Abstract
Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) reverses multidrug resistance (MDR) and induces apoptosis in MDR gastric carcinoma cells. In our previous study, cisplatin proved to be a sensitizing agent for TRAIL. To study the synergistic effects of cisplatin and TRAIL, we investigated the mechanism by which TRAIL reverses multidrug resistance, the role of c-myc in modulating the death receptors DR4 and DR5 and the relationship between cisplatin and cytochrome c (cyt c) release in SGC7901/VCR and SGC7901/DDP cells. We found that after treatment with TRAIL, the DNA-PKcs/Akt/GSK-3β pathway, which is positively correlated with the levels of MDR1 and MRP1, was significantly inhibited and that this tendency can be abolished by Z-DEVD-FMK (a specific caspase 3 inhibitor). We also found that suppression of c-myc by siRNA reduced the expression of DR4 and DR5 and that transfection with a pAVV-c-myc expression vector increased the expression of DR4 and DR5. Moreover, cisplatin increased the expression of c-myc in the presence of TRAIL, and there is a clear increase in cyt c release from mitochondria with the increasing concentrations of cisplatin. Meanwhile, the intrinsic death receptor pathway of caspase 9, as well as the common intrinsic and extrinsic downstream target, caspase 3, was potently activated by the release of cyt c. Together, we conclude that in TRAIL-treated MDR gastric carcinoma cells, cisplatin induces the death receptors DR4 and DR5 through the up-regulation of c-myc and strengthens the activation of caspases via promoting the release of cyt c. These effects would then be responsible for the TRAIL sensitization effect of cisplatin.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gastric cancer is one of the most common malignancies and is the second leading cause of cancer-related death in the world [1, 2]. In China, the morbidity and mortality of gastric carcinoma represent the most common cause of death [3]. At present, surgery combined with postoperative chemotherapy is still the primary method used in the treatment of gastric cancer. However, after receiving multiple courses of chemotherapy, gastric cancer patients often show resistance to the main anti-cancer drugs [4]. Reversing the multidrug resistance of gastric cancer and improving patient prognosis are extremely urgent.
Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL), a novel member of the tumor necrosis factor (TNF) superfamily, is a promising protein for cancer treatment. TRAIL has been shown not only to induce apoptosis in various MDR cancer cells but also to sensitize MDR cells to MDR-related drugs [5]. The mechanism by which TRAIL reverses multidrug resistance has been proved to be correlated with the down-regulation of ATP-dependent transporters known as ATP-binding cassette (ABC) transporters, such as MDR1 and MRP1 [6, 7]. In addition, the levels of MDR1 and MRP1 in MDR variants were positively correlated with the levels of DNA-PKcs, pAkt and pGSK-3β [8].
DNA-PKcs (catalytic subunit of the DNA-dependent protein kinase) is the catalytic subunit of DNA-PK (DNA-dependent protein kinase), a member of the PI3 K-related kinase subfamily of protein kinases [9]. Suk-Bin Seo’s research [8] shows that the expression of MDR1 (P-gp) is positively correlated with the activity of the DNA-PKcs/Akt/GSK-3β signaling pathway. The Akt phosphorylation on Ser473 (S473) is required for the activation of Akt, and a major Akt S473 kinase activity was found to be DNA-PKcs. GSK-3β is a serine/threonine kinase that directly participates in regulating the expression of MDR-related proteins and the induction of cell death; it is also a downstream target of pAkt and is inactivated by phosphorylation on Ser9 by pAkt. The death receptor-induced extrinsic apoptotic signaling is also modulated by GSK-3β activity [10].
In the TRAIL signal transduction pathway [11], there are two important aspects to decide the validity of its function. The first is the expression level of the cell-membrane death receptors DR4 and DR5, which directly determines the capacity for TRAIL-induced downstream signaling; the other is the activation of the caspase cascade inside the cell, which directly influences the effect of TRAIL. The caspase cascade, in which caspase 3 and caspase 9 play key roles, is the main component of the TRAIL signal transduction pathway [12]. TRAIL-induced apoptosis requires the contribution of the mitochondrial pathway that is activated by caspase 8, leading to the activation of caspase 9, which further activates caspase 3 [13]. Caspase 3 is the common downstream target of the intrinsic and extrinsic apoptotic pathways [14–16].
