Abstract
3-Phosphoinositide-dependent protein kinase 1 (PDK1) is centrally involved in cancer progression, including proliferation, apoptosis and invasion. However, its expression pattern and possible cellular functions in human colorectal cancer remain unclear. In the present study, we show that PDK1 expression is up-regulated at both mRNA and protein levels in colorectal cancer clinical specimens and cell lines. Transient knockdown of PDK1 suppresses cellular growth, induces cellular apoptosis and causes abnormal cell cycle distribution. Meanwhile, decreased PDK1 level is closely associated with reduced Akt/cyclin D1 activity. Activating AKT activity and reintroducing cyclin D1 expression significantly compromised the oncogenic activity induced by PDK1. Together, our findings elucidate a key role for PDK1 in colorectal cellular functions trigged by the Akt/cyclin D1 pathway, thus providing a novel insight of PDK1 in colorectal carcinogenesis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
As the second and third most common malignant tumor among females and males worldwide, colorectal cancer (CRC) has been considered as a major public health concern [1, 2]. Although recent therapeutic strategies have significantly improved the diagnosis and treatment for patients with CRC, the overall prognosis for patients with CRC remains poor [3]. This emphasizes an urgent need for investigating the molecular and genetic mechanisms responsible for CRC development.
3-Phosphoinositide-dependent protein kinase 1 (PDK1) has been identified to play a crucial role in driving tumor progression and cellular signaling [4–7]. Increased copy number of PDK1 is present in breast cancer and correlates with the mutation of PI3K class 1 p110 or with the over-expression of ERBB2 [8, 9]. Furthermore, increased levels of PDK1 have been reported in mammary epithelial cells [10], acute myeloid leukemia [11], non-small cell lung cancer (NSCLC) [12] and ovarian cancer [13]. However, the expression pattern and underlying cellular functions of PDK1 in CRC remain vague.
In this paper, we first observed increased expression of PDK1 in CRC clinical specimens as well as colorectal cancer cell lines. To explore the potential roles of PDK1 in CRC cells, we performed in vitro experiments and confirmed that elevated PDK1 is involved in cell proliferation, apoptosis and cell cycle distribution of CRC cells. Mechanistically, the down-regulation of PDK1 expression is closely correlated with the phosphorylation level of Akt/cyclin D1.
Materials and methods
Cell culture
Human CRC cell lines DLD-1, HCT8, HCT116, SW480, SW620 and LOVO were all obtained from American Type Culture Collection (ATCC) and maintained in RPMI 1640 or DMEM (Gibco) supplemented with 10 % fetal bovine serum (FBS) at 37 °C in a humidified incubator with 5 % CO2.
Immunohistochemistry
Colorectal cancer tissue array (CO1501) contained 66 cases of tumor tissues and 5 cases of normal tissues was obtained from Xi-an Alenabio Inc (Xi-an, China). The tissue sections were baked at 65 °C for 2 h and deparaffinized. Antigens were retrieved at 95 °C for 15 min. The slides were incubated in 3 % hydrogen peroxide for 15 min and treated with 0.5 % Triton X-100 for 15 min. Then, the sections were blocked with 10 % BSA for 30 min and incubated with anti-PDK1 antibody (Abcam, USA) overnight. After being washed in PBS for 30 min, the slides were treated with secondary antibody at 37 °C for 30 min. According to the ratio and intensity of positive-staining cells, scoring was conducted by two pathologists.
