Abstract
Hepatocellular carcinoma (HCC) is a leading cause of cancer-related morbidity and mortality. Tumor neovascularization is necessarily required for tumor progression and metastasis. CD105 and vascular endothelial growth factor (VEGF) have separately been identified as important contributors to angiogenesis; however, it is unclear if these factors interact to promote the progression of HCC. The goal of this study was to determine the interaction between CD105 and VEGF in HCC, using HCC tissue samples and the human HCC cell line SMMC-7721. In a survey of 89 HCC tumor samples, we determined that CD105 and VEGF expressions were positively correlated with each other and expressed at a higher level in tumor cells. Furthermore, the expression of CD105 was closely related to the tumor-node-metastasis (TNM) staging of HCC, degree of tumor differentiation, portal vein invasion, and lymph node metastasis (P < 0.05). Next, we used a lentiviral system to stably overexpress CD105 in SMMC-7721 cells, which was confirmed at the messenger RNA (mRNA) and protein level. We observed that VEGF expression was increased in these cells, as was cell motility and migration, as assessed using a wound healing assay and Transwell chamber system, respectively. Using VEGF small interfering RNA (siRNA), we also demonstrated that elevated VEGF expression is required to promote increased cell motility and migration in CD105-overexpressing cells. In conclusion, we interpret our data to prove that CD105 promotes the invasion and metastases of liver cancer cells by increasing VEGF expression. These results provide a new theoretical and experimental basis for the treatment of liver cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hepatocellular carcinoma (HCC) is the fifth most common malignancy in the world, and the third leading cause of cancer-related death after gastric and esophageal cancer [1]. Usually, HCC develops as the result of an underlying chronic liver disease progressing to become cancerous [2]. Currently, surgery and liver transplantation are the preferred treatment choices, but recurrence and metastasis still occur in 30–40 % of patients 5 years after surgery [3]. Therefore, apart from increasing efficacy of curable surgery, an effective therapeutic strategy is highly demanded.
Recent studies have confirmed that neovascularization plays a considerable role in tumor metastasis. Tumor angiogenesis is an extremely complex process, regulated by a plethora of factors, of which CD105 plays an essential role [4]. Quackenbush and Letarte identified the membrane antigen endoglin for the first time in 1985 [5]. In 1993, at the fifth meeting of “the international workshop on human leukocyte differentiation antigens,” all monoclonal antibodies used to detect endoglin were defined as one cluster of differentiation (CD), numbered 105 (i.e., CD105). CD105 is highly expressed on endothelial cells of nascent tumor blood vessels and vascular endothelial cells of the tumor margin and is considered an ideal target in tumor therapy to inhibit tumor angiogenesis [6]. It is known that CD105 is regulated by different environmental factors and cytokines in the process of angiogenesis [7] and is also thought to play an important role in vasculogenesis and maintaining vascular integrity; however, its mechanism of action has not been fully clarified.
Vascular endothelial growth factor (VEGF) has many functions, including the promotion of endothelial cell proliferation, increasing vascular permeability to facilitate migration of endothelial cells, inducing tumor angiogenesis, and maintaining regrowth of tumors [8]. Indeed, VEGF is known to be an angiogenic factor with the greatest activity and specificity. Liver tumors are rich in blood vessels, while VEGF expression is known to play a key role in tumor angiogenesis, making it an attractive focus of research into liver cancer [9]. Previous studies have found an upregulation of CD105 in tumors being treated with anti-VEGF anti-angiogenic therapy as well as an increase in the number of CD105-positive blood vessels, indicating a relationship between CD105 and VEGF [10]. Given this observation, the question remains: What is the role of CD105 in hepatic carcinogenesis? Furthermore, is this potential effect connected with VEGF signaling? To address these questions, we engineered liver cancer cells to overexpress CD105 and measured the effect on cell invasion, metastatic ability, and VEGF expression. We also studied the effect of CD105 on VEGF and subsequent liver cancer invasion by blocking VEGF expression.
Materials and methods
Clinical samples
Liver cancer tissue samples were harvested from 89 patients who had undergone surgery for liver cancer in the Shengjing Hospital of the China Medical University, between March 2008 and September 2010. None of the patients had received any chemotherapy or radiotherapy before surgery. The patient’s clinicopathological data were obtained from their clinical records and pathological reports. The study protocol conformed to the ethical guidelines of the Declaration of Helsinki (1975) and was approved by the ethics committee of the Shengjing Hospital of the China Medical University. All patients provided an informed consent before the study.
