Abstract
Background
Microwave ablation (MWA) is an emerging treatment for treatment of patients with hepatocellular carcinoma (HCC) not amenable of surgical resection.
Patients and Methods
We searched for patients diagnosed as having small-, medium-, and large HCCs treated with MWA under CT guidance between 2010 and 2014. The main outcomes of interest were rates of complete ablation, complications, and overall survival. Rates of complete ablation were compared with Chi-square test, and estimated survival rates were calculated by means of Kaplan-Meier method.
Results
Thirty-two patients with 45 HCC nodules received MWA. Seventeen (37.8%) nodules were <3 cm (small), 15 (33.3%) between 3 and 5 cm (medium), and 13 (28.9%) > 5 cm (large). Complete ablation was obtained in 94.1% of small tumors, 80% of medium tumors, and 53.8% of large tumors (p = 0.03). Two patients had HCC located in risk area (paracardiac position). Minor complications occurred after seven procedures (15.5%). Estimated median survival was 37 months (95% confidence interval 11.97–62.02). One-year OS was 82.7%, 2-year survival 68.9%, and 3-year survival 55.2%.
Conclusion
MWA is a versatile ablative method that can be applied in HCC at various stages, and also in lesions located in risk areas.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hepatocellular carcinoma (HCC) accounts for 5–6% of cancers diagnosed worldwide and represents one of the leading causes of cancer-related death [1, 2]. Surgical resection and orthotopic liver transplantation offer the highest survival rates. However, less than 30% of patients with HCC are surgical candidates [2,3,4,5], as several conditions (e.g., locally advanced or metastatic disease, poor liver function, underlying comorbidities, and poor performance status) preclude surgical treatment. As a result, in the last decade, there has been a surge of ablative treatments for HCC, which have been shown to be effective in patients not suitable for surgery [6,7,8,9].
Among them, microwave ablation (MWA) can be considered a relatively new method, which has showed promising results in ablation of liver tumors. One or more microwave antennas are inserted (generally with percutaneous technique) inside the tumor mass. The electromagnetic microwaves generate thermal energy that results in coagulative necrosis of the tumor and surrounding parenchyma [6, 10,11,12,13].
Literature reports that MWA has been increasingly used for treatment of hepatic malignancies, especially in Asian countries and USA, while data series from European institutions are scarce. The scope of the present article was to report on the CT-guided MWA treatment of HCC at a single European institution, focusing on the technical aspects and short-term outcomes.
Patients and Methods
Patients
In the last 10 years, several techniques were used at our institution for thermal ablation of either primary or metastatic malignancies in the hepato-biliary pancreatic area, including radiofrequency ablation (RFA), cryoablation, and MWA [14]. For the purpose of this study, a retrospective institutional review was conducted on HCC patients receiving MWA between the time period 2010 and 2014. Indications for MWA included the following: unresectable HCC (because of either tumor location or general performance status), multicentric HCC (up to three nodules), or patients’ refusal of surgical operations. Vascular invasion at CT scan or MRI was considered a contraindication to MWA. The following data were extrapolated for the entire study population: age, sex, and tumor characteristics (size, number and localization of lesions, presence of distant metastases, use of postoperative chemotherapy). The operation notes and case notes were used to evaluate the technique of the ablative MWA procedures, associated procedures for other cancer localizations, and complications. Follow-up data evaluated included the time to local recurrence, time to distant mestastases, and death.
Preoperative Evaluation and MWA Procedure
All patients signed informed consent before the interventional radiology treatment. Liver ultrasound (US), 3-phase contrast enhanced liver CT, and total body CT scan were performed preoperatively in all patients. Each case was discussed during a multi-disciplinary tumor board with the involvement of hepato-biliary surgeons, medical oncologists, and interventional radiologists. A Child-Pugh class A or B, a platelet count >100,000/mm3, and a prothrombine time > 65% were required to receive liver MWA. We considered as “small” the HCC nodules measuring 3 cm or smaller, as “medium” those from 3 to 5 cm, and as “large” those larger than 5 cm. Two patients of the latter group were diagnosed as having distant metastases. Both of them were submitted to MWA with the main aim of either obtaining a cytoreduction and relieving of tumor-related symptoms.
