wastes, and municipal wastes (Din et al. 2012; Bhuwal et al. 2014; Munir et al. 2015; Valentino et al. 2015). Bhuwal et al. (2014) reported 66.6% polyhydroxybutyrate production using cardboard industry wastewater as a carbon source. Munir et al. (2015) reported that the production of PHAs using paper mill effluent (50% v/v) significantly increased the polymer production. Bio-on company, Italy, used sugarcane waste and sugar beet to produce PHAs (Dietrich et al. 2017). However, only a few studies have reported that PHAs production using pre-treated agro-industrial effluent is several times higher compared to untreated/raw industrial effluents. Bosco and Chiampo (2010) reported that pre-treated dairy whey and dairy wastewater activated sludge increased both biomass and PHAs production. Similarly, Gomaa (2014) reported that pre-treatment of cane molasses with H2SO4 significantly increased (~ 50%) PHAs production and biomass of Bacillus subtilis and E. coli. Obruca et al. (2015) compiled the importance of agro-industrial pre-treatment and reported that acid- or alkaline-pre-treated lignocellulosic wastes significantly improved the production of PHAs.
PHAs are synthesized by various microbial species such as Ralstonia eutropha, Alcaligenes sp., Aeromonas sp., Pseudomonas sp., Enterococcus sp., Brevundimonas sp., and Bacillus sp. (Bhuwal et al. 2014), of which Bacillus sp. are found to be ideal by numerous industries and academia, as the Bacillus sp. are genetically stable, are fast growing, can use several agro-industrial wastes as a source of carbon, and have the ability to produce endotoxin-free PHAs compared to Gram-negative bacteria (Mohapatra et al. 2017). Desouky et al. (2017) reported 88.0% of PHAs production using Bacillus flexus strain AZU-A2 after 24 h in batch fermentation. Most of the studies suggest that the Bacillus sp. is beneficial to produce PHAs due to their capacity to accumulate higher concentration of PHAs (Khiyami et al. 2011). Hence, the objectives of the present study were to (i) assess the efficiency of untreated/raw and pre-treated sugarcane molasses, paper mill effluent and dairy effluent for PHAs production using B. subtilis RS1 in a laboratory-scale bioreactor, (ii) optimize the experimental variables to maximize PHAs production, and (iii) provide characterization of extracted polymer to understand the polymeric composition.
Materials and methods
Bacterial strain and inoculum preparation
Bacillus subtilis RS1 was procured from the Department of Environmental Science, PSG College of Arts and Science, Coimbatore, Tamil Nadu, India. The culture preservation and PHAs screening by Sudan Black B staining were reported in our previous study (Rathika et al. 2018). For inoculum preparation, the cells were grown on a LB broth for 24 h at 37 °C on a rotary shaker at 150 rpm and log-phase culture was used for the fermentation studies.
Procurement of agro-industrial effluents
The agro-industrial effluents such as dairy effluent, paper mill effluent, and sugarcane molasses were collected from Tamil Nadu, India. The effluents were carefully transported to the laboratory and stored at 4 °C for further use.
Treatment of agro-industrial effluents
The dairy effluent was pre-treated according to Bosco and Chiampo (2010). In brief, the initial pH of the dairy effluent was adjusted to 4.5 with 0.1 N HCl and autoclaved at 121 °C for 15 min. Later, the effluent was cooled, centrifuged at 10,000 rpm for 15 min, and filtered with a glass fibre filter. The filtrate was used as a substrate for the production of PHAs using B. subtilis RS1.
Paper mill effluent: The paper mill effluent was treated according to Munir et al. (2015). In brief, the initial pH of the paper mill effluent was adjusted to 5 with 0.1 N HCl solution and boiled for 10 min. Later, the effluent was centrifuged at 4000 rpm for 10 min, autoclaved at 121 °C for 15 min, cooled, and used as a substrate for the production of PHAs.
Sugarcane molasses: The sugarcane molasses was treated according to Gomaa (2014). In brief, the sugarcane molasses was initially adjusted to pH 3.0 with 0.1 N H2SO4 solution and the effluent was centrifuged at 3000 rpm for 15 min. Later, the effluent was autoclaved at 121 °C for 15 min, cooled, and used as a substrate for the production of PHAs.