Cell lines and reagents
The human hepatocarcinoma cell line SMMC-7721 was obtained from the American Type Culture Collection (Rockville, MD, USA). Dulbecco’s modified Eagle’s medium (DMEM) was purchased from Gibco BRL (Rockville, IN, USA). Fetal bovine serum (FBS) was supplied by Haoyang Biological Manufacturer Company (Shenyang, China). Mouse anti-CD105 was purchased from Abcam (Cambridgeshire, England). Rabbit anti-VEGF was purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Anti-mouse IgG horseradish peroxidase (HRP) antibody was purchased from Zhongshan Golden Bridge (Beijing, China). Cell lysis buffer for Western blotting was purchased from Beyotime Institute of Biotechnology (Jiangsu, China). All other reagents were from Sigma-Aldrich (St. Louis, MO, USA) unless stated otherwise.
Cell culture
SMMC-7721 cells were routinely cultured in DMEM supplemented with 10 % FBS (Gibco BRL) and 100 units of penicillin-streptomycin at 37 °C with 5 % CO2 in a humidified incubator.
Recombinant lentivirus construction
The Homo CD105 gene fragments were amplified by PCR. The following primer sequences were used (GeneChem, Shanghai, China): CD105-AgeI-F, 5′- CCA ACT TTG TGC CAA CCG GTC GCC ACC ATG GAC CGC GGC ACG CTC -3′, and CD105-AgeI-R, 5′- AAT GCC AAC TCT GAG CTT TGC CAT GCC ACC ATG GCT GCT GGT GAG -3′. The lentiviral vector GFP (GeneChem) was digested by the restriction enzyme AgeI, and the CD105 gene fragments were ligated into the lentiviral vector, named Ubi-SMMC-7721-GFP. The primer 5′-CTG ACT ATC CCT GAC ATC ATC-3′, located in the coding sequence of the CD105 gene, was used to identify positive transformants by PCR, which were then sequenced. Recombinant lentiviruses which coexpress enhanced GFP and CD105 were produced by 293 T cells following cotransfection with Ubi-MSC-EGFP-CD105 and the packaging plasmids pHelper1.0 and pHelper2.0 (GeneChem). The virus titer was detected by quantitative real-time PCR after concentrating and harvesting the viral supernatant.
In vitro transcription
The SMMC-7721 cells were cultured in 12-well plates at a density of 5 × 103 cells in 2 ml of DMEM + 10 % FBS per well. Twenty-four hours after plating, transduction was carried out at a multiplicity of infection of 20. After incubation at 37 °C for 12 h, the transduction medium was replaced with fresh DMEM + 10 % FBS. Ad-CD105-SMMC-7721 cells were genetically engineered with recombinant lentivirus coexpressing GFP and CD05 (lentivirus CD105), and Ad-GFP-SMMC-7721 cells were manipulated with lentivirus-expressing GFP (lentivirus GFP) to be used as a negative control. The expression of the GFP transgene in engineered SMMC-7721 cells was confirmed by high-power fluorescence microscopy (Nikon, Tokyo, Japan). In order to explore their viability, engineered SMMC-7721 cells were sequentially expanded for five passages.
Immunohistochemistry
Immunohistochemistry was performed according to standard procedures [11]. Briefly, the sections were initially processed for antigen retrieval (microwaving) and blocking and were incubated with specific primary antibodies overnight at 4 °C. Next, they were incubated with HRP-conjugated secondary antibodies, and the expression signals were revealed using 3,3′-diaminobenzidine buffer. Phosphate-buffered saline (PBS) was used instead of the primary antibodies as a negative control. The results were evaluated according to the method described by Li et al. [11].
Wound healing assay
A wound healing assay was performed according to standard protocols [12]. Briefly, a lesion was created using a plastic pipette tip, and after 24 h, the cells were washed twice with PBS to remove debris. The cell monolayer was then maintained in serum-free DMEM and cultured for a further 24 h. Finally, five randomly selected fields at the border of the lesion were viewed under an inverted microscope (IX71; Olympus, Tokyo, Japan).