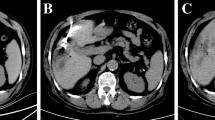
A board-certified interventional radiologist with more than 10 years of experience performed all treatments. MWA was carried out percutaneously under CT guidance (CT device with 5-mm collimation at 80–140 mA SOMATOM Sensation, Siemens, Forcheim, Germany), by using a 2.45 GHz microwave generator (AMICA-GEN; HS Hospital Service, Aprilia, Italy) with energy delivered via one or two 14-gauge, mini-choked, water-cooled interstitial antennae (HSAMICA; HS Hospital Service). In case of synchronous, double-antenna MWA procedures, two generators were used, each driving an individual antenna. The combined action of the patented mini-choke (a miniaturized quarter-wave impedance transformer for reflected wave trapping) and probe shaft cooling system (>50 mL/min of saline solution perfused by an automatic peristaltic pump built into the microwave generator) prevented the back-heating phenomena typical of conventional MWA systems. Throughout the procedure, patients were under conscious sedation, usually achieved through continuous intravenous infusion of fentanyl 0.1 mg/2 mL diluted 1:10 with saline solution and through local anesthesia comprising subcutaneous injection of 2% lidocaine hydrochloride. The choice of probe was at the discretion of the interventional radiologist and was based on several factors, including, but not limited to, tumor size, morphology, location, adjacent structures, and access route. A single14-gauge antenna was used to treat tumors less than 3.5 cm in maximal diameter (51.1% of the ablative sessions), whereas to treat tumors at least 3.5 cm in maximal diameter (48.9%), two antennae were operated simultaneously, according to the operator’s judgment. When the antenna was within the tumor, the introducer was retracted before energy delivery was initiated so as not to interfere with microwave emissions by the active probe tip. We used 20-cm-long microwave antennas to allow a sufficient retraction of the cannula from the ablation area. Two explicative cases of MWA of HCC in risk position are illustrated in Figs. 1 and 2.
MWA complete ablation of paracardiac HCC. a–e Preoperative images. f–i Post-MWA images. CT scan shows a 3.5 cm HCC located in segment 4a, in vicinity of the right heart ventricole. a Contrast enhancement of HCC nodule in the arterial phase; b portale phase (longitudinal section); c portale phase (coronal section); d portale phase (sagittal section); e CT-PET images. f–i CT scan after 1 month from MWA, showing a complete ablation: no contrast enhancement during the arterial (a) and portal (b) phases. h, i The coronal and sagittal section during the portal phase, respectively
MWA ablation of paracardiac HCC measuring 53 mm in its maximum diameter. CT scan shows a 3.5-cm HCC located between segments 4 and 3, abutting the diaphragm, in vicinity of the right heart ventricole. a Contrast enhancement of HCC nodule in the arterial phase; b portale phase; c arterial phase (coronal section); d, e arterial phase 1 month after the first MWA session, showing partial ablation, i.e., residual contrast enhancement. f Second MWA tratment; g CT scan images showing almost complete ablation, i.e., minimal contrast enhancement in the tumor margin
Follow-Up
Efficacy of MWA was evaluated with CT scan at 1, 3, 6, and 12 months for the first year after treatment and every 6 months thereafter. Ablation was considered complete when no contrast enhancement was observed in arterial phase at 1-month follow-up CT. Ablation was considered incomplete when a residual contrast enhancement was observed. Size of the MWA ablation zone measured at 1-month CT scan was used as the basal assessment to which subsequent follow-up imaging was compared. Increase in the diameters of the ablation zone was interpreted as tumor progression, while steadiness or decrease in diameters of the ablation zone was considered as successful ablation. In case of residual ablation, a second MWA session was performed, an example being the case illustrated in Fig. 2.
