Abstract
To retrospectively assess the predictive value of the CT performed at 24 h, compared with the CT performed at 1 month, in the evaluation of the technical success of microwave (MW) ablation of hepatic lesions. In a single center, 50 patients with HCC underwent percutaneous MW ablation between November 2016 and March 2019. Each patient underwent a contrast-enhanced CT exam at 24 h and at 1 month after the procedure. For each patient, was assessed the presence or absence of residual disease, the appearance of a new lesion, complications, and the involvement of the hepatic capsule, both at 24-h and at 1 month. Overall correlation between residual disease, appearance of a new nodule and complications was also assessed. A total of 50 hepatic lesions were treated with US-guided MW ablation. Patients’ mean age was 70.9 years (range 28–87 years). Mean nodule diameter was 17.6 mm (range 7–35 mm). Contingency tables and the χ2 test showed a strong association when looking at capsule involvement (accuracy: 100%), residual disease (accuracy: 90%; p-value 0.003), and the appearance of a new HCC nodule (accuracy: 88%; p-value 0.007); regarding complications, the accuracy was 78% (p-value 0.014). Optimal correlation was reached in 62% of cases, moderate correlation in 26%, minimum correlation in 10% of cases; no cases of zero correlation were recorded. CT at 24 h and 1 month showed comparable efficacy in evaluating residual disease after MW thermal ablation of liver lesions. However, further studies are needed to assess which factors may cause false-negative results at the 24-h CT.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Hepatocellular carcinoma is the fifth most common cancer type, and the second most common cause of cancer-related death globally [1].
The management of HCC is rapidly evolving due to newly introduced novel therapeutic approaches. Imaging-guided ablation gives curative treatment in adequately selected patients or appropriate therapeutic options whether surgical resection or liver transplantation is precluded [2]. Among percutaneous techniques, microwave (MW) ablation is gaining popularity in the ablation of liver tumors with the hope of capitalizing on its potential benefits over RF ablation. These include no need for grounding pads, less susceptibility to the heat sink phenomenon, larger ablation zones, shorter ablation times, and possibly better local tumor control [3].
An early and accurate evaluation of tumor response is essential to assess the efficacy of percutaneous treatments. Current imaging modalities, such as computed tomography (CT) and magnetic resonance (MR) provide reliable and reproducible images to demonstrate tumor burden changes [4]. Traditionally, therapeutic response has been assessed by serial tumor burden measurements according to Response Evaluation Criteria in Solid Tumors (RECIST) or World Health Organization (WHO) criteria [1, 4], which are mainly based on size measurement. However, in liver-directed treatments, simple anatomical changes are not enough informative [4] and there has been a growing interest to monitor an early response of treatment by measuring tumor viability and/or tumor perfusion. Therefore, it was introduced the concept of tumor enhancement in arterial phase of contrast-enhanced imaging studies to characterize only viable target tumors [5] that lead to the drafting of new guidelines by the European Association for the Study of the Liver (EASL) and American Association for the Study of the Liver Diseases (AASLD). Viable tumor should be defined as uptake of contrast agent in the arterial phase. Consequently, a modification of the RECIST criteria (mRECIST criteria) has been adopted [6].
Nevertheless, optimal posttreatment surveillance schedules have been developed for other malignancies, including testicular cancer [7, 8], but not for HCC. In general, guidelines suggest the first follow-up to be at 1 or 2 months after the procedure to evaluate response but there is still no consensus about an ideal schedule and when the patient should undergo the first control to assess the complete response [9,10,11].
The aim of this study is to assess the predictive value of the CT performed at 24 h, compared with the CT performed at 1 month, in the evaluation of the primary technical efficacy (PTE) of microwave (MW) ablation of hepatic lesions.
Materials and methods
Population
This was a retrospective study and the approval of our Internal Review Board was waived.
Data regarding all patients who underwent percutaneous US-guided MWA for HCC nodules at our institution between November 2016 and July 2019 were collected.