Cisplatin is a commonly used anti-cancer drug that is widely used because of its stability, anti-tumor effect and low cost. In our previous study [17], we found that by combining cisplatin and TRAIL, the inhibitory effect on a multidrug resistance gene in gastric carcinoma, MDR1, increased beyond that of the separate use of cisplatin or TRAIL. The precise mechanism of the synergistic effects of cisplatin and TRAIL remains unclear. Here, we investigated whether it correlates with the intracellular drug concentrations of cisplatin, which might strengthen the efficiency of TRAIL. It has been reported that TRAIL inhibited the efflux function of ABC transporters in MDR cells by cleaving those transporters via caspase activation, which might contribute to the accumulation of the effective concentration of cisplatin in MDR cells [18].
The mechanism of a TRAIL-sensitizing agent should be clarified before further studies using it in combination with TRAIL in clinical trials. In our study, we investigated the mechanism underlying the synergistic effects of cisplatin and TRAIL in multidrug-resistant gastric cancer cell lines. We demonstrate the mechanism by which TRAIL reverses multidrug resistance, identifies the role of c-myc in modulating the death receptors DR4 and DR5 and describes the involvement of cisplatin in the acceleration of cytochrome c (cyt c) release and the activation of caspases.
Materials and methods
Cell lines
The human gastric cancer cell line SGC7901, the vincristine-resistant cell line SGC7901/VCR and the cisplatin-resistant cell line SGC7901/DDP used in our experiments were donated by Prof. Fan Daiming of the Institute of Digestive Diseases, Xijing Hospital, Fourth Military Medical University.
Reagents and antibodies
3-(4,5-Dimethyl-thiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) was purchased from Sigma-Aldrich (St. Louis, MO). The DNA-PKcs, MDR1, MRP1, DR4, DR5, c-myc and β-actin primers were designed and compounded by Sangon Biotech Co., Ltd. (Shanghai, China). The RevertAid First Strand cDNA Synthesis Kit and PCR amplification reagent kit were both purchased from Thermo Scientific. The QuantiFast SYBR Green PCR kit was purchased from QIAGEN. The human TRAIL protein was purchased from Peprotech (USA). Mouse anti-human monoclonal antibodies against DR4, DR5, c-myc, DNA-PKcs, MDR1, MRP1, cyt c, caspase 3, caspase 9 and β–actin and rabbit anti-human monoclonal antibodies against Akt, P-Akt (Ser 473), GSK-3, P-GSK-3β (Ser9) were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Lipofectamine™ 2000 was purchased from Invitrogen (Carlsbad, UA). Control siRNA, c-myc siRNA and transfection reagent were purchased from GenePharma Co., Ltd. (Shanghai, China). Vincristine (VCR) was purchased from Shenzhen Main Luck Pharmaceuticals Inc.; cisplatin (DDP) was purchased from Nanjing Pharmaceutical Factory Co., Ltd.
Cell culture
SGC7901, SGC7901/VCR and SGC7901/DDP cells were maintained in complete RPMI 1640 culture medium containing 10 % fetal bovine serum (FBS), 100 units/ml penicillin and 100 μg/ml streptomycin in a humidified chamber at 37 °C in 5 % CO2 with saturated humidity. For SGC7901/VCR cells, 1 μg/ml VCR was added to the culture solution and 1 μg/ml DDP was added to the culture solution supplied for SGC7901/DDP to maintain the drug resistance. VCR and DDP were removed 2 weeks before the experiment.
Cell viability assay
Cell viability after treatment was analyzed using the MTT assay. Briefly, 1 × 104 cells were plated into each well in 96-well plates and cultured overnight. The cells were cultured with 100 μl/well culture media containing test compound for 48 h. Then, the medium was replaced, and cells were incubated with 100 μl/well fresh culture media containing MTT (0.5 mg/ml) for another 4 h. The supernatants were then discarded and replaced with 150 μl of DMSO to dissolve crystals. Plates were protected from light and shaken e at low speed for 10 min, and then the absorbance (A) of each well was measured at 490 nm in a microplate reader. The cell viability was calculated as (drug group A/control group A) × 100 %. The IC50 value was calculated using SPSS 13.0 software based on the absorbance (A) of each well.