Statistical Analysis
Continuous variables were presented as mean ± SD. Percentages of complete ablation were compared in the three size groups (small, medium, and large) and compared by means of Chi-square test; values <0.05 were considered statistically significant. Overall survival (OS) analysis was conducted with the Kaplan-Meier method after eliminating data of one patient lost to follow-up and of the two patients with distant metastases at the time of the MWA procedure. Analysis was conducted using IBM SPSS Statistics version 20 (IBM Corporation 2011).
Results
Demographics and Operative Outcomes
Our database included 32 consecutive patients with 45 HCC nodules who underwent MWA during the considered period. Demographics and tumor data are resumed in Table 1. Out of 45 HCC lesions, 37.8% were classified as “small,” 33.3% as “medium,” and 28.9% as “large.”
A total of 45 MWA sessions was performed (Table 2). Two patients had metastatic disease (bone and lungs, respectively) at the time of first MWA treatment; both of them underwent simultaneous ablation of the metastatic lesions. No cases of perioperative mortality were observed. Complications occurred after seven procedures (15.5%). All of them were of minor entity and resolved spontaneously (Table 3).
Overall, ablation was complete after the initial MWA treatment in 32/45 lesions (71.1%) and incomplete in 13/45 (28.9). When considering ablation rates in the three study groups, we observed complete ablation in 94.1% of small tumors, in 80% of medium tumors, and in 53.8% of large tumors (p = 0.03) (Fig. 3).
Oncological Outcomes
After a median follow-up of 30 months (range 3–48), 13 patients died. Overall median survival was 37 months (95% confidence interval 11.97–62.02). One-year OS was 82.7%, 2-year survival 68.9%, and 3-year survival 55.2% (Fig. 3). Local recurrence was observed in four patients and distant metastases in four patients. Patients developing local recurrence of HCC were treated with a new MWA session (three patients) or trans-arterial chemoembolization (TACE) (one patient).
Discussion
Our study shows that CT-guided MWA can be used successfully in treatment of patients with HCC at different stages. Our rates of complete ablation of HCC compare favorably with previous published data.
Today, minimally invasive thermal ablation techniques, such as MWA, RFA, and cryotherapy, are being increasingly used for both primary and metastatic liver cancers. RFA has been the most used method for ablation of nonresectable liver tumors [6, 15,16,17,18] and may offer same results of radical surgery for treatment of very early HCC [15].
However, there are several limitations and disadvantages associated with the use of RFA. In fact, efficacy of RFA may be reduced by increased impedance from temperature higher than 100 °C, heat sink effect, and the unreliable use of multiple antennae simultaneously. MWA offers some advantages compared to RFA. Efficacy of ablation is not affected by high temperatures and seems less affected by heat sink effect. More than one antenna can be used when necessary in order to obtain a larger ablation zone in shorter time [5, 6, 11, 16, 19, 20]. Hence, MWA has been proposed as an effective alternative to RFA in patients with hepatic tumors up to 3 cm. To date, only one randomized trial has compared MWA with and RFA for treatment of HCC [21]. In this study, the authors demonstrated no significant differences between these two techniques with regard to therapeutic effects, complications, and rates of residual disease. Similarly, a retrospective study comparing MWA and RFA in 53 HCC patients showed no significant differences in completion of ablation, rates of residual disease, and recurrence rate between the two techniques [22]. A recent meta-analysis on percutaneous thermal ablation for HCC reported that although RFA and MWA are equally effective and safe in tumor treatment, MWA may be more effective with respect to RFA in preventing local tumor progression when larger tumors are treated [7].