Eligible patients were at least 18 years of age with primary non-extrahepatic liver disease only.
Patients whose preoperative CT or MRI exams that could not be reviewed were excluded.
Patients who could not come to our Institution to perform post-procedural follow-up examinations either at 24 h or at 1 month were also excluded.
The resulting population was composed of 50 patients with 1 nodule for person.
For each patient, the following data were registered: age, sex, liver status (normal, hepatitis, or cirrhosis), etiology of liver disease (alcohol, HBV, HCV, non-alcoholic steatohepatitis), and Child Pugh classification.
Images from the pre-procedural exam, either CT or MRI within 1 month prior to procedure, were reviewed for each patient, and the following data were registered for each HCC nodule: maximum dimension, hepatic segment, and distance from capsule.
MWA procedure
Informed consent was obtained from all patients before procedure.
An Anesthesiologist assisted the patient during the whole ablation session.
Moderate sedation was achieved in each patient through intravenous injection of propofol (0.5–2.0 mg/kg/h), fentanyl (1–2 mcg/kg), and midazolam (0.07–0.08 mg/kg). Vital parameters (heart rate, respiratory rate, blood pressure), together with oxygen saturation and electrocardiographic tracing, were continuously monitored during the procedure.
All patients received an antibiotic prophylaxis according to an “ultra-short-term” scheme that consists in preprocedural intravenous administration of cefazolin 1 g, to obtain the drug’s plasmatic concentration peak at the time of ablation and derived cellular necrosis.
10.0 ml of lidocaine 2% solution was injected to obtain local anesthesia in correspondence of the entrance site of the antenna that was positioned under US-guidance (Emprint™ Microwave Ablation System, Covidien, Boulder, CO, USA) in all cases.
The ablation system consisted of a microwave generator, capable of producing a power of 100 W at 2450 MHz, connected to a 13.5-gauge straight microwave antenna with a 2.8-cm radiating section by coaxial cable. Continuous perfusion with saline solution at 60 ml/min and at room temperature was provided by the system along the proximal part of the antenna to avoid any thermal damage.
Ablation power and time were decided according to data given by the manufacturer and to operator’s preference. The ablation field was assessed continuously by US during the whole procedure.
Ablation power and time were registered for each patient.
CT protocol
Each patient underwent a contrast-enhanced CT exam 24 h and 1 month (range 24–37 days) after the procedure.
CT protocol consisted in a standard multiphase contrast-enhanced CT (LightSpeed VCT 64; GE Medical Systems, Milwaukee, Wisconsin, USA).
The scans were acquired as routine follow-up with the following parameters: 210 mA, 120 kV, 2.5 mm collimation, and pitch of 1.0. For contrast-enhanced CT scans, a body weight adjusted amount of contrast agent (Ultravist 370, Bayer Vital GmbH, Leverkusen, Germany) was injected intravenously with a flow rate of 4 ml/s followed by a 30 ml saline chaser bolus. Scans were started with an 18 s delay for the arterial phase and 45 s delay for the venous phase using the bolus tracking method (threshold, 100 HU).
Reports by three radiologists with respectively 4, 10, and 18 years of experience in hepatobiliary imaging were subsequently reviewed.
Outcome and analysis of predictors of outcomes and risk factors for complications
The presence/absence of Residual Disease, appearance of a new HCC nodule, complications, and hepatic capsule involvement were evaluated.
Primary technical effectiveness (PTE) was defined as the absence of any residual disease at follow-up images.
Residual Disease (RS) was defined as the presence of enhancing tissue into or about the ablation field.
“Optimal correlation” was defined as the complete correspondence between the CT scans at 24 h and at 1 month of all of the following: residual disease/PTE, appearance of a new nodule, and complications.
The images at 24 h and at 1 month were compared to the preprocedural ones to assess the involvement of the hepatic capsule, which occurred if there was no apparent cleavage plane between the ablated area and the hepatic capsule.