Rt-PCR and Rt-qPCR experiment
Total RNA was extracted with Trizol Reagent, and the concentration and purity of RNA were detected by a spectrophotometer. 2.5 μg of the total cellular RNA was used to synthesize first-strand cDNA by using first-strand cDNA Synthesis Kit. β-actin was used as the internal reference. The indicated genes primer sequence is show in Table 1 below. RT-PCR for indicated genes was done with the GeneAmp PCR System 9700 using Thermo PCR Master Mix; the PCR products were visualized on 5 % agarose gels with ethidium bromide staining under UV transillumination with a digital camera system. RT-qPCR was performed with the 7500 Real-time PCR System using the QuantiFast SYBR Green PCR kit. The expression levels of the indicated genes were normalized to β-actin, and the cycle threshold (Ct) value of the sample was used to calculate the relative gene expression level = 2−(Ct target−Ct actin). The relative change in gene expression compared with the control group was expressed as fold change, calculated by the 2−ΔΔCt method. The sequences of primers used in reverse transcription PCR are listed in Table 1.
Western blot experiment
Cells were lysed in ice-cold RIPA buffer containing protease inhibitors. The supernatants of all samples were collected after centrifugation. The BCA protein assay kit was used to measure the protein concentration. Equal amounts of proteins were separated on 10–12 % SDS-PAGE and transferred onto PVDF membranes. The membranes were blocked for 4 h at 4 °C using blocking buffer. Then, membranes were incubated with primary antibody overnight at 4 °C, followed by incubation with a horseradish peroxidase-conjugated secondary antibody for 4 h at room temperature. Then, the protein bands were visualized on the ImageQuant LAS 4000 mini (GE Healthcare Sweden) using the SuperSignal West Femto Maximum Sensitivity Substrate (Thermo Fisher Scientific, US). To verify equal protein loading and transfer, β-actin was used as a protein loading control.
RNA interference
The expression of c-myc was lowered using predesigned target-specific siRNA oligofectamine; the sequence of c-myc siRNA is 5′-CACAUCUCAUUUUUCCGUAdTdT-3′. In brief, 2 × 105 cells/well were seeded in 6-well plates, and then, c-myc or control siRNA/oligofectamine complex was transfected into cells using 12 µl HiPerFect Transfection Reagent. After 48 h, the cells were collected for RT-PCR and Western blot analysis.
Transfection
The pAVV-c-myc expressed vectors were employed for transfection using Lipofectamine™ 2000. Cells were seeded at 2 × 105 cells/well in 6-well plates in complete medium overnight and then transfected with 20 μg of control or c-myc expression vectors in serum-free medium for 48 h. After transfection, the cells were collected for RT-PCR and Western blot analysis.
Statistical analysis
Each experiment was repeated at least three times. The results obtained are expressed as the mean ± S.D. Differences between control and different treatment groups were analyzed using two-tailed Student’s t tests. *P < 0.05, **P < 0.01 and ***P < 0.001 were considered statistically significant in all experiments.
Result
TRAIL potentiated the cytotoxicity of cisplatin in SGC7901/VCR and SGC7901/DDP cells
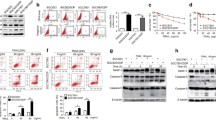
To examine the cytotoxicity of cisplatin following treatment with TRAIL in human MDR gastric cancer cells, the SGC7901/VCR and SGC7901/DDP cell lines were analyzed by morphological analysis and MTT assays. Treatment with 0–2 μg/ml cisplatin combined with 5 ng/ml TRAIL for 48 h induced abnormal cellular morphology, which was observed using phase-contrast in an inverted microscope for both SGC7901/VCR and SGC7901/DDP cells (Fig. 1a). Figure 1b shows that the cytotoxicity of cisplatin (0–2 μg/ml) was significantly enhanced in both SGC7901/VCR and SGC7901/DDP cells by pre-treatment with a low dose of TRAIL (5 ng/ml) in a concentration-dependent manner.