In general, single lesions greater than 5 cm are not considered amenable to ablation. However, MWA has been shown to be effective in treatment HCC larger than 3 cm [6, 17]. Medhat et al. obtained complete MWA ablation in 73.1% of HCC measuring 5–7 cm in larger diameter [23]. Alexander and coworkers treated with MWA single hepatic malignancies up to 12 cm in maximum diameter [6]. One of the largest published series analyzed data of more than 2000 liver malignancies treated with US-guided MWA, ranging from 0.9 to 8 cm [24].
In recent studies, MWA has been considered an effective ablative option when dealing with medium-large HCC nodules [16, 17]. To note, 62% of lesions in our series have a diameter > 3 cm, and 4 of them (8.9%) were >8 cm. We used two antennas to treat the main part of tumors larger than 3.5 cm and observed that the likelihood to obtain a complete ablation was inversely correlated with tumor size. To date, few authors reported on the use of MWA for large HCC [6, 17]. Zhang and coworkers treated by means of MWA 60 HCC lesions measuring 3–8 cm, obtaining complete ablation rates of 82% for the first ablation and 100% for the second ablation [17]. In the present report, including 4 HCC lesions >8 cm, the rates of complete and partial ablation were 78.1 and 28.9%, respectively, with a statistically significant difference linked to the tumor size.
Treatment protocols suggest caution when using thermoablation for lesions in proximity of major vessels, major bile ducts, intra-abdominal organs, or diaphragm [25,26,27]. The reason for this is that ablation destroys a small amount of normal tissue around the tumor; thus, there could be a risk for serious complications. Nevertheless, recent reports have highlighted a progressive broadening of indications for MWA of liver malignancy located near those structures. For example, MWA of liver tumors abutting the diaphragm has been suggested to be safe and effective in expert hands [26]. Of the 45 patients treated by Zhang and coworkers, 17 had tumors that were within 5 mm of risk areas [17]. Our experience corroborates the safety of MWA of HCC located in the risk areas. In particular, we treated two cases of HCC abutting the diaphragm, in vicinity to pericardium, without any complications.
In patients with HCC, the presence of distant metastases has been usually considered as an exclusion criteria for MWA and other ablative treatments. In that setting, systemic chemotherapy and/or supportive therapy are considered the most efficacious management. However, MWA may have a role in palliative treatment of symptomatic metastatic HCC. We used MWA for large HCC in two patients with lung and bone metastases, complaining of severe flank pain and difficult digestion, likely secondary to the mass effect of the liver tumors with compression of nearby organs. We opted for ablative technique with the aim to obtain a local control of the disease by reducing the tumor volume and diminish the risks of complications such as bleeding or rupture.
In the present report, 1-, 2-, and 3-year OS rates were 82.7, 68.9, and 55.2%, respectively. Those data compare favorably with previous published data on MWA in patients with HCC. To date, only one published study reported on long-term outcomes following MWA for liver malignancies [5]. On a total of 176 patients including metastases, HCC, and primary biliary cancer, they observed an overall survival at 4 years of 58.3% for colorectal metastases and 79.4% for other pathology. They concluded that MWA of liver malignancies resulted in good long-term survival, either combined or not with surgical resection and regional/systemic treatments. They also observed that recurrence rates were low after treatment of tumors smaller than 3 cm in diameter, and those remote from vessels. Sun et al. reported cumulative OS rates of 89, 74, and 60% at 1, 2, and 3 years, respectively, in patients undergoing MWA for medium sized HCC [16]. In a report of MWA in large HCC, the 1- and 2-year OS rates were 95.5 and 86.7% [17]. Those survival rates are higher than ours; however, it should be noted that the authors treated HCC with maximum diameter of 8 cm, whereas 8.9% of our patients had tumors >8 cm.
Side effects and serious complications related to percutaneous thermoablation can be considered as uncommon, but possible [27]. Optimal tumor ablation and prevention of injuries to major vascular and biliary structures can be obtained with intraprocedural monitoring using CT. In the present report, we observed complications in 15.5% of MWA procedures, all of whom were of minor entity and resolved spontaneously after few days. We did not observe any skin burns, a complication which has been reported after percutaneous MWA of large HCC.
