Abstract
The biological role of fibroblast growth factor receptor 3 (FGFR3) in tumor angiogenesis of hepatocellular carcinoma (HCC) has not been discussed before. Our previous work had indicated FGFR3 was overexpressed in HCC, and silencing FGFR3 in Hu7 cells could regulate tumorigenesis via down-regulating the phosphorylation level of key members of classic signaling pathways including ERK and AKT. In the present work, we explored the role of FGFR3 in angiogenesis-dependent metastasis by using SMMC-7721 and QGY-7703 stable cell lines. Our results indicated FGFR3 could regulate in vitro cell migration ability and in vivo lung metastasis ability of HCC, which was in accordance with increased angiogenesis ability in vitro and in vivo. Using the supernatant from SMMC-7721/FGFR3 cells, we conducted a human angiogenesis protein microarray including 43 angiogenesis factors and found that FGFR3 modulated angiogenesis and metastasis of HCC mainly by promoting the protein level of monocyte chemotactic protein 1 (MCP-1). Silencing FGFR3 by short hairpin RNA (shRNA) could reduce MCP-1 level in lysates and supernatant of QGY-7703 cells and SMMC-7721 cells. Silencing MCP-1 in QGY-7703 or SMMC-7721 cells could induce similar phenotypes compared with silencing FGFR3. Our results suggested FGFR3 promoted metastasis potential of HCC, at least partially if not all, via facilitating MCP-1-mediated angiogenesis, in addition to previously found cell growth and metastasis. MCP-1, a key medium between HCC cells and HUVECs, might be a novel anti-vascular target in HCC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hepatocellular carcinoma (HCC) remains the fifth death-related tumor worldwide with intricate etiologies and carcinogenesis [1]. Metastasis at early stage is a main factor for unfavorable prognosis of HCC patients while the exact mechanism remains elusive [2]. Lung is the most common extrahepatic organ for HCC metastasis [3]. Many efforts have been made to arrest metastasis of HCC; however, there is still an urgent need for discovering novel therapeutic measurements due to lacking effective targets [1, 4, 5]. Tumor growth and metastasis are based on glorious angiogenesis to provide necessary nutrition, and tumor diameter will be limited to 2 mm without continuous vascular formation [6]. Despite several mechanisms of tumor angiogenesis including endothelial-dependent angiogenesis, vasculogenic mimicry (VM) and their transitional form mosaic vessel have been reported, and endothelium-dependent vessels are the most classic mechanism for solid tumor angiogenesis [7–10]. Therefore, recent studies have identified endothelium as a potential anti-angiogenesis target.
Receptor tyrosine kinases (RTKs) comprise a member of enzyme-linked receptors which mainly involve in tyrosine phosphorylation of target proteins [11, 12]. The classic structure of RTKs including a ligand-binding site localized to extracellular domain, a single hydrophobic transmembrane α-helix region and an intracellular domain containing tyrosine kinase (RTK) activity [13]. Recently, the overactivation of FGFR signaling in HCC had drawn much attention and our previous work had identified overexpression of FGFR3 correlated with aggressive clinicopathological parameters and biological behaviors [14–16]. In the present study, we reported new findings that the biological functions, especially angiogenesis ability of HCC cells induced by FGFR3 overexpression, were mainly depended on monocyte chemotactic protein 1 (MCP-1), a member of the CC chemokine family. Although MCP-1 had been identified as an oncogene in multiple human solid tumors, little was known about the biological function of MCP-1 in HCC, especially on the pro-angiogenic effect and the relationship between FGFR3 and MCP-1. Our present work might contribute to the knowledge expansion of angiogenic mechanism and FGFR3-mediated anticancer strategy of HCC.
Materials and methods
Cell culture
The human HCC cell lines SMMC-7721, QGY-7703 and human umbilical vein endothelial (HUVECs) cells were purchased from Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences. All cells were cultured in high glucose DMEM and 10 % fetal bovine serum (FBS) at 37 °C and 5 % CO2.