Cisplatin sensitizes SGC7901/VCR and SGC7901/DDP cells to TRAIL-induced apoptosis. a SGC7901/VCR and SGC7901/DDP cells were treated with 0–2 μg/ml cisplatin with or without 5 ng/ml TRAIL for 48 h, and then, the cell morphology was observed using an inverted microscope. Representative cell morphology data are shown from one of the three separate experiments with similar findings. b The cell viability was measured using the MTT assay. The results were obtained from three independent experiments, and the bar represents the mean ± SD. ***P < 0.001 indicates a significant difference between TRAIL-treated and TRAIL-untreated groups
The enhancement of the TRAIL-induced reversal of MDR in drug-selected cell lines by cisplatin
To determine the effect of TRAIL-induced reversal of multidrug resistance in drug-selected cell lines, the IC50 values (the concentration of 50 % inhibition of cell viability) of multiple anti-cancer drugs were determined using MTT assays in SGC7901, SGC7901/VCR and SGC7901/DDP cells and these cells pre-treated with the indicated compounds. As shown in Table 2, compared with their parental cell line (SGC7901), SGC7901/VCR and SGC7901/DDP showed higher resistance to VCR, DDP, ADM and 5-FU. TRAIL at 5 ng/ml decreased the IC50 values of VCR, DDP, ADM and 5-FU in SGC7901/VCR and SGC7901/DDP cells. However, in combination with 0.1 μg/ml cisplatin, TRAIL significantly reversed the resistance to VCR, DDP, ADM and 5-FU in both the SGC7901/VCR and SGC7901/DDP cell lines.
Increased expression levels of DNA-PKcs, MDR1 and MRP1 were associated with multidrug resistance in MDR gastric cancer cells
To demonstrate the decisive effect of ABC transporters on multidrug resistance, we compared the expression levels of MDR1 and MRP1 in SGC7901/VCR and SGC7901/DDP cells with their parental cell line SGC7901. Our results show that the expression levels of MDR1, MRP1 and DNA-PKcs in SGC7901/VCR and SGC7901/DDP cells were significantly higher than in their parental cell line SGC7901, making them more efficient at pumping the anti-cancer drugs out of the cytoplasm and avoiding cytotoxicity from anti-cancer drugs (Fig. 2).
mRNA and protein levels of DNA-PKcs, MDR1, MRP1, DR4, DR5, c-myc and β-actin in SGC7901, SGC7901/VCR, SGC7901/DDP cells. Total RNA was isolated from each sample and then converted to cDNA. The mRNA levels of the indicated genes were assessed by RT-PCR (RT), using β-actin as the internal control. Total cellular protein extracts were subjected to Western blot (WB) analysis using anti-DNA-PKcs, anti-MDR1, anti-MRP1, anti-DR4, anti-DR5, anti-c-myc and anti-β-actin antibodies. β-actin protein was the internal control. The bands in the Western blots were quantified using Quantity One software. Relative protein expression was normalized to β-actin. Representative data are shown from one of the three independent experiments with similar findings, and the bar represents the mean ± SD. ***P < 0.001 indicates a significant difference compared to the SGC7901 cells
The cisplatin-induced enhancement of TRAIL-induced down-regulation of the DNA-PKcs/Akt/GSK-3β pathway, of the expression levels of MDR1 and MRP1 and of the activation of caspase 3 in MDR gastric cancer cells
To examine the induction of DNA-PKcs/Akt/GSK-3β pathway and its downstream targets following treatment with TRAIL or TRAIL plus cisplatin, the mRNA and protein levels of caspase 3, DNA-PKcs, Akt, pAkt, pGSK-3β, GSK-3, MDR1 and MRP1 in SGC7901/VCR and SGC7901/DDP cells were analyzed by real-time PCR and Western blot assays, respectively. As shown in Fig. 3b, the level of the active form of caspase 3 was increased following treatment with 0–50 ng/ml of TRAIL for 24 h; after TRAIL combined with 0.4 μg/ml cisplatin, the activation of caspase 3 was significantly enhanced. We also found that treatment with 0–50 ng/ml of TRAIL for 24 h inhibited the DNA-PKcs/Akt/GSK-3β pathway and reduced the expression of MDR1 and MRP1; in combination with 0.4 μg/ml cisplatin, these functions of TRAIL were markedly amplified (Fig. 3).