Quantitative real-time PCR
Twenty-five paired freshly frozen CRC tissues and corresponding non-cancerous tissues were also obtained from Shenzhen People’s Hospital, Second Clinical Medical College of Jinan University, China. Collection of clinical specimens was performed in full accordance with the Medicine Institutional Guidelines of Second Clinical Medical College of Jinan University. All the patients joined this study have signed informed consent. Total RNA exacted from cells or clinical specimens using Trizol reagent (Invitrogen, USA) according to manufacturer’s instructions. cDNA was synthesized using PrimeScript RT reagent kit (Takara, Japan). Real-time PCR was carried out using SYBR Green PCR master mix (Takara, Japan). Thermal cycling was performed by an initial denaturing step at 95 °C for 3 min followed by 40 cycles of denaturation at 95 °C for 10 s and then 60 °C for 1 min for annealing. Amplification and detection were performed using ABI7500 system. PDK1 was amplified using the sense primer 5′-GCGAATTCGACTTGGGGCTCATGGCC-3′ and antisense primer 5′-GCTCTAGACTGGTGCCAAGGGTTTCC-3′. GAPDH was used as control and amplified using sense primer 5′-GTCAGTGGTGGACCTGACCT-3′ and antisense primer 5′-GACTTGACAAAGTGGTCG-3′.
siRNA interference and gene cloning transfection
Short interfering RNA (siRNA) designed for PDK1 was used to knockdown expression of PDK1. HCT116 and SW480 cells were transfected with PDK1-specific siRNA using RNAi Mate Transfection Agent (GenePharma). After indicated incubation time, interfere efficiency was analyzed in qRT-PCR or western blot experiments. The pcDNA3.CCND1 plasmid (Life Technologies, MD), the pcDNA3.BTBD10 plasmid (Life Technologies, MD) and pcDNA3.1Neo vector control plasmid (Life Technologies, MD) were transfected into HCT116 and SW480 cells according to the manufacturer’s protocol. The cell culture medium was refreshed 8 h after transfection.
Western blot analysis
Cells were lysed in RIPA lysis buffer, and supernatant was collected after centrifuging. Equal amount of proteins were separated by SDS-PAGE and transferred to nitrocellulose membranes (BioRad, CA). After blocking in 5 % skimmed milk in PBS for 1 h at room temperature, membranes were incubated with primary antibodies overnight: anti-PDK1 (Abcam), anti-p-Akt (T308), anti-p-Akt (S473), anti-Akt, anti-cyclin D1, anti-cyclin D2, anti-cyclin E (Cell Signaling Technology, MA) and GAPDH (Abcam, MA) overnight at 4 °C. The membranes were then probed by horseradish peroxidase-conjugated goat anti-rabbit IgG in 1:5000 dilution (GE Healthcare). The blots were developed using enhanced chemiluminescence solution (Thermo Scientific), and images were captured by Image J software.
MTT assay
Proliferation of colorectal cells was evaluated by MTT assay. Cells were seeded in duplicate in 96-well plates at 3 × 103 cells per well overnight followed by addition of MTT (5 mg/ml) to a final concentration of 0.5 mg/ml and incubation of the plate at room temperature for 3 h. Cell viability was obtained by measuring the absorbance at 595 nm wavelength with Fluostar Optima plate reader (BIO-TEK).
Colony formation assay
PDK1 siRNA-treated and control siRNA-treated cells were seeded into 6-cm2 plates, and after 2 weeks, the cells were fixed with methyl alcohol for 15 min and stained with Gimsa for 30 min. Then, the colonies were counted. Each sample was measured in triplicate to ensure quantitative accuracy.
Apoptosis assay
The apoptosis of HCT116 and SW480 cells was analyzed using Annexin V/PI apoptosis detection kit I (BD Pharmingen). PDK1 siRNA-treated and control siRNA-treated cells were seeded into 6-cm2 plates and harvested for 24 h. After washing with cold PBS for three times, the cells were incubated in 300 μL binding buffer containing 5 μL of Annexin V and PI for 20 min. Samples were analyzed by flow cytometry (BD Biosciences Clontech).
Cell cycle assay
PDK1 siRNA-treated and control siRNA-treated cells were seeded into 6-cm2 plates and incubated for 24 h. Then, the cells were trypsinized, washed with PBS and resuspended in 300 μL binding buffer containing 0.1 % Triton X-100 and 90 units RNase A in −20 °C overnight. Then, the cells were incubated in 300 μL binding buffer containing 5 μL of PI for 20 min and analyzed by flow cytometry (BD Biosciences Clontech).