Lentiviral product
Lentivirus expressing shFGFR3 or shNC was produced in HEK-293T cells using the corresponding shRNA expressing pLKO.1 vector with the aid of packaging plasmids pMD-VsVg and pCMV-delta 8.9 by Lipofectamine 2000 (Invitrogen). As to overexpression of FGFR3, full-length FGFR3 cDNA was obtained by RT-PCR from total RNA extracted from HCC samples and the primers used were 5′- gacGGATCC atgggcgcccctgcctgcgccctc - 3′ (constructed with BamHI site) and 5′- gacACTAGT tcacgtccgcgagcccccactgct - 3′(constructed with Spe I site). Then, FGFR3 cDNA was inserted into lentivirus plasmid pWPI.1 (Addgene, Cambridge, MA, USA) to obtain a pWPI.1-FGFR3 construct. Then, pWPI.1-FGFR3, psPAX2 and pMD were co-transfected into HEK-293T cells in the presence of Lipofectamine 2000 for obtaining lentivirus supernatants. Then, the supernatants were collected and stable cell lines were constructed by the standard protocol.
Western blotting
Cell protein extraction and western blotting were performed according to established protocols [17]. The antibodies used were: GAPDH (Kangchen, China); FGFR3 (Santa Cruz, USA); MCP-1 (Abcam, USA).
Cell proliferation analysis
Cell Counting Kit-8 (Dojindo) was employed for in vitro cell proliferation assay. In brief, cells (800 cells/well) of each group were seeded in 96-well culture plates for 7 days. Cell viability was detected at the same time of each day by microplate reader (Epoch, BioTek).
Cell migration analysis
Cell migration ability was analyzed by transwell according to the manufacture’s protocol. Briefly, 200 ul non-serum culture medium containing 1 × 105 cells was placed in the upper chamber of transwell and 600 ul medium/10 % fetal bovine serum was placed in lower chamber. After incubated at 37 °C and 5 % CO2 for 24 h, cells attached inside the chambers were cleared softly and cells at the bottom of culture inserts were stained in 1 % crystal violet for 30 min. Then, cells migrated to the underside of upper chamber were counted with 5 random fields.
Endothelial tube formation analysis
Endothelial tube formation analysis was employed for in vitro angiogenesis assay. HUVECs were cultured in tumor supernatant of each group in 96-well plate coated with 40 ul Matrigel (BD Bioscience) at a density of 3 × 104 cells per well after Matrigel was polymerized at 37 °C for 1 h. After 6-h incubation at 37 °C with 5 % CO2, tubules were photographed by microscopy and tubular numbers, length and intersections were counted according to published protocols by Image-Pro Plus software [18].
Enzyme-linked immunosorbent assay
For enzyme-linked immunosorbent assay, concentrations of MCP-1 in supernatants of each group were analyzed by Quantikine ELISA kits (Boster, Wuhan, China) according to the manufacturer’s protocol.
Nude mice xenograft and lung metastasis analysis
All animal experiments were performed in accordance with the official recommendations of the Chinese animal community and had been approved by the Ethical Committee of Ruijin Hospital, Shanghai Jiao Tong University School of Medicine. For tumor growth assay, QGY-7703/shNC or QGY-7703/shFGFR3 cells (1 × 106 in 0.2 ml of sterilized PBS buffer) were implanted subcutaneously into the right flank of 4-week-old male nude mice (5 mice per group). Tumor nodules were measured every week by the formula: tumor volume = (Width2 × Length)/2. Subcutaneous tumors were weighed and fixed with 10 % buffered formalin for immunohistochemistry analysis. As to in vivo metastasis assay, QGY-7703/shNC or QGY-7703/shFGFR3 cells (2 × 106 in 0.2 ml of sterilized PBS buffer) were injected into nude mice via tail vein. After 8 weeks, the pulmonary metastatic masses were dissected out and fixed with 10 % buffered formalin for HE staining.
Immunohistochemistry
For immunohistochemistry assay, paraffin-embedded specimens were cut into 5 mm sections and mounted on slides before staining. After sections were incubated overnight and washed, antigen retrieval was performed with microwave retrieval method; 3 % hydrogen peroxide was used to inactive endogenous peroxidase. Then, the sections were subjected to immunohistochemical staining. The antibodies used were Ki-67 (Abcam, USA) and CD34 (Abcam, USA). Then, the slides were viewed with light microscope. Thereafter, the IOD of all each photograph (200×) was measured using Image-Pro Plus software and the relative intensity was calculated.
Human angiogenesis array
The supernatants from SMMC-7721/NC and SMMC-7721/FGFR3 were collected for human angiogenesis array analysis. The protein levels of 43 angiogenesis-associated molecules were analyzed by using the Human Angiogenesis Antibody Array 1 (RayBio®) according to the manufacturer’s protocol.