A new HCC nodule was defined as appearance of a lesion with HCC features in a different area from the treated zone.
Complications were classified according to Common Terminology Criteria for Adverse Events [12].
Major complications were defined as events that could have threatened life if left untreated, or that caused substantial morbidity and disability, or hospital re-admission, or a significant lengthening of the patient’s hospital stay [13].
Minor complications included characteristic symptoms of the post-ablation syndrome (fever, pain, nausea, and vomiting, perihepatic fluid collection, when < 3.0 cm) if lasting more than 4 days after the ablation procedure.
Statistical analysis
The concordance between CT scan result at 24 h and 1 month was evaluated by contingency table, Fisher's exact test and χ2 test.
True and False Positive (TP, FP), True and False Negative (TN, FN), Sensibility, Specificity, Positive Predictive Value (PPV), Negative Predictive Value (NPV) and Accuracy were calculated for all nominal variables. SPSS v25.0.0 (IBM, Armonk, New York, USA) was used for all statistical analyses. p-value were considered significant when < 0.05.
Results
Descriptive data regarding patients, tumors, procedure, and complications are reported in Table 1.
Mean patients’ age was 70.6 years old (range 28–88 years old). Regarding Child Pugh score, patients were classified as A in 83.3% of cases (40/48), and B in 16.6% (8/48); for two patients (2/48), Child Pugh score data were not available. Mean nodule diameter resulted 17.6 mm (range 7–35 mm).
Mean ablation time was 03:37 min (range 06:30–01:30 min). The generator was always used at maximum power, 100 W.
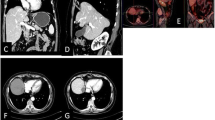
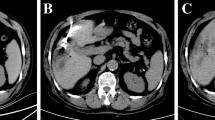
Sign of residual disease (Figs. 1,2) were reported in 8% of cases (4/50) at 24 h CT and in 10% of cases (5/50) at 1 month CT.
Overall complications rate was 26% (14/50) at 24 h and 18% (9/50) at 1 month. No signs of severe complications were reported at 24 h (0/50); 1 case of severe complications 2% (1/50) was reported at 1 month (duodenal perforation) (Fig. 3).
A new nodule appearance was reported in 6% of cases (3/50) at 24 h CT and in 14% of cases (7/50) at 1 month CT.
Capsule involvement was reported in 18% of cases (9/50) both at 24 h and at 1 month CT.
Contingency table and Fisher's exact test results showed a strong association between CT scan at 24 h and at 1 month from treatment when looking at capsule involvement (accuracy: 100%), residual disease (accuracy 90.0%, p-value 0,005) and appearance of a new HCC nodule (accuracy 88.0%, p-value 0.007). Accuracy for complications was 76% (p-value 0.073) (Table 2); statistical analysis showed no association for severe complication rate.
Optimal correlation was reached in 62% of cases, moderate correlation in 26%, minimum correlation in 10% of cases; no cases of zero correlation were recorded (Table 3).
Discussion
The goal of follow-up imaging after liver-directed therapy for hepatocellular carcinoma (HCC), including trans-arterial chemoembolization, radioembolization, and MW ablation, is to detect residual or recurrent disease that requires additional treatment [14]. Earlier detection has proved to result in better outcomes [1, 15], but no surveillance schedule has demonstrated to be effective and reliable for HCC surveillance.
In general, proposed surveillance schedules after liver-directed therapy include surveillance at 1 month and every 3 months thereafter, or with the interval stretched to every 6 months after 1 year after treatment [16, 17].
When the guidelines are analyzed individually, however, what is evident is a lack of uniformity among them.
Some authors [14, 18, 19] suggest to perform the first follow-up CT approximately 1 month after ablation. Others prefer to perform the first CT scan immediately after ablation and then 1 month after the procedure [20].
CIRSE guidelines for RFA [10], which are currently under revision, recommend to perform contrast-enhanced CT or MRI 4–6 weeks after treatment to assess successful ablation.