Statistical analysis
Multiple comparisons were assessed by the use of SPSS 16.0 software (SPSS Inc, Chicago, IL) for statistical analysis. Difference between groups was assessed by Student’s t test or one-way ANOVA. All values were shown as mean ± SD. P < 0.05 was considered statistically significant.
Results
Increased expression of PDK1 in colorectal cancer specimens and cell lines
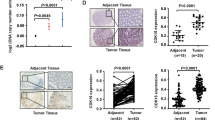
It has been reported that PDK1 has an essential role in human malignancies, such as breast cancer [8, 9] and ovarian cancer [11, 13]. To explore the possible role of PDK1 in CRC development, we examined the expression of PDK1 in 25 pairs of colorectal tumor samples and normal samples by qRT-PCR. As shown in Fig. 1a, the mRNA expression levels of PDK1 in colorectal tumor samples were significantly higher than those in normal samples (P < 0.001). Then, PDK1 expression in colorectal cell lines was examined by qRT-PCR and western blot, and the result showed that high mRNA (Fig. 1b) and protein (Fig. 1c) expression levels of PDK1 in six colorectal cells were found. Next, the expression of PDK1 in colorectal tissue assays was evaluated by immunohistochemistry. Indeed, PDK1 was over-expressed in 72.7 % (48/66) of all detected specimens (Fig. 1d). These results indicate that PDK1 expression dysregulated in CRC and might play a role in pancreatic cancer development and progression.
Expression of PDK1 was increased in CRC patients and cells. a Quantitative analysis of the expression levels of PDK1 in CRC samples and matched normal colon samples. b mRNA expression level of PDK1 in CRC cells by qRT-PCR. c Protein expression level of PDK1 in CRC cell lines was detected by western blot. d Representative immunohistochemistry photographs showing the expression status of PDK1 protein in CRC tissues and adjacent normal tissues (scale bar 50 μm)
Transient knockdown of PDK1 inhibits CRC cell growth
To determine the roles of PDK1 in CRC, we chosen HCT116 and SW480 cells to transient knockdown PDK1 expression. The efficiency was measured by western blot (Fig. 2a) and qRT-PCR, respectively (Fig. 2b). Thereafter, MTT and plate colony formation assays were performed to assess the cell proliferation after suppression of PDK1. Significantly decreased proliferation rate was observed in HCT116 (P < 0.001, Fig. 2c) and SW480 (P < 0.05, Fig. 2d) cells transfected with PDK1 siRNA as compared to cells transfected with control siRNA by MTT assay. The plate colony formation assay showed similar result that the number of the colonies formed was markedly decreased in HCT116 and SW480 cells transfected with PDK1 siRNA as compared to cells transfected with control siRNA (P < 0.01, Fig. 2e). The observation was further confirmed by the expression of Ki67, a proliferation index, as demonstrated by western blot. We observed that the protein expression of Ki67 in HCT116 cells down-regulated compared with control cells (P < 0.01, Fig. 2f). Taken together, these data suggest that elevated PDK1 was indeed involved in the regulation of colorectal cancer cell growth.
Effects of PDK1 on CRC cell proliferation. PDK1 expression of HCT116 and SW480 cells transfected with siRNA specifically targeting PDK1 by western blot (a) and qRT-PCR (b). Growth curves for HCT116 (c) and SW480 (d) cells were measured after transfected with siRNA specifically targeting PDK1. e Quantitative results for plate colony formation assays. HCT116 and SW480 cells transfected with siRNA specifically targeting PDK1 and their control cells were cultured in 6-cm2 plates for 2 weeks. f Western blot results for proliferation marker of Ki67 in HCT116 cells after silencing of PDK1. NC versus si1 or si2; **P < 0.01; ***P < 0.001
Transient knockdown of PDK1 induces cellular apoptosis and causes abnormal cell cycle distribution
Annexin V/PI staining was applied to quantify the number and stage of apoptotic cells. As illustrated in Fig. 3a, b, the early apoptotic proportions were increased in HCT116 and SW480 cells transfected with PDK1 siRNA as compared to control cells. Then, we examined the impact of PDK1 transient knockdown on cell cycle distribution in HCT116 and SW480 cells by flow cytometry. We found that PDK1 transient knockdown dramatically increased cell percentage at G0/G1 and G2/M phase as well as correspondingly reduced cell percentage at S phase (Fig. 3c, d).