Fermentation studies
PHAs were produced aerobically in a 5-L laboratory-scale bioreactor manufactured by LARK, India. Log-phase culture of B. subtilis RS1 (5% v/v) was carefully inoculated into a bioreactor vessel containing 2 L of raw/untreated or pre-treated effluents and incubated at 37 °C for 24 h with an agitation speed of 500 rpm. The pH of the substrate medium was controlled automatically by the addition of 0.5 N NaOH and 0.5 N HCl. After incubation, cell growth (OD at 660 nm) and PHAs production were determined according to Rathika et al. (2018). Experimental variables considered were (i) type of industrial effluent on cell growth and PHAs production and (ii) the effect of incubation time (24–96 h), pH (5, 7, and 9), and inoculum dosage (5–25%) on PHAs production.
Extraction of PHAs
The biomass was collected, and PHAs were extracted by sodium hypochlorite digestion method (Heinrich et al. 2012). Briefly, the biomass was mixed with diluted sodium hypochlorite solution (3% v/v), digested at 37 °C for 2 h, and centrifuged at 8000 rpm for 15 min. Later, the cell pellets were washed with water, acetone, and diethyl ether and were dissolved in hot chloroform. The dissolved pellets were evaporated to collect PHAs granules.
Quantification of PHAs
Determination of dry cell weight (DCW) and PHAs: The biomass was separated from the samples by centrifugation (10,000 rpm for 10 min), washed with saline solution (0.9 g/L), and dried to estimate DCW. The percentage of PHAs accumulation was estimated using the following equation (Desouky et al. 2017).
UV–Visible spectrophotometer analysis of PHAs
The extracted PHAs and commercial PHAs (Sigma-Aldrich) were acid-hydrolysed (Law and Slepecky (1961)) using 10 ml concentrated H2SO4 and heated for 20 min at 100 °C in a water bath. The PHA crystals were converted into crotonic acid by dehydration. The resultant brown colour crotonic acid solution was cooled, and the absorbance was measured at 230–400 nm in an UV Spectrophotometer (UV 1700 PharmaSpec, Shimadzu).
Fourier transform infrared spectroscopy (FTIR)
The extracted PHAs (10 mg) and commercial PHAs were mixed individually with dried potassium bromide (KBr) in a sufficient ratio to make transparent pellet, and it was ground in a mortar and pestle using hydraulic pressure instrument. The pellet was kept in sample holder (FTIR-Miracle 10 Single Reflection ATR Accessory, Shimadzu), and IR rays were passed through it at 400–4000 cm−1 (Getachew and Woldesenbet. 2016). The obtained results were analysed for the determination of functional groups present in the PHAs.
Gas chromatography–mass spectrophotometer (GC–MS) analysis
The monomeric composition of the extracted PHAs was determined by GC–MS analysis as described by Mohandas et al. (2018). Briefly, 10 mg of the extracted polymer was suspended in 1 ml of chloroform and 1 ml of methanol/sulphuric acid (85:15v/v) mixture in a screw capped tube and incubated at 100 °C for 2 h. After cooling, the bottom chloroform phase was separated and used for GC–MS analysis (GC–MS, QP2010 plus, Shimadzu). Helium (1 ml/min) was used as carrier gas. The injector and detector were kept at 250 and 200 °C, respectively.
Results and discussion
Bacterial growth and PHAs yield
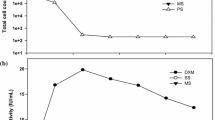
The present study explored the potential of carbon-rich raw/untreated and pre-treated agro-industrial effluents as a substrate for the production of PHAs using B. subtilis RS1. The growth rate of the strain RS1 in raw/untreated and pre-treated agro-industrial effluents was evaluated, and the results are presented in Fig. 1. Results indicated that RS1 was capable of utilizing carbohydrates and polyols present in industrial effluents as a nutrient source for cell growth and PHAs synthesis. The log-phase was attained within 6 h of incubation, and the maximum growth was obtained at 48 h. The decline phase started at 72 h and could be due to lack of adequate nutrients. Most of the Bacillus sp. have intrinsic ability to exploit various inexpensive carbon substrates as nutrients owing to their metabolic activity. Santimano et al. (2009) reported that the Bacillus sp strain COLI/A6 isolated from humus utilized carbon-rich agro-wastes such as starch, wafer residues, citrus pulp waste, and cane molasses as growth supplements and produced higher quantities of PHAs (65.25 ± 0.91%) with 24 h of incubation time. A difference in the growth rate of the strain RS1 was observed between pre-treated and raw/untreated industrial effluents. Increased growth rate in pre-treated industrial effluents could be due to the alteration of pH and removal of residues. The results are consistent with previous study reporting that the alteration of the pH of cane molasses significantly increased the growth rate of B. subtilis and E. coli (Gomaa 2014).