AASLD, EASL, NCCN, The Italian Association for the Study of the Liver (AISF), ESMO guidelines and mRECIST [1, 6, 9, 11, 21, 22] do not mention any specific follow-up schedule after thermal ablation to evaluate response to treatment.
Although some papers state that at about 1–3 weeks after the ablation portal venous phase imaging allows better assessment of the treatment because there is sufficient time for the ablation zone to mature and become better defined [23,24,25], we believe that CT scan 24 h after ablation can be as informative as the one performed at 4–6 weeks.
The aim of this study is to assess the predictive value of the CT performed at 24 h, compared with the CT performed at 1 month, in the evaluation of primary technical effectiveness of MW ablation of hepatic lesions.
To the best of our knowledge, this is the first study that tries to evaluate the predictive value of the CT scan performed at 24 h compared with the CT scan at 1 month, which nowadays can be considered as the standard of care to evaluate the response to treatment.
This retrospective analysis consists of a series of 50 patients, all with a diagnosis of HCC.
The results showed a strong correlation between the CT scan at 24 h and at 1 month regarding the assessment of the residual disease. Of 50 patients treated with MW, in five cases the 24-h and 1-month CT scans were inconsistent in the assessment of treatment response. In particular, CT scan at 24 h shows an NPV of 93% with a 90% accuracy (p-value 0.005) in excluding a residual disease.
Despite being only a preliminary result, this finding may represent a major change in the management of the patient with HCC treated with MW ablation.
These patients, at present, must wait at least 1 month to be assured regarding the technical success. With this particular protocol, instead, after only 24 h it is possible to get a very precise indication. The next follow-up image would be then scheduled directly after three months, to proceed then with the regular follow-up as indicated by the international guidelines.
This may represent a significant change in the first place for the interventional radiologist who carried out the procedure who after only 24 h, may become aware of the technical result of the intervention and, in case of signs of residual disease, set up a different strategy to perform a radical intervention (i.e., new percutaneous ablation, chemoembolization).
Also for the patient itself there are some potential benefits: during the course of the admission, he may become aware of the possible success of the procedure and the subsequent check-up would be at three months, thus guaranteeing him more time for himself without anticipatory anxiety for the close follow-up.
The analysis of complications between the two groups did not yield statistically significant results. In particular, the NPV of CT at 24 h in the identification of complications was 87% with an accuracy of 76% (p-value 0.073).
This result, however, should not be at all surprising, particularly if we highlight the fact that in our case history there was only one case of severe complication (duodenal perforation).
In that case, the 24-h CT scan had already shown edema of the duodenal walls, but the clinical picture manifested itself with acute abdominal pain only after 35 days.
All other complications were classified as minor, which did not require any further treatment. Therefore, in our opinion, it is entirely justifiable fact that some of them are no longer evident after 1 month.
Another parameter that we considered was capsular involvement. Subcapsular nodules are considered harder to treat because no safety margin along the capsule is possible. In the treatment of subcapsular tumors, ablation of the capsule is recommended when there is evidence of capsular involvement by the tumor [26, 27]. Therefore, we chose to analyze this parameter very closely.
In all cases with capsule involvement at the 1-month CT scan (9 patients) that was also evident at 24 h, thus, resulting in a NPV and an accuracy of 100%.
The appearance of a new nodule in a different part of the liver, not present at previous imaging was also considered. The analysis of this parameter between the two groups did yield statistically significant results. In particular, the NPV of CT at 24 h in the identification of a new nodule was 89% with an accuracy of 88% (p-value 0.007). Still, PPV was only 67% and five nodules were missed at the 24 h CT scan. This factor could have serious implications since it could delay the diagnosis by two months if the 1-month CT scan were omitted to directly perform the one at 3 months.