Effects of PDK1 on CRC cell apoptosis and cell cycle. Quantification of early cell apoptosis ratio in HCT116 (a) and SW480 (b) cells transfected with siRNA specifically targeting PDK1 and their control cells. Cell cycle distribution in HCT116 (c) and SW480 (d) cells was measured after transfected with siRNA specifically targeting PDK1. NC versus si1 or si2; **P < 0.01; ***P < 0.001
Akt/cyclin D1 signaling pathways mediate the oncogenic activity of PDK1
Akt is a critical survival kinase that controls cell proliferation and death. PDK1 membrane localization is essential for Akt phosphorylation at T308 [14], and S473 is phosphorylated by the mammalian target of rapamycin [15, 16]. To investigate possible downstream signaling related to PDK1-induced proliferation, apoptosis and abnormal cycle distribution, Akt was examined by western blot assay. The down-regulation in phosphorylation of both Akt (T308) and Akt (S473) was detected in HCT116 and SW480 cells transfected with PDK1 siRNA compared to control cells (Fig. 4a). It was reported that PDK1 regulates cell proliferation and cell cycle progression through control of cyclin D1 expression [17]. So we examined cyclin D1, cyclin D2 and cyclin E expression after PDK1 was silenced. As shown in Fig. 4b, silencing of PDK1 resulted in significant decrease expression of cyclin D1 and cyclin D2, but not cyclin E. To further confirm the role of Akt/cyclin D1 in PDK1-mediated cellular functions, we activated AKT activity by over-expressing BTBD10 [18] (Fig. 5a) and found that the activator significantly compromised the ability of PDK1 to promote proliferation (Fig. 5c), inhibit apoptosis (Fig. 5e) and cause abnormal cell cycle distribution (Fig. 5g). In addition, similar results were observed by over-expressing cyclin D1 (Fig. 5b, d, f, h). Collectively, these data above indicate that altered Akt/cyclin D1 signaling is involved in PDK1-mediated cellular functions in colorectal cancer.
Suppression of PDK1 resulted in decreased Akt/cyclin D1 activity. a Western blot results for Akt expression at Thr308 and Ser573 in HCT116 and SW480 cells transfected with siRNA specifically targeting PDK1. b Western blot results for cyclin D1, cyclin D2 and cyclin E expression in HCT116 and SW480 cells transfected with siRNA specifically targeting PDK1
Akt/cyclin D1 signaling pathways mediated the oncogenic activity of PDK1. a Akt activity was measured after transfected with pcDNA3.BTBD10 plasmid. b Cyclin D1 expression was measured after transfected with pcDNA3.CCND1 plasmid. Growth curves for HCT116 (c) and SW480 (d) cells, colony formation assay (e), cell apoptosis (f), as well as cell cycle distribution of HCT116 (g) and SW480 (h) were measured after transfected with pcDNA3.BTBD10 plasmid and pcDNA3.CCND1 plasmid. si1 or si1 + BTBD10 or si1 + CCND1; ***P < 0.001
Discussion
CRC is the third most common cancer worldwide and more common in developed countries than developing countries [19–21]. The molecular genesis of CRC and therapies promises major advances [22]. However, achievements in targeted therapy have not dramatically improved the outcomes of patients with colorectal cancer. PDK1 belongs to the AGS kinases family [23]. It is a protein of 556 amino acids [24] and is discovered as the kinase for the phosphorylation of the Akt activation loop [25]. It has been reported that PDK1 plays a pivotal role in breast cancer, acute myeloid leukemia and ovarian cancer progression [8, 10, 24]. PDK1 has an essential role in regulating cell migration and invasion [26–28]. Nagashima et al. [29] have reported pharmacological but not genetic inhibition of PDK1 resulted in tumor cell growth arrest. Although extensive researches show that PDK1 plays a crucial role in various cancer progressions, the functions of PDK1 in CRC are not well studied.