Growth of B. subtilis RS1 in raw and pre-treated agro-industrial effluents (SR raw sugarcane molasses, ST pre-treated sugarcane molasses, DR raw dairy effluents, DT pre-treated dairy effluents, PR raw paper mill effluents, PT pre-treated paper mill effluents). The bacteria exhibited maximum growth in pre-treated sugarcane molasses
DCW and PHAs yield in raw/untreated and pre-treated industrial effluents were evaluated, and the results are presented in Table 1. In general, maximum DCW was observed in pre-treated agro-industrial effluents compared to untreated/raw industrial effluents. DCW of pre-treated sugarcane molasses was 4.5 g/L followed by dairy effluent (3.8 g/L) and paper mill effluent (3.6 g/L), whereas in untreated/raw industrial effluents, it was 2.4, 1.8, and 1.6 g/L, respectively. The increased DCW in pre-treated molasses could be due to the increased availability of nutrients to the bacteria. This was supported by the results from growth studies where increased growth rate of the strain RS1 was observed in pre-treated industrial effluents compared to untreated/raw industrial effluents. The results are consistent with earlier study reporting that the DCW and PHAs yield increased in pre-treated agro-industrial effluents. Gomaa (2014) reported that H2SO4-treated molasses increased the dry cell biomass of bacteria (B. subtilis 10.98 g/L and E. coli 7. 63 g/L) compared to crude (B. subtilis 5.66 g/L and E. coli 3.55 g/L), centrifuged (B. subtilis 7.63 g/L and E. coli 4.22 g/L), and calcium phosphate-treated (B. subtilis 9.44 g/L and E. coli 6.18 g/L) molasses.
The dark-coloured intracellular granules (Fig. 2) in Sudan Black B staining confirmed the production of PHAs by the strain RS1 (Gomaa 2014). The results were further confirmed by crotonic acid assay where a sharp UV absorbance peak was observed at 233 nm (Fig. 3a). The UV absorbance was consistent with the commercial PHAs where the maximum absorbance was observed at 231 nm (Fig. 3b). Ojha and Das (2018) and Salgaonkar and Braganca (2017) reported similar UV absorbance peak (235 nm) for PHAs extracted from Wickerhamomyces anomalus VIT-NN01 and Halogeometricum borinquense E3.
The production of PHAs in raw/untreated and pre-treated agro-industrial effluents using the strain RS1 was evaluated, and the results are shown in Table 1. Results indicated that PHAs production was high in pre-treated effluents compared to raw/untreated effluents. The maximum PHAs yield was observed in pre-treated sugarcane molasses (44.7%) followed by dairy effluent (31.6%) and paper mill effluent (30.6%). However, in raw/untreated effluents, the PHAs yield in sugarcane molasses was 41.7% followed by paper mill effluent (37.5%) and dairy effluent (33.3%). The decreased PHAs yield was due to the limited growth of the strain RS1 in raw/untreated effluents. Excessive proteins and residues present in the raw/untreated effluents may limit the production of PHAs by the strain RS1. The results are in accordance with the previous studies reporting excessive production of PHAs in pre-treated agro-industrial effluents. Acid treatment of cane molasses increased the PHAs yield from 22.61 to 48.26% (Gomaa 2014). Bhattacharyya et al. (2012) reported that 25–50% (v/v) pre-treatment of vinasse maximized PHAs production by 70%.
The influence of incubation time on PHAs production was evaluated, and the results are shown in Table 2. The results revealed that the DCW and PHAs production of the strain RS1 were increased progressively and attained maximum yield (67.6%) at 48 h with a DCW and PHAs accumulation of 8.0 and 5.4 g/L. However, the yield was gradually decreased at 72 (47.6%) and 96 h (44.9%). The decreased PHAs yield at 72 and 96 h could be due to decay in enzyme system for the synthesis of PHAs and the intracellular consumption of PHAs as energy and carbon source. The results are in agreement with previous study reporting the maximum production (8.4 mg/L) of polyhydroxybutyrate after 48 h of incubation, and the production rate was gradually decreased with increase in incubation time (Muralidharan et al. 2013). Based on these results, pre-treated sugarcane molasses and the incubation time of 48 h were used for further optimization studies.