Statistical analysis
Student t test and one-way analysis of variance were used when appropriate. Data were presented as mean ± SD from three independent experiments. SPSS 15.0 software was used, and p < 0.05 was considered as statistically significant.
Results
FGFR3 regulated metastatic potential of hepatocellular carcinoma in vitro and in vivo
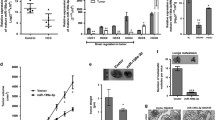
FGFR3 overexpression and knockdown effect were confirmed in SMMC-7721 and QGY-7703 cell lines by western blot (Fig S1A and Fig S1B). In our previous literature and the work performed by Jakob Paur and the collaborators, FGFR3 was overexpressed in HCC tissues and silencing FGFR3 could inhibit tumor growth of hepG2 cells, which was firstly verified by our present work in SMMC-7721 and QGY-7703 cells (Fig S1C and Fig S1D) [15, 19]. Then, we observed that silencing FGFR3 in SMMC-7721 and QGY-7703 cells could inhibit cell migration ability by transwell assay (Fig. 1a–d, left). The data were comforted by the observed opposite effects following FGFR3 overexpression so could be trustable (Fig. 1a–d, right). Furthermore, we conducted lung metastasis model by tail vein injection of QGY-7703/shNC or QGY-7703/shFGFR3 cells. As the result, 4 out of 5 mice showed tumorigenicity in lung in QGY-7703/shNC group, which is significantly higher than QGY-7703/shFGFR3 group (Fig. 2a–c).
FGFR3 regulated metastatic potential of SMMC-7721 and QGY-7703 cells in vitro. a, b Silencing FGFR3 in SMMC-7721 cells could reduce the migrated cells through transwell while FGFR3 overexpression could increase the migrated cells. c, d Silencing FGFR3 in QGY-7703 cells could reduce the migrated cells through transwell while FGFR3 overexpression could increase the migrated cells. The data represent the mean ± S.D. of three independent experiments. *p < 0.05 and **p < 0.01
FGFR3 regulated metastatic potential of HCC in vivo. a QGY-7703/shNC or QGY-7703/shFGFR3 cells were injected into nude mice via tail vein and the QGY-7703/shFGFR3 group yield a significant decrease in pulmonary metastatic masses compared with QGY-7703/shNC group. b The metastatic masses of the two group were counted for statistics, none of the 5 mice showed tumorigenicity in lung of QGY-7703/shFGFR3 group, which was significantly lower than that in NC group (4/5). c Metastatic cancer mass is easily observed microscopically in QGY-7703/shNC group compared with QGY-7703/shFGFR3 group. *p < 0.05 and **p < 0.01
FGFR3 regulated in vitro tubular formation of HUVECs and vasculogenic mimicry
Next, we wanted to know the potential mechanisms through which FGFR3 regulated tumor growth and metastasis. Endothelium-dependent angiogenesis was well-established evidences for tumor progression. We collected supernatants from each group of SMMC-7721 and QGY-7703 cells for tubular formation assay, respectively. Interestingly, after incubated for 6 h, HUVECs added with supernatant from QGY-7703/shFGFR3 or SMMC-7721/shFGFR3 cells showed decreased tubular numbers, length and intersections compared with shNC group, while the QGY-7703/FGFR3 and SMMC-7721/FGFR3 cells showed increased angiogenesis ability compared with NC group (Fig. 3a–d). To further investigate the role of FGFR3 in angiogenesis, 10 nude mice (5 mice each group injected with QGY-7703/shNC or QGY-7703/shFGFR3, respectively) were enrolled in our present study for subcutaneous tumor experiments and the xenografts was fixed with 10 % buffered formalin for further analysis. As a result, silencing FGFR3 in QGY-7703 cells could be significantly inhibited in vivo tumor growth (Fig S2A–Fig S2C). Furthermore, tumor angiogenesis marker Ki-67, and VM marker CD34 by immunohistochemistry were significantly decreased in QGY-7703/shFGFR3 group (Fig. 4a–c).