To investigate the potential role of PDK1 in CRC development, we first examined the expression of PDK1 in clinical specimens by qRT-PCR and immunohistochemistry, respectively. We found that PDK1 expression at mRNA and protein levels was significantly higher in CRC tissues than in its matched non-tumor tissues. Han et al. [12] reported that aberrant expression of PDK1 mRNA and miR-138 in NSCLC patients’ sera correlated with patient’s survival, TNM stage and positive lymph node metastasis. Combined with the observation that elevated expression of PDK1 in colorectal cell lines, we speculated that PDK1 might contribute to colorectal tumorigenesis.
Aberrant PDK1 expression is a strong indicator of invasion and metastasis, especially with deficient PTEN [26]. PDK1 controls migration and malignant transformation [30, 31]. Furthermore, it has been shown that PDK1 regulates cell motility [32]. Different from the implications of PDK1 on these previous reports, suppression of PDK1 significantly decreased proliferation and plate colony formation assays, promoted cell apoptosis and caused abnormal cell cycle distribution. These observations indicate that PDK1 affects both tumor growth and metastasis in CRC.
Regulation of PDK1-activated signaling involves in various mechanisms. It is still controversial whether PDK1 translocates to or is localized to the plasma membrane [33]. PDK1 membrane localization is essential for Akt phosphorylation [34, 35]. To precise mechanisms by which PDK1 impacts on the functions in CRC cell lines, our study focused on Akt pathway and showed that phosphorylation of both Akt (T308) and Akt (S473) was down-regulated after knockdown of PDK1. Dennis et al. report that cyclin D1 performs a critical cell cycle regulation during G2 phase and is a normal part of cell cycle progression [36]. Consistent with this, our data revealed that reduced expression of cyclin D1 and cyclin D2 induced by inhibition of PDK1. Furthermore, gain-of-function studies confirmed the roles of AKT activity and cyclin D1 in the oncogenic activity induced by PDK1.
In conclusion, in this study, we provide evidence that PDK1 promotes tumor growth through altered Akt/cyclin D signaling and indicate that PDK1-related pathway might represent a novel strategic therapy for colorectal cancer.
References
Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64(1):9–29. doi:10.3322/caac.21208.
Siegel R, Desantis C, Jemal A. Colorectal cancer statistics, 2014. CA Cancer J Clin. 2014;64(2):104–17. doi:10.3322/caac.21220.
Wood LD, Parsons DW, Jones S, Lin J, Sjoblom T, Leary RJ, et al. The genomic landscapes of human breast and colorectal cancers. Science. 2007;318(5853):1108–13. doi:10.1126/science.1145720.
Mora A, Komander D, van Aalten DM, Alessi DR. PDK1, the master regulator of AGC kinase signal transduction. Semin Cell Dev Biol. 2004;15(2):161–70.
Biondi RM. Phosphoinositide-dependent protein kinase 1, a sensor of protein conformation. Trends Biochem Sci. 2004;29(3):136–42. doi:10.1016/j.tibs.2004.01.005.
Bayascas JR. Dissecting the role of the 3-phosphoinositide-dependent protein kinase-1 (PDK1) signalling pathways. Cell Cycle. 2008;7(19):2978–82.
Bayascas JR. PDK1: the major transducer of PI 3-kinase actions. Curr Top Microbiol Immunol. 2010;346:9–29. doi:10.1007/82_2010_43.
Lin HJ, Hsieh FC, Song H, Lin J. Elevated phosphorylation and activation of PDK-1/AKT pathway in human breast cancer. Br J Cancer. 2005;93(12):1372–81. doi:10.1038/sj.bjc.6602862.