Most of current literature on use of MWA for HCC originates from Asia and North America. The present study is one of the few reports that focus on outcomes of HCC treated with MWA at a European Institution. Our research has some limitations, including a retrospective design and the small cohort size. Moreover, we included patients with different disease stage.
Conclusions
Our data confirms that percutaneous MWA under CT guidance represents a valid ablative management of patients with HCC unsuitable for surgical treatment. In particular, MWA is a versatile method that can be applied also in medium and large HCC and in lesions at a risk location.
References
McGlynn KA, Petrick JL, London WT. Global epidemiology of hepatocellular carcinoma: an emphasis on demographic and regional variability. Clin Liver Dis. 2015;19:223–38. doi:10.1016/j.cld.2015.01.001.
Fancellu A, Rosman AS, Sanna V, et al. Meta-analysis of trials comparing minimally-invasive and open liver resections for hepatocellular carcinoma. J of Surg Res. 2011;171:e33–45. doi:10.1016/j.jss.2011.07.008.
Kang TW, Rhim H. Recent advances in tumor ablation for hepatocellular carcinoma. Liver Cancer. 2015;4:176–87. doi:10.1159/000367740.
Memeo R, de Blasi V, Cherkaoui Z, et al. New approaches in Locoregional therapies for hepatocellular carcinoma. J Gastrointest Cancer. 2016;47:239–46. doi:10.1007/s12029-016-9840-6.
Leung U, Kuk D, D’Angelica MI. Long-term outcomes following microwave ablation for liver malignancies. Br J Surg. 2015;102:85–91. doi:10.1002/bjs.9649.
Alexander ES, Wolf FJ, Machan JT, et al. Microwave ablation of focal hepatic malignancies regardless of size: a 9-year retrospective study of 64 patients. Eur J Radiol. 2015;84:1083–90. doi:10.1016/j.ejrad.2015.02.027.
Chinnaratha MA, Chuang MY, Fraser RJ, Woodman RJ, Wigg AJ. Percutaneous thermal ablation for primary hepatocellular carcinoma: a systematic review and meta-analysis. J Gastroenterol Hepatol. 2016;31:294–301. doi:10.1111/jgh.13028.
Kalra N, Gupta P, Chawla Y, Khandelwal N, et al. Locoregional treatment for hepatocellular carcinoma: the best is yet to come. World J Radiol. 2015;7:306–18. doi:10.4329/wjr.v7.i10.306.
Shi J, Sun Q, Wang Y, et al. Comparison of microwave ablation and surgical resection for treatment of hepatocellular carcinomas conforming to Milan criteria. Journal Gastroenterol Hepatol. 2014;29:1500–7. doi:10.1111/jgh.12572.
North DA, Groeschl RT, Sindram D, et al. Microwave ablation for hepatic malignancies: a call for standard reporting and outcomes. Am J Surg. 2014;208:284–94. doi:10.1016/j.amjsurg.2014.02.002.
Eng OS, Tsang AT, Moore D, et al. Outcomes of microwave ablation for colorectal cancer liver metastases: a single center experience. J Surg Oncol. 2015;111:410–3. doi:10.1002/jso.23849.
Pusceddu C, Sotgia B, Fele RM, Ballicu N, Melis L. Combined microwave ablation and Cementoplasty in patients with painful bone metastases at high risk of fracture. Cardiovasc Intervent Radiol. 2016;39:74–80. doi:10.1007/s00270-015-1151-y.
Pusceddu C, Sotgia B, Fele RM, Melis L. Treatment of bone metastases with microwave thermal ablation. J Vasc Intervent Radiol. 2013;24:229–33. doi:10.1016/j.jvir.2012.10.009.
Pusceddu C, Melis L, Sotgia B, Fancellu A, Meloni GB. Computed tomography-guided Cryoablation of local recurrence after primary resection of pancreatic adenocarcinoma. Clin Pract. 2015;5:741. doi:10.4081/cp.2015.741.