FGFR3 regulated in vitro angiogenesis ability of HCC. a Silencing FGFR3 in SMMC-7721 cells could decrease the tubular formation ability of HUVEC while FGFR3 overexpression could increase tubular formation ability. b The tubular numbers, length and intersections of SMMC-7721/shFGFR3 and SMMC-7721/shNC group were counted for statistics. c Silencing FGFR3 in QGY-7703 cells could decrease the tubular formation ability of HUVEC while FGFR3 overexpression could increase tubular formation ability. d The tubular numbers, length and intersections of QGY-7703/shFGFR3 and QGY-7703/shNC group were counted for statistics. The data represent the mean ± S.D. of three independent experiments. *p < 0.05 and **p < 0.01
FGFR3 regulated in vivo angiogenesis ability of HCC. The xenografts of QGY-7703/shFGFR3 and QGY-7703/shNC group were fixed and stained for Ki-67, CD34 and double-staining immunohistochemistry of CD34 and PAS. a Expression of Ki-67 in nude mice by immunohistochemistry (left). The relative intensity of Ki-67 level was counted (right). b Expression of CD34 in nude mice by immunohistochemistry (left). The relative intensity of CD34 level was counted (right). c Histograms showed the numbers of VM tubules (left) and the VM structures were counted (right). *p < 0.05 and **p < 0.01
FGFR3 regulated HUVEC proliferation and migration ability
Cell proliferation and migration abilities were essential for tubular formation of HUVECs. We first wanted to understand the proliferation ability of HUVECs of each group by CCK-8 assay. Tumor supernatants of each group were collected and co-incubated with HUVECs for 24 h. HUVEC proliferation was increased in QGY-7703/FGFR3 group and decreased in QGY-7703/shFGFR3 group compared with control group, respectively. However, we did not detect significant difference between FGFR3/shNC group and FGFR3/shRNA group of SMMC-7721 cells despite the former showed a growth advantage from the third day (Fig S3). Interestingly, we detected a sharp decrease in HUVECs through transwell in FGFR3/shRNA group compared with FGFR3/shNC group and a significant increase in FGFR3 overexpression group compared with NC group in both cell lines, respectively (Fig. 5a–d). These results indicated FGFR3 could regulate tumor angiogenesis, at least partially if not all, by stimulating HUVECs proliferation and migration.
FGFR3 regulated migration ability of HUVECs. The supernatants of each group were collected to evaluate the migration ability of HUVECs. a, b HUVECs resuspended in the supernatant of SMMC-7721/shFGFR3 cells yield a significant decrease in migration ability compared with SMMC-7721/shNC group while FGFR3 overexpression could enhance the migration ability of HUVECs. c, d HUVECs resuspended in the supernatant of QGY-7703/shFGFR3 cells yield a significant decrease in migration ability compared with QGY-7703/shNC group while FGFR3 overexpression could enhance the migration ability of HUVECs. The data represent the mean ± S.D. of three independent experiments. *p < 0.05 and **p < 0.01
FGFR3 overexpression could increase MCP-1 level and influence multiple biological behaviors
Next, we wanted to explore the potential mechanisms behind which FGFR3 regulated tumor angiogenesis. Supernatants of SMMC-7721/FGFR3 and SMMC-7721/NC cells were collected for human angiogenesis array including 43 angiogenesis-associated molecules (Fig S4). As indicated in Fig. 6a, the results of angiogenesis array showed the concentration of MCP-1 in supernatant had a sharp increase after FGFR3 overexpression, which was verified by the result of ELISA (Fig. 6b, c). Next, we wanted to identify whether the difference in MCP-1 level in supernatant is caused by expression level change in tumor cells or singly by increase in secretion. Then, we detected the protein level of MCP-1 in the cell lysates of each group and the change was consistent with the result of ELISA (Fig. 6d). These results indicated MCP-1 may act as an appropriate medium between HCC cells and HUVECs for angiogenesis. Taken together, FGFR3 could regulate MCP-1 level in QGY–7703 and SMMC-7721 cells and tumor supernatant.
FGFR3 overexpression could increase the protein level of multiple angiogenesis factors. a Human angiogenesis array was utilized to profile the angiogenesis factors of SMMC-7721/FGFR3 or SMMC-7721/NC cells. b, c Up-regulated concentration of MCP-1 level was verified by ELISA in the supernatant of SMMC-7721/FGFR3 and QGY-7703/FGFR3 cells. d The protein level of MCP-1 in each group was evaluated by western blotting. *p < 0.05 and **p < 0.01
Next, we explored whether silencing MCP-1 in QGY-7703 and SMMC-7721 cells could induce similar phenotypes, especially angiogenesis-related tumor progression compared with silencing FGFR3. Interestingly, we observed that silencing MCP-1 in HCC cells could significantly decreased MCP-1 secretion (Fig. 7b) and partly inhibit cell migration (positive in SMMC-7721 cells and negative in QGY-7703 cells, Fig. 7c). In addition, silencing MCP-1 could inhibit angiogenesis ability of both cell lines (Fig. 7d, e). These results were similar to the effects of silencing FGFR3 expression in HCC cells.