Maurer M, Su T, Saal LH, Koujak S, Hopkins BD, Barkley CR, et al. 3-Phosphoinositide-dependent kinase 1 potentiates upstream lesions on the phosphatidylinositol 3-kinase pathway in breast carcinoma. Cancer Res. 2009;69(15):6299–306. doi:10.1158/0008-5472.CAN-09-0820.
Zeng X, Xu H, Glazer RI. Transformation of mammary epithelial cells by 3-phosphoinositide-dependent protein kinase-1 (PDK1) is associated with the induction of protein kinase Calpha. Cancer Res. 2002;62(12):3538–43.
Pearn L, Fisher J, Burnett AK, Darley RL. The role of PKC and PDK1 in monocyte lineage specification by Ras. Blood. 2007;109(10):4461–9. doi:10.1182/blood-2006-09-047217.
Han L, Zhang G, Zhang N, Li H, Liu Y, Fu A, et al. Prognostic potential of microRNA-138 and its target mRNA PDK1 in sera for patients with non-small cell lung cancer. Med Oncol. 2014;31(9):129. doi:10.1007/s12032-014-0129-y.
Ahmed N, Riley C, Quinn MA. An immunohistochemical perspective of PPAR beta and one of its putative targets PDK1 in normal ovaries, benign and malignant ovarian tumours. Br J Cancer. 2008;98(8):1415–24. doi:10.1038/sj.bjc.6604306.
Osaki M, Oshimura M, Ito H. PI3K-Akt pathway: its functions and alterations in human cancer. Apoptosis Int J Program Cell Death. 2004;9(6):667–76. doi:10.1023/B:APPT.0000045801.15585.dd.
Guertin DA, Sabatini DM. Defining the role of mTOR in cancer. Cancer Cell. 2007;12(1):9–22. doi:10.1016/j.ccr.2007.05.008.
Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129(7):1261–74. doi:10.1016/j.cell.2007.06.009.
Nakamura K, Sakaue H, Nishizawa A, Matsuki Y, Gomi H, Watanabe E, et al. PDK1 regulates cell proliferation and cell cycle progression through control of cyclin D1 and p27Kip1 expression. J Biol Chem. 2008;283(25):17702–11. doi:10.1074/jbc.M802589200.
Nawa M, Kanekura K, Hashimoto Y, Aiso S, Matsuoka M. A novel Akt/PKB-interacting protein promotes cell adhesion and inhibits familial amyotrophic lateral sclerosis-linked mutant SOD1-induced neuronal death via inhibition of PP2A-mediated dephosphorylation of Akt/PKB. Cell Signal. 2008;20(3):493–505. doi:10.1016/j.cellsig.2007.11.004.
Sharma RA, Dalgleish AG, Steward WP, O’Byrne KJ. Angiogenesis and the immune response as targets for the prevention and treatment of colorectal cancer (review). Oncol Rep. 2003;10(5):1625–31.
Russo MW, Wei JT, Thiny MT, Gangarosa LM, Brown A, Ringel Y, et al. Digestive and liver diseases statistics, 2004. Gastroenterology. 2004;126(5):1448–53.
Littlejohns P, Tamber S, Ranson P, Campbell B. Treatment for liver metastases from colorectal cancer. Lancet Oncol. 2005;6(2):73.
Weitz J, Koch M, Debus J, Hohler T, Galle PR, Buchler MW. Colorectal Cancer. Lancet. 2005;365(9454):153–65. doi:10.1016/S0140-6736(05)17706-X.
Pearce LR, Komander D, Alessi DR. The nuts and bolts of AGC protein kinases. Nat Rev Mol Cell Biol. 2010;11(1):9–22. doi:10.1038/nrm2822.
Wick MJ, Ramos FJ, Chen H, Quon MJ, Dong LQ, Liu F. Mouse 3-phosphoinositide-dependent protein kinase-1 undergoes dimerization and trans-phosphorylation in the activation loop. J Biol Chem. 2003;278(44):42913–9. doi:10.1074/jbc.M304172200.