Transwell invasion and migration assays
Cell invasion and migration assays were performed using a Transwell system (Corning, Grand Island, NY, USA), according to the manufacturer’s protocol. To assess invasion ability, membranes were precoated with Matrigel (BD Biosciences, Franklin Lakes, NJ, USA). Approximately 5 × 104 cells in serum-free media were added to the top chamber, and the bottom chamber was filled with DMEM containing 10 % FBS. After incubation for 24 h, the cells on the upper surface of the membrane were gently removed with a cotton swab, and the membrane was fixed in 4 % methanol for 30 min and stained with 0.1 % crystal violet for 30 min. Cells that migrated to the bottom surface of the membrane were captured and counted. The same experimental design was used for the migration assay, but the membranes were not precoated with Matrigel.
RNA interference of VEGF
Expression of human VEGF was knocked down with small interfering RNA (siRNA) duplexes targeting the sequence gatccc AAGGAGTACCCTGATGAGATC ttcaagaga GATCTCATCAGGGTACTCCTT ttttttggaaa (sense) 5′-agcttttccaaaaaa AGGAGTACCCTGATGAGATC tctcttgaa GATCTCATCAGGGTACTCCTT g-3′ (antisense). The negative control (NC) siRNA 5′-UUC UCC GAA C-G UGU CAC GUT T-3′ and 5′-ACG UGA CAC GUU CGG –AG AAT T-3′ targeting an unknown messenger RNA (mRNA) sequence was used as a control. Both siRNAs were synthesized by Sigma-Aldrich. A BLAST search of the human genome verified that the selected sequences were specific for the target genes. Exponential growth-phase cells were plated in six-well plates at a density of 0.5 × 105 cells/ml, cultured for 24 h, and transfected with 1 μg siRNA in reduced serum medium at 30–50 % confluence.
Quantitative reverse transcription PCR
Total RNA was extracted with TRIzol reagent according to the manufacturer’s protocol. Next, cDNA was synthesized from 1 μg of total RNA using a PrimeScript RT Reagent kit (TaKaRa, Dalian, China). Real-time quantitative RT-PCR was performed using a SYBR green PCR master mix in an Applied Biosystems StepOne and StepOnePlus Real-Time PCR system. The gene expression ΔCt values of mRNA from each sample were calculated by normalizing with the reference gene glyceraldehyde 3-phosphate dehydrogenase (GAPDH). All experiments were repeated in triplicate to confirm the findings.
Western blotting
Western blotting was performed to confirm the results of our immunohistochemistry. Proteins were extracted from the fresh liver cancer tissues according to the manufacturer’s instructions (Yafa, Wuhan). After induction, cells were harvested and prepared for protein extraction [13]. The extracted proteins were separated by 10 % SDS-PAGE and then transferred to polyvinylidene fluoride membranes [13]. After being incubated with 10 % nonfat milk, the membranes were probed with primary antibodies raised against CD105 (1:500), VEGF (1:500), and GAPDH (1:3000) overnight at 4 °C and then incubated with HRP-labeled secondary antibodies. The relative expression level was quantified according to the reference protein GAPDH.
Statistical analyses
The correlation between tumor protein expression and the clinicopathological features was performed using chi-square or Fisher’s exact tests. A paired sample t test was used to compare the protein and mRNA expression in liver tumors with that of their paired adjacent normal liver tissue samples. Overall survival curves were calculated with the Kaplan-Meier method and were analyzed with the log-rank test. Differences or correlations between the two groups were assessed using Student’s t test and Pearson’s correlation test. All analyses were performed using SPSS 13.0 (SPSS, Inc., Chicago, USA). Statistical significance was defined as P < 0.05.
Results
CD105 and VEGF expression in human liver cancer tissue
We assessed the CD105 and VEGF expression in tumor tissue samples collected from 89 patients with liver cancer by immunohistochemistry. Our results show that CD105 was primarily expressed in the cell membrane, while VEGF was primarily expressed in the cytoplasm (Fig. 1a). Furthermore, there was a positive correlation between CD105 and VEGF expression (r = 0.32, P = 0.002). Importantly, CD105 and VEGF expression appeared to be higher in liver cancer tumors vs. normal liver tissue. This observation was confirmed by Western blotting, which shows that CD105 and VEGF expression was higher in liver cancer tissue compared to normal liver tissue (Fig. 1b).