Influence of pH on PHAs yield by B. subtilis RS1
pH has a significant influence on bacterial growth and PHAs yield. The change in the pH is susceptible to the growth of organisms and PHAs yield. The data (Table 3) revealed that the strain RS1 (pre-treated sugarcane molasses; incubation time 48 h) exhibited maximum growth (8.2 g/l DCW) and PHAs yield (56.4%) at pH 7.0 compared to acidic and alkaline pH. pH 7.0, i.e. being neutral, is the most favourable state for the growth and accumulation of PHAs by the strain RS1. Gomaa 2014 reported that pH 7.0 was optimal for PHAs production by B. subtilis using sugarcane molasses as the growth medium. In our study, the PHAs yield decreased to 32.1% and 38.2% at pH 5 and 9. This decreased growth rate and PHAs yield at acidic and alkaline pH could be due to reduced enzyme activities. This observation is in accordance with the results obtained by Nehra et al. (2015) who reported pH 7 as optimal for PHB production. As a result, pH 7.0 was considered as the ideal pH for achieving high yield of PHAs by the strain RS1.
Influence of inoculum dosage on PHAs yield B. subtilis RS1
Inoculum dosage is an important factor that must be carefully optimized in fermentation studies. The effect of inoculum dosage on DCW and PHAs yield by the strain RS1 was evaluated using five different inoculum sizes (5, 10, 15, 20, and 25%) under optimized conditions (raw material, pre-treated sugarcane molasses; incubation time 48 h; pH 7), and the results are presented in Table 4. There was a significant increase in the yield of PHAs from 66.7 to 70.5% as the inoculum size increased from 5 to 10%, followed by a gradual decline in inoculum size. DCW and PHAs yield at 10% inoculum size were 9.5 and 6.7 g/L, respectively. Decreased DCW and PHAs yields at higher inoculum size could be due to the limited availability of oxygen to the bacteria. The results were in accordance with the results obtained by Desouky et al. (2017). Alternatively, the bacterial cells might utilize the accumulated PHAs as a source of carbon and energy after the nutrients in the production media are depleted. However, this phenomenon needs more study.
FTIR and GC–MS
FTIR spectra of commercial and extracted PHAs are shown in Fig. 4. The extracted polymer exhibited the characteristic PHAs signature (Fig. 4a). The broad transmittance peak at 3281 cm−1 could be ascribed to the stretching of O–H groups (Vega-Castro et al. 2016). Peaks observed at 2924 and 2852 cm−1 were associated with the C-H stretching of bonds of methyl (CH3) and methylene (CH2) groups (Shamala et al. 2009). The intense absorption peak at 1722 cm−1 was the characteristic carbonyl (C=O) stretching of ester groups in the extracted PHAs (Vega-Castro et al. 2016; Salgaonkar and Braganca 2017). The peak (1722 cm−1) was comparable with that of the commercial PHAs (Fig. 4b), which further confirms that the extracted polymer was a copolymer of PHAs. The peak at 1635 cm−1 indicates a weak carbonyl (C=O) stretching for conjugated carbonyl or amide group, and a peak at 1539 cm−1 indicates the presence of N–H amide protein in the polymer (Getachew and Woldesenbet. 2016). The series of peaks from 1150 to 1300 cm−1 indicate C–O–C stretching (Vega-Castro et al. 2016), and a peak at 979 cm−1 corresponds to the presence of alkyl halides in the extracted polymer (Shamala et al. 2009). These all prominent absorbance peaks confirmed that the extracted polymer is PHAs. These results were in accordance with the previous study reporting the presence of carbonyl and ester groups in PHAs (Khadeejah et al. 2016).
GC–MS chromatogram of the methanolysis of PHAs extracted from B. subtilis RS1 cultured and processed in a medium of sugarcane molasses (Fig. 5) revealed significant peaks at retention times 20.9, 23.1, and 23.8 min, corresponding to methyl esters of pentadecanoic acid and hexadecanoic acid, respectively. Furthermore, the results revealed that hexadecanoic acid methyl ester is the predominant monomer of PHAs produced from B. subtilis RS1. This observation was in agreement with Gholamveisi et al. (2018) who reported that 3HB (hexadecanoic acid methyl ester) is the major constituent of PHAs extracted from Bacillus thuringiensis strain NG. The PHAs accumulated by the strain RS1 have good functional properties similar to commercial PHAs and thus is a promising candidate for commercial production and applications.