Silencing FGFR3 in HCC cells could induce similar phenotypes compared with FGFR3 down-regulation. *p < 0.05 and **p < 0.01. a The silencing effect of MCP-1 was verified by western blot. b The concentration of MCP-1 in the supernatant of SMMC-7721 and QGY-7703 cells was evaluated by ELISA. c The migration ability of SMMC-7721 and QGY-7703 cells was decreased after MCP-1 down-regulation. d–e The tubular numbers, length and intersections were decreased after MCP-1 down-regulation in SMMC-7721 and QGY-7703 cells. The data represent the mean ± S.D. of three independent experiments. *p < 0.05 and **p < 0.01
Discussion
FGFRs were important members of protein tyrosine kinase (PTK) family. Activating FGFR3 mutations could induce skeletal disorders and multiple aggressive behaviors of human solid tumors [20–22]. Furthermore, changes in expression level of FGFR3 had also been confirmed in human oral cancer, multiple myeloma and HCC [15, 23, 24]. Different splicing isoforms of FGFR3 had also been observed in solid tumors including HCC, and we focused on the novel splicing variant of FGFR3 in another study [15, 16, 25]. Overactivation of FGFR3 signaling could induce hypochondroplasia via inhibiting the growth of chondrocytes [26]. However, it seems unlikely that FGFR3 activation by overexpression or mutation could induce inhibition of tumor growth. In bladder cancer, FGFR3 overexpression correlated with adverse outcomes and biological behaviors [27]. Our previous work had initially revealed the clinical role of FGFR3 in HCC. Furthermore, we found that silencing FGFR3 in Huh7 cells could suppress tumor proliferation and metastasis ability via down-regulating p-ERK and p-AKT level. In this study, we wanted to explore the angiogenesis-related biological roles of FGFR3 in HCC and the potential mechanisms.
In the present work, we first determined FGFR3 promoted metastatic potential of HCC in vitro and in vivo. Considering tumor metastasis was based on flourish angiogenesis to provide essential nutrients, we detected the functional role of FGFR3 on endothelium-dependent angiogenesis. As the result, FGFR3 could regulate in vitro tubular formation and in vivo CD34 level, a marker of endothelial cells. Next, we determined that the metastasis ability of HUVECs in each group was coincided with tubular formation ability. To further understand the mechanisms through which FGFR3 regulated metastasis and angiogenesis, an angiogenesis array was employed to identify downstream signaling of FGFR3 and MCP-1 was considered as a potential angiogenic medium between tumor cells and HUVECs. MCP-1 was a member of the CC chemokine family which was abundantly produced in inflammation diseases including rheumatoid arthritis and atherosclerosis. Various human solid tumors and cell lines could produce MCP-1, thus attracting immunosuppressive macrophages. Recently, the clinical and biological function of MCP-1 overexpression in human solid tumors had been gradually identified. In oral squamous cell carcinoma, MCP-1 had been identified as a downstream gene regulated by galectin-1 and correlated with tumor progression and metastasis. As to hepatocellular carcinoma, MCP-1 played as a medium between myofibroblasts and tumor cells: Hepatic myofibroblasts could secret MCP-1 and promote invasion and migration ability of HCC cells. Furthermore, the pro-angiogenic role of MCP-1 had been identified in breast cancer and melanoma. However, whether MCP-1 could directly regulate metastasis and angiogenesis of HCC remained elusive.
As to tumor angiogenesis, previous studies mainly focused on “classical” genes such as vascular endothelial growth factor (VEGF) and matrix metalloproteinases, which were all well-established angiogenic factors. In the present study, these factors did not show significant change after FGFR3 overexpression and we detected sharp change in a novel factor MCP-1 in supernatants of SMMC-7721 cells after restoring FGFR3. To verify the regulatory role of FGFR3 on MCP-1, we detected the protein level of MCP-1 in cell lysates and supernatants by western blot and ELISA, respectively. As the result, silencing FGFR3 by shRNA could down-regulate protein level in cell lysates and concentration of MCP-1 in supernatants. Silencing MCP-1 in QGY-7703 or SMMC-7721 cells could induce similar changes in metastasis and angiogenesis ability with silencing FGFR3. These results indicated FGFR3 could regulate angiogenesis-dependent tumor metastasis via targeting MCP-1.