Nevertheless, this may not impact patients’ prognosis, as based on the actual guidelines [1] and on the available knowledge on mean HCC volume doubling time [28, 29], a 6-month interval represents a reasonable choice of follow-up schedule, since a shorter interval did not translate into any clinical benefit [1].
Last parameter that was considered was the so-called “optimal correlation”. Complete agreement at 24 h and at 1 month for residual disease, complications, and appearance of a new nodule was reached in 64% of cases; moderate agreement (2/3 parameters) was reached in 26% of cases, minimum agreement (1/3 parameters) in 10% of cases (Table 3.).
This finding shows that the 24-h CT images cannot be easily superimposed on the 1-month CT images, in terms of overall pathological findings, despite the reliable result regarding the residual disease.
This study has several limitations, first of all the small population. Second, no comparison was made with other treatments, like RFA or laser ablation, and larger studies are required to confirm or contradict our results that can only be considered preliminary.
In addition, the images were reviewed by three radiologists with different level of experience, which could also impact our results. Also, only CT scan was considered and other imaging modality like MRI or contrast-enhanced US have not been taken into account. Last, other parameters like the nodule location, distance to the hilum or patients’ specific laboratory and clinical characteristics were not considered for the statistical analysis.
In conclusion, CT at 24-h and 1 month showed comparable results in evaluating residual disease/primary technical efficacy after MW thermal ablation of liver lesions. Further studies are necessary to understand what may cause false-negative results at 24-h and whether the 24 h CT scan may be considered as a fully legitimate follow-up image after thermal ablation of hepatic lesions, waiving the need to perform the CT scan after 1 month and waiting 3 months to execute the second follow-up.
References
Galle PR, Forner A, Llovet JM, Mazzaferro V, Piscaglia F, Raoul JL, Schirmacher P, Vilgrain V. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69:182–236. https://doi.org/10.1016/j.jhep.2018.03.019.
Galun D, Basaric D, Zuvela M, Bulajic P, Bogdanovic A, Bidzic N, Milicevic M. Hepatocellular carcinoma: from clinical practice to evidence-based treatment protocols. World J Hepatol. 2015;7:2274. https://doi.org/10.4254/wjh.v7.i20.2274.
Shady W, Petre EN, Do KG, Gonen M, Yarmohammadi H, Brown KT, Kemeny NE, D’Angelica M, Kingham PT, Solomon SB, Sofocleous CT. Percutaneous microwave versus radiofrequency ablation of colorectal liver metastases: ablation with clear margins (A0) provides the best local tumor control. J Vasc Interv Radiol. 2018;29:268–275.e1. https://doi.org/10.1016/j.jvir.2017.08.021.
Arora A, Kumar A. Treatment response evaluation and follow-up in hepatocellular carcinoma. J Clin Exp Hepatol. 2014;4:S126–S129129. https://doi.org/10.1016/j.jceh.2014.05.005.
Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, Burroughs AK, Christensen E, Pagliaro L, Colombo M, Rodés J. Clinical Management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. J Hepatol. 2001;35:421–30. https://doi.org/10.1016/S0168-8278(01)00130-1.
Lencioni R. New data supporting modified RECIST (mRECIST) for hepatocellular carcinoma. Clin Cancer Res. 2013;19:1312–4. https://doi.org/10.1158/1078-0432.CCR-12-3796.
Smith TJ. Breast cancer surveillance guidelines. J Oncol Pract. 2013;9:65–7. https://doi.org/10.1200/JOP.2012.000787.
Lieng H, Warde P, Bedard P, Hamilton RJ, Hansen AR, Jewett MAS, O’malley M, Sweet J, Chung P. Recommendations for followup of stage I and II seminoma: the Princess Margaret Cancer Centre approach. Can Urol Assoc J. 2018;12:59–66. https://doi.org/10.5489/cuaj.4531.
Vogel A, Cervantes A, Chau I, Daniele B, Llovet JM, Meyer T, Nault JC, Neumann U, Ricke J, Sangro B, Schirmacher P, Verslype C, Zech CJ, Arnold D, Martinelli E. Hepatocellular carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2019;30:871–3. https://doi.org/10.1093/annonc/mdy510.