TRAIL reduces the protein expression of MDR1 and MRP1, inhibits the DNA-PKcs/Akt/GSK-3β pathway and reduces the levels of cleaved caspase 3 in SGC7901/VCR and SGC7901/DDP cells. Cisplatin can strengthen those functions of TRAIL. a SGC7901/VCR and SGC7901/DDP cells were treated with 0–50 ng/ml TRAIL, with or without 0.4 μg/ml cisplatin, for 24 h. Total RNA was isolated from each sample and then converted to cDNA. The mRNA levels of indicated genes were detected using real-time PCR. Relative mRNA expression was calculated using the 2−ΔΔCt method. Fold change was calculated by normalizing all values to the untreated group. The bar represents the mean ± SD from three independent experiments. Comparisons between groups are indicated by brackets. NS indicates no significant difference (P > 0.05). **P < 0.01 and ***P < 0.001 indicate significant differences compared to the control. b Total cellular protein extracts were subjected to Western blot (WB) analysis using anti-caspase 3, anti-DNA-PKcs, anti-Akt, anti-pAkt, anti-pGSK-3β, anti-GSK-3, anti-MDR1, anti-MRP1 and anti-β-actin antibodies. β-actin was used as the internal control. Representative data are shown from one of the three independent experiments with similar findings. c Relative protein expression was normalized to β-actin. The bar represents the mean ± SD. Comparisons between groups are indicated by brackets. NS no significant difference (P > 0.05). ***P < 0.001 indicates a significant difference compared to the control
TRAIL-induced caspase 3 activation shows its key role in the regulation of the DNA-PKcs/Akt/GSK-3β pathway
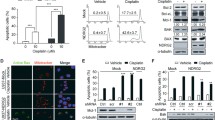
To further determine the role of caspase 3 in regulating the DNA-PKcs/Akt/GSK-3β pathway, the SGC7901/VCR cells were pre-treated with 50 μm Z-DEVD-FMK, a specific caspase 3 inhibitor, for 3 h and then with TRAIL (50 ng/ml) for 24 h. Then, the protein levels of DNA-PKcs and caspase 3 were detected by Western blotting. Our data show that the levels of activated caspase 3 induced by TRAIL were reduced by treatment with Z-DEVD-FMK in SGC7901/VCR cells; meanwhile, the protein levels of DNA-PKcs were significantly increased (Fig. 4).
TRAIL inhibits the DNA-PKcs/Akt/GSK-3β pathway by activating caspase 3. a The cell lysates of the SGC7901/VCR cells treated with TRAIL (50 ng/ml) for 24 h or pre-treated with 50 μm Z-DEVD-FMK, a specific caspase 3 inhibitor, for 3 h and then with TRAIL (50 ng/ml) for 24 h were subjected to Western blot analysis to monitor levels of DNA-PKcs, caspase 3 and β-actin. β-actin protein was used as the internal control. Representative data are shown from one of the three independent experiments with similar findings. b The bands from the Western blot were quantified using Quantity One software. Relative protein expression was normalized to β-actin. The bar represents the mean ± SD from three independent experiments. ***P < 0.001 indicates a significant difference between the Z-DEVD-FMK-treated and Z-DEVD-FMK-untreated groups
The expression of the death receptors DR4 and DR5 was positively correlated with the levels of c-myc
As shown in Fig. 2, the basal levels of c-myc, DR4 and DR5 were somewhat higher in SGC7901/VCR and SGC7901/DDP cells than those in SGC7901 cells. We then examined whether the increased levels of the death receptors DR4 and DR5 were well correlated with the level of c-myc in the SGC7901/VCR and SGC7901/DDP cells. To further evaluate the direct role of c-myc in regulating the expression of the death receptors DR4 and DR5, we used siRNA to knock-down c-myc expression and assessed the effect of c-myc suppression on the expression of the death receptors DR4 and DR5 in SGC7901/VCR and SGC7901/DDP cells. After transfecting SGC7901/VCR and SGC7901/DDP cells with siRNA against c-myc, using scrambled siRNA as a control, the expression levels of DR4, DR5 and c-myc were detected by RT-PCR and Western blot analysis. The results show that suppression of c-myc expression significantly attenuated the expression of the death receptors DR4 and DR5 in both SGC7901/VCR and SGC7901/DDP cells (Fig. 5a, b). Next, we transfected a c-myc expression vector (pAVV-c-myc) into SGC7901/VCR and SGC7901/DDP cells to overexpress c-myc and evaluate the effects of c-myc overexpression on the expression of DR4 and DR5. RT-PCR and Western blot analysis were used to detect the expression levels of DR4, DR5 and c-myc. Our results show that the overexpressed c-myc significantly increased the expression of DR4 and DR5 (Fig. 5c, d). These results suggest that the expression of DR4 and DR5 was positively regulated by c-myc.