The protein and mRNA expression level of CD105 and VEGF in liver tumors. Representative photomicrographs of normal tissue, well-differentiated hepatic cancer, and poorly differentiated hepatic cancer are shown. CD105 was detected in the plasmalemma and cytoplasm. VEGF was detected in the cytoplasm. Original magnification, ×200. Representative Western blot data showing the protein expression level of CD105 and VEGF are also presented
Relationship between CD105, VEGF, and clinicopathological parameters
Next, we analyzed the relationship between the CD105 and VEGF expression and the clinicopathological parameters of patients with liver cancer (Table 1). Interestingly, we found that CD105 and VEGF expression was closely related to the tumor-node-metastasis (TNM) staging of HCC, the differentiation degree, and lymph node metastasis (P < 0.05), while it was independent of gender, age, tumor size, and other clinical parameters. The results suggest that CD105 has close relation with VEGF in the progress of liver cancer. Meanwhile, the group results suggest that CD105 and VEGF protein can be used as indicators of clinical staging.
Lentivirus CD105 upregulates the expression of CD105 in SMMC-7721
We designed a lentivirus construct to increase the CD105 expression in SMMC-7721 cells. Our results demonstrate that we successfully engineered these cells to express higher levels of CD105, as confirmed at the mRNA and protein expression level (Fig. 2a, b). In contrast, the control construct had a little effect on CD105 mRNA and protein expression level.
Successful overexpression of CD105 in SMMC-7721 cells. Real-time PCR analyses of CD105 expression in vector- and wild-type CD105-transfected SMMC-7721 cells (a). Wild-type transfectants exhibited significantly increased CD105 expression. Representative Western blot analysis of wild-type GFP-fusion proteins in SMMC-7721 cells (b). Column 1 positive control, column 2 negative control using untransfected SMMC-7721 cells, columns 3 and 4 wild-type GFP-fusion proteins of CD105. Phase contrast of the transfected SMMC-7721 cells (c, d). **P < 0.05
CD105 enhanced invasion and metastatic ability of SMMC-7721 cells
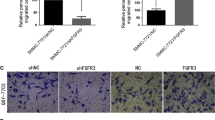
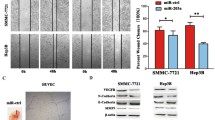
In order to determine the effect of CD105 overexpression on invasion and metastasis of liver cancer cells, we measured these parameters in SMMC-7721 stably overexpressing CD105. Cell motility was investigated by wound healing assay, revealing that 48 h after scratching, CD105-SMMC-7721 cells had healed the wound to a greater extent than the NC group cells (Fig. 3). We then conducted a Transwell chamber-based three-dimensional cell migration assay to investigate the impact of CD105 on directional motility. Consistent with the aforementioned results, the CD105-overexpressing cells exhibited an augmented ability to migrate from the top of the chamber to the bottom during a 48-h period (Fig. 3). Taken together, these results demonstrate that CD105 overexpression promotes hepatocarcinoma cell migration.
CD105 overexpression promotes HCC cell invasion. The effect of CD105 overexpression on cell invasion ability, as examined by wound healing assay and Transwell invasion assay (a, b). The effect of CD105 on the expression of VEGF as evaluated by real-time PCR and Western blot, respectively (c–e). The results are expressed as the mean ± SD from three independent experiments. **P < 0.05
Increased VEGF expression in CD105-SMMC-7721 cells
VEGF mRNA and protein expression was measured in SMMC-7721 cells stably overexpressing CD105. Our mRNA data indicate that VEGF expression was increased by eightfold in CD105-SMMC-7721 cells compared to the NC group. Similarly, we also found that VEGF expression was significantly higher in CD105-SMMC-7721 cells at the protein level (P < 0.05).
Blocking VEGF inhibits the invasion and metastasis of CD105-SMMC-7721 cells
In order to verify that increased CD105 expression promoted hepatocarcinoma cell invasion and metastases via VEGF, we used siRNA to block the VEGF expression in CD105-SMMC-7721 cells. First, we confirmed that VEGF siRNA effectively blocked the expression of VEGF at the mRNA and protein level (Fig. 4). Next, our wound healing and cell migration assay revealed that blocking VEGF could effectively inhibit the CD105-mediated potentiation of invasion and metastasis in CD105-SMMC-7721 cells (Fig. 4).
Downregulation of VEGF by siRNA partially reverses the effect of CD105 overexpression on cell invasion. The protein and mRNA expression of VEGF which was analyzed by Western blotting and real-time PCR is shown in cells transfected with VEGF siRNA (a, b). Results from the wound healing assay and Transwell invasion assay show that the invasion was partially abrogated in VEGF-silenced cells (c, d)
Discussion
Increased fat metabolism and high rates of proliferation are unique characteristics of tumor cells leading to a much higher demand on oxygen, glucose, and other energy substrates compared to normal cells [14]. Meanwhile, increasing tumor volume increases the distance between tumor cells and the oxygen- and nutrient-supplying blood vessels, which can lead to nutritional deficiencies in the tumor microenvironment [15, 16]. To overcome these deficiencies, neovascularization of tumors is stimulated by tumor cells to ensure adequate supply of the required factors [4].