In conclusion, our study suggested FGFR3 promoted lung metastasis and angiogenesis of hepatocellular carcinoma via targeting MCP-1. Anti-MCP-1 might serve as an effective therapeutic approach for HCC.
References
Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53(3):1020–2.
Shiina S, Tateishi R, Arano T, Uchino K, Enooku K, Nakagawa H, et al. Radiofrequency ablation for hepatocellular carcinoma: 10-year outcome and prognostic factors. Am J Gastroenterol. 2012;107(4):569–77.
Katyal S, Oliver JH III, Peterson MS, Ferris JV, Carr BS, Baron RL. Extrahepatic metastases of hepatocellular carcinoma 1. Radiology. 2000;216(3):698–703.
Fang Y, Xue JL, Shen Q, Chen J, Tian L. MicroRNA-7 inhibits tumor growth and metastasis by targeting the phosphoinositide 3-kinase/Akt pathway in hepatocellular carcinoma. Hepatology. 2012;55(6):1852–62.
Yau WL, Lam CSC, Ng L, Chow AKM, Chan STC, Chan JYK, et al. Over-expression of miR-106b promotes cell migration and metastasis in hepatocellular carcinoma by activating epithelial-mesenchymal transition process. PloS one. 2013;8(3):e57882.
Shojaei F. Anti-angiogenesis therapy in cancer: current challenges and future perspectives. Cancer Lett. 2012;320(2):130–7.
Seftor RE, Hess AR, Seftor EA, Kirschmann DA, Hardy KM, Margaryan NV, et al. Tumor cell vasculogenic mimicry: from controversy to therapeutic promise. Am J Pathol. 2012;181(4):1115–25.
Kirschmann DA, Seftor EA, Hardy KM, Seftor RE, Hendrix MJ. Molecular pathways: vasculogenic mimicry in tumor cells: diagnostic and therapeutic implications. Clin Cancer Res. 2012;18(10):2726–32.
Liu J, Huang J, Yao W-Y, Ben Q-W, Chen D-F, He X-Y, et al. The origins of vacularization in tumors. Front Biosci. 2012;17(1):2559–65.
Young EW. Cells, tissues, and organs on chips: challenges and opportunities for the cancer tumor microenvironment. Integr Biol. 2013;5(9):1096–109.
Casaletto JB, McClatchey AI. Spatial regulation of receptor tyrosine kinases in development and cancer. Nat Rev Cancer. 2012;12(6):387–400.
Takeuchi K, Ito F. Receptor tyrosine kinases and targeted cancer therapeutics. Biol Pharm Bull. 2011;34(12):1774–80.
Hunter T, Manning G. The eukaryotic protein kinase superfamily and the emergence of receptor tyrosine kinases, chap 1. In: Wheeler DL, Yarden Y, editors. Receptor tyrosine kinases: structure, functions and role in human. New York: Springer-Verlag; 2015.
Hagel M, Miduturu C, Sheets M, Rubin N, Weng W, Stransky N, et al. First selective small molecule inhibitor of FGFR4 for the treatment of hepatocellular carcinomas with an activated FGFR4 signaling pathway. Cancer Discov. 2015;5(4):424–37.
Qiu W-H, Zhou B-S, Chu PG, Chen W-G, Chung C, Shih J, et al. Over-expression of fibroblast growth factor receptor 3 in human hepatocellular carcinoma. World J Gastroenterol. 2005;11(34):5266.
Qiu W, Yang W, Jing X, Wang B, Liu X, Ma D, et al. The phenotypic and signaling consequences of a novel aberrantly spliced transcript of fibroblast growth factor receptor 3 in hepatocellular carcinoma. Cancer Res. 2015;75(15 Supplement):3958.
Qiu W, Zhou B, Chu PG, Luh F, Yen Y. The induction of growth arrest DNA damage-inducible gene 45 β in human hepatoma cell lines by S-adenosylmethionine. Am J Pathol. 2007;171(1):287–96.
Jiang J, Liu W, Guo X, Zhang R, Zhi Q, Ji J, et al. IRX1 influences peritoneal spreading and metastasis via inhibiting BDKRB2-dependent neovascularization on gastric cancer. Oncogene. 2011;30(44):4498–508.