Peng Z, Lin XJ, Zhang YJ, et al. Radiofrequency ablation versus hepatic resection for the treatment of hepatocellular carcinomas 2 cm or smaller: a retrospective comparative study. Radiology. 2012;262:1022–33. doi:10.1148/radiol.11110817.
Sun AX, Cheng ZL, Wu PP, et al. Clinical outcome of medium-sized hepatocellular carcinoma treated with microwave ablation. W J Gastroenterol. 2015;21:2997–3004. doi:10.3748/wjg.v21.i10.2997.
Zhang NN, Lu W, Cheng XJ, Liu JY, Zhou YH, Li F. High-powered microwave ablation of larger hepatocellular carcinoma: evaluation of recurrence rate and factors related to recurrence. Clin Radiol. 2015;70:1237–43. doi:10.1016/j.crad.2015.06.092.
Thandassery RB, Goenka U, Goenka MK. Role of local ablative therapy for hepatocellular carcinoma. J Clin and Exp Hepatol. 2014;4(Suppl 3):104–11. doi:10.1016/j.jceh.2014.03.046.
Poggi G, Tosoratti N, Montagna B, Picchi C. Microwave ablation of hepatocellular carcinoma. World J Hepatol. 2015;7:2578–89. doi:10.4254/wjh.v7.i25.2578.
Poulou LS, Botsa E, Thanou I, Ziakas PD, Thanos L. Percutaneous microwave ablation vs radiofrequency ablation in the treatment of hepatocellular carcinoma. World J Hepatol. 2015;7:1054–63. doi:10.4254/wjh.v7.i8.1054.
Shibata T, Iimuro Y, Yamamoto Y, et al. Small hepatocellular carcinoma: comparison of radio-frequency ablation and percutaneous microwave coagulation therapy. Radiology. 2002;223:331–7.
Vogl TJ, Farshid P, Naguib NN, et al. Ablation therapy of hepatocellular carcinoma: a comparative study between radiofrequency and microwave ablation. Abdom Imaging. 2015;40:1829–37. doi:10.1007/s00261-015-0355-6.
Medhat E, Abdel Aziz A, Nabeel M, et al. Value of microwave ablation in treatment of large lesions of hepatocellular carcinoma. J Digest Dis. 2015;16:456–63. doi:10.1111/1751-2980.12259.
Yu J, Liang P, Yu XL, Cheng ZG, et al. Local tumour progression after ultrasound-guided microwave ablation of liver malignancies: risk factors analysis of 2529 tumours. Eur J Radiol. 2015;25:1119–26. doi:10.1007/s00330-014-3483-4.
Orlacchio A, Chegai F, Del Giudice C et al (2014) Radiofrequency thermoablation of HCC larger than 3 cm and less than 5 cm proximal to the gallbladder without gallbladder isolation: a single center experience. Biomed Res Int 896527. doi:10.1155/2014/896527.
Smolock AR, Lubner MG, Ziemlewicz TJ, et al. Microwave ablation of hepatic tumors abutting the diaphragm is safe and effective. Am J Roentgenol. 2015;204:197–203. doi:10.2214/AJR.14.12879.
Lahat E, Eshkenazy R, Zendel A, et al. Complications after percutaneous ablation of liver tumors: a systematic review. Hepatobiliary Surg Nutr. 2014;3:317–23. doi:10.3978/j.issn.2304-3881.2014.09.07.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interests
The authors declare that they have no conflict of interest.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Financial Support
No financial support was received for this submission.
Rights and permissions
About this article
Cite this article
Pusceddu, C., Melis, L., Ballicu, N. et al. Percutaneous Microwave Ablation Under CT Guidance for Hepatocellular Carcinoma: a Single Institutional Experience. J Gastrointest Canc 49, 295–301 (2018). https://doi.org/10.1007/s12029-017-9951-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12029-017-9951-8