Tumor angiogeneses play an important role in the tumor metastasis and growth and provide essential nutrient sources and transfer pathways for tumor tissue metabolism. Studies have shown that tumor angiogenesis is affected by regulating a variety of factors, including CD105 focused.
CD105-encoding gene is located in human chromosome 9q34-qter24, containing 633 amino acids, and is a hypoxia-inducible protein associated with proliferation. CD105 protein is distributed into intracellular, transmembrane, and extracellular proteins, which contains 47, 25, and 561 amino acids, respectively. Most studies have shown that CD105 plays a major role in tumor angiogenesis diagnosis and treatment [18, 19]. Although the function of CD105 is not entirely clear, many studies have demonstrated that it plays an important role in the process of growth and development of blood vessels and maintenance of vascular integrity. CD105 also has relevance with tumor growth, proliferation, and metastasis. In this respect, previous research has shown that the function of CD105 is closely related to angiogenesis [17]. Because of this, CD105 expression is thought to be a good molecule for assessing the genesis and development of malignant tumors as well as tumor prognosis [18, 19]. Currently, several studies have shown that CD105 and VEGF play an important role in the diagnosis of tumor angiogenesis, therapy, and other processes [20–24]. Vascular endothelial growth factor (VEGF) is an effective human angiogenic factor and promotes angiogenesis by participating in the angiogenesis process directly or indirectly. VEGF gene is located in chromosome 6p21.3, a total length of 14 kb, containing eight exons and seven introns. VEGF as a major angiogenesis and vascular permeability factor plays an extremely important role in the biological behavior of a variety of tumors. Thus, inhibition of VEGF can inhibit the pathological angiogenesis, tumor growth, and metastasis. It is significant in the gene therapy of tumors.
Because of this link between CD105 and angiogenesis and the known importance of VEGF in this process, we sought to determine the relationship between VEGF and CD105 in HCC.
Here, we report that CD105 and VEGF expression is positively correlated in human liver tumor tissue and that the expression of both factors was closely related to clinicopathological parameters, such as TNM, tumor size, and lymph node metastasis. We interpret these data to indicate that CD105 and VEGF are involved in the occurrence and development of liver cancer; however, our data did not definitely establish that these factors were linked. To address this issue, we engineered SMMC-7721 cells to stably overexpress CD105. The invasion capacity of hepatoma cell SMMC-7721-overexpressed CD105 is enhanced through permeable membrane and scratches. CD105 promote the invasion of hepatoma cell SMMC-7721 whether has related with VEGF. Western blot and real-time PCR results showed that the expression of VEGF protein and mRNA-overexpressed CD105 was significantly higher than that of the control group and increased by sixfold and eightfold, respectively, suggesting that the ability of CD105 to promote the invasion and metastases of liver cancer cells might be associated with increased VEGF expression. To further demonstrate the CD105 promotes invasion and metastasis of hepatocellular carcinoma cells either through VEGF or not, siRNA technology is used to block VEGF and then to test CD105 impacting the invasion and metastasis of liver cancer cells. Our results showed that the invasion and metastasis of hepatocellular carcinoma cells was significantly inhibited by overexpressed CD105 after blocking the expression of VEGF. The results suggest that CD105 promotes the invasion of liver cancer cell by promoting the expression of VEGF achieved.
This interpretation was confirmed by using siRNA to block VEGF expression in CD105-overexpressing SMMC-7721 cells, demonstrating that the ability of CD105 to promote liver cancer cell invasion and metastasis required elevated levels of VEGF expression.
In conclusion, our experiments prove that CD105 and VEGF are involved in the occurrence and development of liver cancer and that CD105 promotes the invasion and metastases of liver cancer cells by increasing VEGF expression. These results provided a new theoretical and experimental basis for the treatment of liver cancer.
References
Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29.
El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557–76.
Han ZB, Chen HY, Fan JW, et al. Up-regulation of microRNA-155 promotes cancer cell invasion and predicts poor survival of hepatocellular carcinoma following liver transplantation. J Cancer Res Clin Oncol. 2012;138:153–61.