Crocetti L, De Baere T, Lencioni R. Quality improvement guidelines for radiofrequency ablation of liver tumours. Cardiovasc Intervent Radiol. 2010;33:11–7. https://doi.org/10.1007/s00270-009-9736-y.
Bolondi L, Cillo U, Colombo M, Craxì A, Farinati F, Giannini EG, Golfieri R, Levrero M, Daniele Pinna A, Piscaglia F, Raimondo G, Trevisani F, Bruno R, Caraceni P, Ciancio A, Coco B, Fraquelli M, Rendina M, Squadrito G, Toniutto P. Position paper of the Italian Association for the Study of the Liver (AISF): the multidisciplinary clinical approach to hepatocellular carcinoma. Dig Liver Dis. 2013;45:712–23. https://doi.org/10.1016/j.dld.2013.01.012.
D. NCI, NIH (2009) Common terminology criteria for adverse events v4.0. NIH Publ. pp 0–71
Filippiadis DK, Binkert C, Pellerin O, Hoffmann RT, Krajina A, Pereira PL. Cirse quality assurance document and standards for classification of complications: the cirse classification system. Cardiovasc Intervent Radiol. 2017;40:1141–6. https://doi.org/10.1007/s00270-017-1703-4.
Boas FE, Do B, Louie JD, Kothary N, Hwang GL, Kuo WT, Hovsepian DM, Kantrowitz M, Sze DY. Optimal imaging surveillance schedules after liver-directed therapy for hepatocellular carcinoma. J Vasc Interv Radiol. 2015;26:69–73. https://doi.org/10.1016/j.jvir.2014.09.013.
Santi V, Trevisani F, Gramenzi A, Grignaschi A, Mirici-Cappa F, Del Poggio P, Di Nolfo MA, Benvegnù L, Farinati F, Zoli M, Giannini EG, Borzio F, Caturelli E, Chiaramonte M, Bernardi M. Semiannual surveillance is superior to annual surveillance for the detection of early hepatocellular carcinoma and patient survival. J Hepatol. 2010;53:291–7. https://doi.org/10.1016/J.JHEP.2010.03.010.
Lim HK, Choi D, Lee WJ, Kim SH, Lee SJ, Jang H-J, Lee J-H, Lim JH, Choo IW. Hepatocellular carcinoma treated with percutaneous radio-frequency ablation: evaluation with follow-up multiphase helical CT. Radiology. 2001;221:447–54. https://doi.org/10.1148/radiol.2212010446.
Lewandowski RJ, Kulik LM, Riaz A, Senthilnathan S, Mulcahy MF, Ryu RK, Ibrahim SM, Sato KT, Baker T, Miller FH, Omary R, Abecassis M, Salem R. A comparative analysis of transarterial downstaging for hepatocellular carcinoma: chemoembolization versus radioembolization. Am J Transpl. 2009;9:1920–8. https://doi.org/10.1111/j.1600-6143.2009.02695.x.
Liu W, Zheng Y, Zou R, Shen J, He W, Yang Z, Zhang Y, Li B, Yuan Y. Impact of follow-up interval on patients with hepatocellular carcinoma after curative ablation. BMC Cancer. 2018;18:1–9. https://doi.org/10.1186/s12885-018-5069-z.
Yaghmai V, Besa C, Kim E, Gatlin JL, Siddiqui NA, Taouli B. Imaging assessment of hepatocellular carcinoma response to locoregional and systemic therapy. Am J Roentgenol. 2013;201:80–96. https://doi.org/10.2214/AJR.13.10706.