Paur J, Nika L, Maier C, Moscu-Gregor A, Kostka J, Huber D, et al. Fibroblast growth factor receptor 3 isoforms: novel therapeutic targets for hepatocellular carcinoma? Hepatology. 2015;62(6):1767–78.
Colvin JS, Bohne BA, Harding GW, McEwen DG, Ornitz DM. Skeletal overgrowth and deafness in mice lacking fibroblast growth factor receptor 3. Nat Genet. 1996;12(4):390–7.
Yamashita A, Morioka M, Kishi H, Kimura T, Yahara Y, Okada M, et al. Statin treatment rescues FGFR3 skeletal dysplasia phenotypes. Nature. 2014;513(7519):507–11.
Gauglhofer C, Sagmeister S, Schrottmaier W, Fischer C, Rodgarkia-Dara C, Mohr T, et al. Up-regulation of the fibroblast growth factor 8 subfamily in human hepatocellular carcinoma for cell survival and neoangiogenesis. Hepatology. 2011;53(3):854–64.
Henson B, Gollin S. Overexpression of KLF13 and FGFR3 in oral cancer cells. Cytogenet Genome Res. 2010;128(4):192–8.
Keats JJ, Reiman T, Maxwell CA, Taylor BJ, Larratt LM, Mant MJ, et al. In multiple myeloma, t (4; 14)(p16; q32) is an adverse prognostic factor irrespective of FGFR3 expression. Blood. 2003;101(4):1520–9.
Paur J, Nika L, Maier C, Moscu-Gregor A, Kostka J, Huber D, et al. Fibroblast growth factor receptor 3 isoforms: novel therapeutic targets for hepatocellular carcinoma? Hepatology. 2015;62:1767–78.
Linnankivi T, Mäkitie O, Valanne L, Toiviainen-Salo S. Neuroimaging and neurological findings in patients with hypochondroplasia and FGFR3 N540 K mutation. Am J Med Genet Part A. 2012;158(12):3119–25.
Turo R, Harnden P, Thygesen H, Fleischmann A, Thalmann GN, Seiler R, et al. FGFR3 expression in primary invasive bladder cancers and matched lymph node metastases. J Urol. 2015;193(1):325–30.
Acknowledgments
This study was supported by Nature Science Foundation of China (30872511) and Shanghai Charity Foundation for Cancer Research.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest or financial disclosures of this study.
Additional information
Xinyu Liu, Xiaoqian Jing and Xi Cheng contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig S1
FGFR3 regulated in vitro proliferation of HCC. a The silencing effect of FGFR3 in SMMC-7721 and QGY-7703 cells was confirmed by western blot with 40 ug total protein per well. b The overexpression effect of FGFR3 in SMMC-7721 and QGY-7703 cells was confirmed by western blot with 15 ug total protein per well. *p<0.05 and **p<0.01 (TIFF 2097 kb)
Fig S2
FGFR3 regulated in vivo proliferation of HCC. a Knockdown of FGFR3 inhibited tumor growth in vivo. b The volume of xenografts tumors was measured every 5 days. c Tumor weight was measured after the xenografts tumors were removed. *p<0.05 and **p<0.01 (TIFF 1874 kb)
Fig S3
The effect of FGFR3 overexpression on the proliferation of HUVEC cells. a FGFR3 overexpression in SMMC-7721 cells could significantly increase the proliferation ability of HUVECs while silencing FGFR3 had no significant effect on the proliferation ability of HUVECs. b FGFR3 overexpression in QGY-7703 cells could significantly increase the proliferation ability of HUVECs and silencing FGFR3 impaired the proliferation ability of HUVECs. *p<0.05 and **p<0.01 (TIFF 1327 kb)
Fig S4
Human angiogenesis array. Molecules shown in red were up-regulated in SMMC-7721/FGFR3 group compared with SMMC-7721/NC group. Pos, positive control; Neg, negative control (TIFF 4157 kb)
Rights and permissions
About this article
Cite this article
Liu, X., Jing, X., Cheng, X. et al. FGFR3 promotes angiogenesis-dependent metastasis of hepatocellular carcinoma via facilitating MCP-1-mediated vascular formation. Med Oncol 33, 46 (2016). https://doi.org/10.1007/s12032-016-0761-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-016-0761-9