Alessi DR, James SR, Downes CP, Holmes AB, Gaffney PR, Reese CB, et al. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Balpha. Current Biol. 1997;7(4):261–9.
Leslie NR, Yang X, Downes CP, Weijer CJ. The regulation of cell migration by PTEN. Biochem Soc Trans. 2005;33(Pt 6):1507–8. doi:10.1042/BST20051507.
Lim MA, Yang L, Zheng Y, Wu H, Dong LQ, Liu F. Roles of PDK-1 and PKN in regulating cell migration and cortical actin formation of PTEN-knockout cells. Oncogene. 2004;23(58):9348–58. doi:10.1038/sj.onc.1208147.
Rodriguez OC, Lai EW, Vissapragada S, Cromelin C, Avetian M, Salinas P, et al. A reduction in Pten tumor suppressor activity promotes ErbB-2-induced mouse prostate adenocarcinoma formation through the activation of signaling cascades downstream of PDK1. Am J Pathol. 2009;174(6):2051–60. doi:10.2353/ajpath.2009.080859.
Nagashima K, Shumway SD, Sathyanarayanan S, Chen AH, Dolinski B, Xu Y, et al. Genetic and pharmacological inhibition of PDK1 in cancer cells: characterization of a selective allosteric kinase inhibitor. J Biol Chem. 2011;286(8):6433–48. doi:10.1074/jbc.M110.156463.
Liu Y, Wang J, Wu M, Wan W, Sun R, Yang D, et al. Down-regulation of 3-phosphoinositide-dependent protein kinase-1 levels inhibits migration and experimental metastasis of human breast cancer cells. Mol Cancer Res. 2009;7(6):944–54. doi:10.1158/1541-7786.MCR-08-0368.
Finlay DK, Sinclair LV, Feijoo C, Waugh CM, Hagenbeek TJ, Spits H, et al. Phosphoinositide-dependent kinase 1 controls migration and malignant transformation but not cell growth and proliferation in PTEN-null lymphocytes. J Exp Med. 2009;206(11):2441–54. doi:10.1084/jem.20090219.
Pinner S, Sahai E. PDK1 regulates cancer cell motility by antagonising inhibition of ROCK1 by RhoE. Nat Cell Biol. 2008;10(2):127–37. doi:10.1038/ncb1675.
Currie RA, Walker KS, Gray A, Deak M, Casamayor A, Downes CP, et al. Role of phosphatidylinositol 3,4,5-trisphosphate in regulating the activity and localization of 3-phosphoinositide-dependent protein kinase-1. Biochem J. 1999;337(Pt 3):575–83.
Milburn CC, Deak M, Kelly SM, Price NC, Alessi DR, Van Aalten DM. Binding of phosphatidylinositol 3,4,5-trisphosphate to the pleckstrin homology domain of protein kinase B induces a conformational change. Biochem J. 2003;375(Pt 3):531–8. doi:10.1042/BJ20031229.
Calleja V, Ameer-Beg SM, Vojnovic B, Woscholski R, Downward J, Larijani B. Monitoring conformational changes of proteins in cells by fluorescence lifetime imaging microscopy. Biochem J. 2003;372(Pt 1):33–40. doi:10.1042/BJ20030358.
Stacey DW. Cyclin D1 serves as a cell cycle regulatory switch in actively proliferating cells. Curr Opin Cell Biol. 2003;15(2):158–63.
Conflict of interest
The authors declare that there is no conflict of interests.
Author information
Authors and Affiliations
Corresponding author
Additional information
Zhenglei Xu and Bihong Liao have contributed equally to this article.
Rights and permissions
About this article
Cite this article
Xu, Z., Liao, B., Zhang, R. et al. Expression of 3-phosphoinositide-dependent protein kinase 1 in colorectal cancer as a potential therapeutic target. Med Oncol 32, 198 (2015). https://doi.org/10.1007/s12032-015-0645-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-015-0645-4