Mittal K, Ebos J, Rini B. Angiogenesis and the tumor microenvironment: vascular endothelial growth factor and beyond. Semin Oncol. 2014;41:235–51.
Quackenbush EJ, Letarte M. Identification of several cell surface proteins of non-T, non-B acute lymphoblastic leukemia by using monoclonal antibodies. J Immunol. 1985;34:1276–85.
Seon BK, Haba A, Matsuno F, et al. Endoglin-targeted cancer therapy. Curr Drug Deliv. 2011;8:135–43.
Henry-Berger J, Mouzat K, Baron S, et al. Endoglin (CD105) expression is regulated by the liver X receptor alpha (NR1H3) in human trophoblast cell line JAR. Biol Reprod. 2008;78:968–75.
Hsueh C, Lin JD, Wu IC, et al. Vascular endothelial growth factors and angiopoietins in presentations and prognosis of papillary thyroid carcinoma. J Surg Oncol. 2011;103:395–99.
Scartozzi M, Faloppi L, Svegliati Baroni G, et al. VEGF and VEGFR genotyping in the prediction of clinical outcome for HCC patients receiving Sorafenib: the ALICE-1 study. Int J Cancer. 2014;135:1247–56.
Wood LM, Pan ZK, Guirnalda P, et al. Targeting tumor vasculature with novel Listeria-based vaccines directed against CD105. Cancer Immunol Immunother. 2011;60:931–42.
Li H, Sui C, Kong F, et al. Expression of HSP70 and JNK-related proteins in human liver cancer: potential effects on clinical outcome. Dig Liver Dis. 2007;39:663–70.
Darakhshan S, Bidmeshkipour A, Mansouri K, et al. The effects of tamoxifen in combination with tranilast on CXCL12-CXCR4 axis and invasion in breast cancer cell lines. Iran J Pharm Res. 2014;13:683–93.
Biao W, Wei X, Yan Z, Xin D. Effects of chronic aluminum exposure on memory through multiple signal transduction pathways. Environ Toxicol Pharmacol. 2010;29:308–13.
Jain RK. Normalizing tumor microenvironment to treat cancer: bench to bedside to biomarkers. J Clin Oncol. 2013;31:2205–18.
Gacche RN, Meshram RJ. Targeting tumor micro-environment for design and development of novel anti-angiogenic agents arresting tumor growth. Prog Biophys Mol Biol. 2013;113:333–54.
Hagberg CE, Mehlem A, Falkevall A, et al. Targeting VEGF-B as a novel treatment for insulin resistance and type 2 diabetes. Nature. 2012;490:426–30.
Kumagai Y, Sobajima J, Higashi M, et al. Angiogenesis in superficial esophageal squamous cell carcinoma: assessment of microvessel density based on immunostaining for CD34 and CD105. Jpn J Clin Oncol. 2014;44:526–33.
Nassiri F, Cusimano MD, Scheithauer BW, et al. Endoglin (CD105): a review of its role in angiogenesis and tumor diagnosis, progression and therapy. Anticancer Res. 2011;31:2283–90.
Saroufim A, Messai Y, Hasmim M, et al. Tumoral CD105 is a novel independent prognostic marker for prognosis in clear-cell renal cell carcinoma. Br J Cancer. 2014;110:1778–84.
Cwiklnska A, Sobstyl M, Kwasniewski W, Bednarek W. Microtissue density prognostic factor evaluation based on antigens CD34 and CD 105 in ovarian cancer patients. Ann Agric Environ Med. 2013;20:838–42.
Hsieh MC, Hsu HT, Hsiao PC, et al. Role of VEGF-C gene polymorphisms in susceptibility to hepatocellular carcinoma and its pathological development. J Clin Lab Anal. 2014;28:237–44.
Benazzi C, Al-Dissi A, Chau CH, et al. Angiogenesis in spontaneous tumors and implications for comparative tumor biology. Scientific World Journal. 2014;2014:919570.
Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473:298–307.
Benedito R, Rocha SF, Woeste M, et al. Notch-dependent VEGFR3 upregulation allows angiogenesis without VEGF-VEGFR2 signalling. Nature. 2012;484:110–4.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (No. 81071955) and the Scientific Research from Educational Department of Liaoning Province, China (No. 2011225019).
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, Y., Zhai, Z., Liu, D. et al. CD105 promotes hepatocarcinoma cell invasion and metastasis through VEGF. Tumor Biol. 36, 737–745 (2015). https://doi.org/10.1007/s13277-014-2686-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-014-2686-2