Goldberg SN, Grassi CJ, Cardella JF, Charboneau JW, Dodd GD, Dupuy DE, Gervais DA, Gillams AR, Kane RA, Lee FT, Livraghi T, McGahan J, Phillips DA, Rhim H, Silverman SG, Solbiati L, Vogl TJ, Wood BJ, Vedantham S, Sacks D. Image-guided tumor ablation: standardization of terminology and reporting criteria. J Vasc Interv Radiol. 2009;20:S377–S390390. https://doi.org/10.1016/j.jvir.2009.04.011.
Heimbach JK, Kulik LM, Finn RS, Sirlin CB, Abecassis MM, Roberts LR, Zhu AX, Murad MH, Marrero JA. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67:358–80. https://doi.org/10.1002/hep.29086.
Benson AB, D’Angelica MI, Abbott DE, Abrams TA, Alberts SR, Anaya DA, Are C, Brown DB, Chang DT, Covey AM, Hawkins W, Iyer R, Jacob R, Karachristos A, Kelley RK, Kim R, Palta M, Park JO, Sahai V, Schefter T, Schmidt C, Sicklick JK, Singh G, Sohal D, Stein S, Tian GG, Vauthey J-N, Venook AP, Zhu AX, Hoffmann KG, Darlow S. NCCN guidelines insights: hepatobiliary cancers, version 1.2017. J Natl Compr Cancer Netw. 2017;15:563–73. https://doi.org/10.6004/jnccn.2017.0059.
Puijk RS, Ruarus AH, Scheffer HJ, Vroomen LGPH, van Tilborg AAJM, de Vries JJJ, Berger FH, van den Tol PMP, Meijerink MR. Percutaneous liver tumour ablation: image guidance, endpoint assessment, and quality control. Can Assoc Radiol J. 2018;69:51–62. https://doi.org/10.1016/j.carj.2017.11.001.
Liang P, Yu J, De Lu M, Dong BW, Yu XL, Zhou XD, Hu B, Xie MX, Cheng W, He W, Jia JW, Lu GR. Practice guidelines for ultrasound-guided percutaneous microwave ablation for hepatic malignancy. World J Gastroenterol. 2013;19:5430–8. https://doi.org/10.3748/wjg.v19.i33.5430.
Patel N, King AJ, Breen DJ. Imaging appearances at follow-up after image-guided solid-organ abdominal tumour ablation. Clin Radiol. 2017;72:680–90. https://doi.org/10.1016/j.crad.2017.01.014.
Venkatesan AM, Gervais DA, Mueller PR. Percutaneous radiofrequency thermal ablation of primary and metastatic hepatic tumors: current concepts and review of the literature. Semin Intervent Radiol. 2006;23:73–84. https://doi.org/10.1055/s-2006-939843.
Rhim H, Goldberg SN, Dodd GD, Solbiati L, Lim HK, Tonolini M, Cho OK. Essential techniques for successful radio-frequency thermal ablation of malignant hepatic tumors. RadioGraphics. 2001;21:S17–S35. https://doi.org/10.1148/radiographics.21.suppl_1.g01oc11s17.
Ebara M, Hatano R, Fukuda H, Yoshikawa M, Sugiura N, Saisho H. Natural course of small hepatocellular carcinoma with underlying cirrhosis. A study of 30 patients. Hepatogastroenterology. 1998;45(Suppl 3):1214–20.
Barbara L, Benzi G, Gaiani S, Fusconi F, Zironi G, Siringo S, Rigamonti A, Barbara C, Grigioni W, Mazziotti A, Bolondi L. Natural history of small untreated hepatocellular carcinoma in cirrhosis: A multivariate analysis of prognostic factors of tumor growth rate and patient survival. Hepatology. 1992;16:132–7. https://doi.org/10.1002/hep.1840160122.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fumarola, E.M., Ierardi, A.M., Biondetti, P. et al. Follow-up of percutaneous microwave (MW) ablation of hepatic lesion: predictive value of CT at 24-h compared with CT at 1 month. Med Oncol 37, 41 (2020). https://doi.org/10.1007/s12032-020-01364-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-020-01364-y