GC–MS chromatograph of PHAs extracted from B. subtilis RS1. The results indicated that the PHAs were composed of methyl esters of pentadecanoic acid and hexadecanoic acid. a GC-MS chromatograph, b retention time, 20.9 min: compound name, pentadecanoic acid methyl ester, c retention time, 23.1 min: compound name, hexadecanoic acid methyl ester, and d retention time, 23.8 min: compound name, hexadecanoic acid methyl ester
Conclusion
The obtained results indicated that pre-treated agro-industrial wastes like sugarcane molasses could be used as an inexpensive substrate for PHAs production using B. subtilis RS1. Optimization studies revealed that under optimized conditions (incubation time 48 h; pH 7; inoculum size 10%) the PHAs yield was increased to 70.5%. The characterization studies confirmed that the polymer was composed of methyl esters of pentadecanoic acid and hexadecanoic acid. Further studies will address film synthesis from extracted PHAs and its characterization and biodegradation.
References
Bhattacharyya A, Pramanik A, Maji SK, Haldar S, Mukhopadhyay UK, Mukherjee J (2012) Utilization of vinasse for production of poly-3-(hydroxybutyrate-co-hydroxyvalerate) by Haloferax mediterranei. AMB Express 2:34–44
Bhuwal AK, Singh G, Aggarwal NK, Goyal V, Yadav A (2014) Poly-β-hydroxybutyrate production and management of cardboard industry effluent by new Bacillus sp. NA10. Bioresour and Bioprocess 1:9–19
Bosco F, Chiampo F (2010) Production of polyhydroxyalcanoates (PHAs) using milk whey and dairy wastewater activated sludge: production of bioplastics using dairy residues. J Biosci Bioeng 109:418–421
Desouky SES, Abdel-Rahman MA, Azab MS, Esmael ME (2017) Batch and fed-batch production of polyhydroxyalkanoates from sugarcane molasses by Bacillus flexusAzu-A2. J Innov Pharm Biol Sci 4:55–66
Dietrich K, Dumont MJ, Del Rio LF, Orsat V (2017) Producing PHAs in the bioeconomy—Towards a sustainable bioplastic. Sustain Prod Consum 9:58–70
Din MFM, Mohanadoss P, Ujang Z, van Loosdrecht M, Yunus SM, Chelliapan S, Olsson G (2012) Development of Bio-PORec® system for polyhydroxyalkanoates (PHA) production and its storage in mixed cultures of palm oil mill effluent (POME). Biores Technol 124:208–216
Getachew A, Woldesenbet F (2016) Production of biodegradable plastic by polyhydroxybutyrate (PHB) accumulating bacteria using low cost agricultural waste material. BMC Res Notes 9:509–518
Gholamveisi N, Azar SM, Moravej R (2018) Bacillus thuringiensis strain NG, a novel isolated strain for production of various polyhydroxyalkanoates. Biol J Microorg 6:13–20
Gomaa EZ (2014) Production of polyhydroxyalkanoates (PHAs) by Bacillus subtilis and Escherichia coli grown on cane molasses fortified with ethanol. Braz Arch Biol Technol 57:145–154
Heinrich D, Madkour MH, Al-Ghamdi MA, Shabbaj II, Steinbüchel A (2012) Large scale extraction of poly (3-hydroxybutyrate) from Ralstonia eutropha H16 using sodium hypochlorite. AMB Express 2:59–64
Khadeejah ONN, Shittu KO, Kabiru AY (2016) Production and characterization of polyhydroxyalkanoate (PHA) using mango seed kernel as an alternative to glucose. Br Biotechnol J 13:1–11
Khiyami MA, Al-Fadual SM, Bahklia AH (2011) Polyhydroxyalkanoates production via Bacillus plastic composite support (PCS) biofilm and date palm syrup. J Med Plants Res 5:3312–3320
Kourmentza C, Koutra E, Venetsaneas N, Kornaros M (2017) Integrated biorefinery approach for the valorization of olive mill waste streams towards sustainable biofuels and bio-based products. Microbial Appl 1:211–238
Law JH, Slepecky RA (1961) Assay of poly-β-hydroxybutyric acid. J Bacteriol 82:33–36
Mohandas SP, Balan L, Jayanath G, Anoop BS, Philip R, Cubelio SS, Singh IB (2018) Biosynthesis and characterization of polyhydroxyalkanoate from marine Bacillus cereus MCCB 281 utilizing glycerol as carbon source. Int J Biol Macromol 119:380–392
Mohapatra S, Sarkar B, Samantaray DP, Daware A, Maity S, Pattnaik S, Bhattacharjee S (2017) Bioconversion of fish solid waste into PHB using Bacillus subtilis based submerged fermentation process. Environ Technol 38:3201–3208
Muhammadi Shabina, Afzal M, Hameed S (2015) Bacterial polyhydroxyalkanoates-eco-friendly next generation plastic: production, biocompatibility, biodegradation, physical properties and applications. Green Chem Lett Rev 8:56–77
Munir S, Iqbal S, Jamil N (2015) Polyhydroxyalkanoates (PHA) production using paper mill wastewater as carbon source in comparison with glucose. J Pure Appl Microbiol 9:453–460
Muralidharan R, Sindhuja PB, Sudalai A, Radha KV (2013) Polyhydroxybutyrate production accompanied by the effective reduction of chemical oxygen demand (COD) and biological oxygen demand (BOD) from industrial effluent. Korean J Chem Eng 30:2191–2196
Nehra K, Jaglan A, Shaheen A, Yadav J, Lathwal P (2015) Manpret, production of poly-β-hydroxybutyrate (PHB) by bacteria isolated from rhizospheric soils. Int J Microbial Resour Technol 2:38–48
Obruca S, Benesova P, Marsalek L, Marova I (2015) Use of lignocellulosic materials for PHA production. Chem Biochem Eng Q 29:135–144
Ojha N, Das N (2018) A statistical approach to optimize the production of polyhydroxyalkanoates from Wickerhamomyces anomalus VIT-NN01 using response surface methodology. Int J Biol Macromol 107:2157–2170
Pandian SR, Deepak V, Kalishwaralal K, Rameshkumar N, Jeyaraj M, Gurunathan S (2010) Optimization and fed-batch production of PHB utilizing dairy waste and sea water as nutrient sources by Bacillus megaterium SRKP-3. Biores Technol 101:705–711
Rathika R, Soumiyamithran Saraswathi U, Manonmani S, Kamala Kannan S, Shanthi K (2018) Optimization of polyhydroxyalkanote (PHA) synthesis from Bacillus subtilis RS1. Asian J Microbiol Biotechnol Environ Sci 20:165–171
Salgaonkar BB, Braganca JM (2017) Utilization of sugarcane bagasse by Halogeometricum borinquense strain E3 for biosynthesis ofpoly(3-hydroxybutyrate-co-3-hydroxyvalerate). Bioengineering 4:50–68
Santimano MC, Prabhu NN, Garg S (2009) PHA production using low cost agro industrial wastes by Bacillus sp. strain COL1/A6. Res J Microbiol 4:89–96
Shamala TR, Divyashree MS, Davis R, Kumari KL, Vijayendra SV, Raj B (2009) Production and characterization of bacterial polyhydroxyalkanoate copolymers and evaluation of their blends by Fourier transform infrared spectroscopy and scanning electron microscopy. Indian J Microbiol 49:251–258
Valentino F, Riccardi C, Campanari S, Pomata D, Majone M (2015) Fate of β-hexachlorocyclohexane in the mixed microbial cultures (MMCs) three-stage polyhydroxyalkanoates (PHA) production process from cheese whey. Biores Technol 192:304–311
Vega-Castro O, Contreras-Calderon J, Leon E, Segura A, Arias M, Perez L, Sobral PJ (2016) Characterization of a polyhydroxyalkanoate obtained from pineapple peel waste using Ralsthonia eutropha. J Biotechnol 231:232–238
Acknowledgement
This paper was supported by research funds of Chonbuk National University in 2017. The author (SKK) is grateful to the Head and faculties of Department of Environmental Sciences, PSG College of Arts and Science, Coimbatore, Tamil Nadu, India for their valuable support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they do not have any conflict of interest.
Additional information
Editorial responsibility: J Aravind.
Rights and permissions
About this article
Cite this article
Rathika, R., Janaki, V., Shanthi, K. et al. Bioconversion of agro-industrial effluents for polyhydroxyalkanoates production using Bacillus subtilis RS1. Int. J. Environ. Sci. Technol. 16, 5725–5734 (2019). https://doi.org/10.1007/s13762-018-2155-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-018-2155-3