c-myc regulates the expression of the death receptors DR4 and DR5 in SGC7901/VCR and SGC7901/DDP cells. a The mRNA/protein levels of DR4, DR5, c-myc and β-actin in SGC7901/VCR, SGC7901/DDP, SGC7901/VCR and SGC7901/DDP cells transfected with scrambled siRNA and in SGC7901/VCR and SGC7901/DDP cells transfected with c-myc siRNA were determined by RT-PCR (RT) and Western blotting (WB). β-actin was used as the internal control. Representative data are shown from one of the three independent experiments with similar findings. b The protein expression of the death receptors DR4 and DR5 and of c-myc was normalized to β-actin. The bars represent the mean ± SD from three independent experiments. Comparisons between groups are indicated by brackets. NS no significant difference (P > 0.05). ***P < 0.001 indicates a significant difference compared to the control. c The mRNA and protein levels of DR4, DR5, c-myc and β-actin in SGC7901/VCR and SGC7901/DDP cells, in SGC7901/VCR and SGC7901/DDP cells transfected with pAVV-c-myc, and in SGC7901/VCR and SGC7901/DDP cells transfected with pAVV-Blank were determined by RT-PCR (RT) and Western blotting (WB). β-actin was used as the internal control. Representative data are shown from one of the three independent experiments with similar findings. d The protein expression levels of the death receptors DR4 and DR5 and of c-myc were normalized to β-actin. The bars represent the mean ± SD from three independent experiments. Comparisons between groups are indicated by brackets. NS no significant difference (P > 0.05). ***P < 0.001 indicates a significant difference compared to the control
Cisplatin increased the expression of the death receptors DR4 and DR5 by up-regulating c-myc expression in TRAIL-treated MDR cells
To further examine the relationship between cisplatin and c-myc, we treated the SGC7901/VCR and SGC7901/DDP cells with 0–0.5 μg/ml cisplatin, with or without 10 ng/ml TRAIL, for 24 h. Then, the mRNA and protein levels of DR4, DR5 and c-myc were detected by real-time PCR and Western blot assays. The results showed that cisplatin in combination with TRAIL increased the expression of c-myc compared with cisplatin alone in a dose-dependent manner in both SGC7901/VCR and SGC7901/DDP cells. Further, as expected, the cisplatin-mediated up-regulation of c-myc resulted in significant increases in both DR4 and DR5 expression in SGC7901/VCR and SGC7901/DDP cells (Fig. 6). Based on these results, it could be suggested that the cisplatin-mediated c-myc overexpression, which resulted in the increased expression of DR4 and DR5, would be responsible for the TRAIL sensitization effect of cisplatin.
Cisplatin increases the expression of the death receptors DR4 and DR5 and of c-myc in TRAIL-treated SGC7901/VCR and SGC7901/DDP cells. a SGC7901/VCR and SGC7901/DDP cells were treated with 0–0.5 μg/ml cisplatin, with or without 10 ng/ml TRAIL, for 24 h. The mRNA levels of the indicated genes were detected using real-time PCR. Relative mRNA expression levels were calculated using the 2−ΔΔCt method. Fold change was calculated by normalizing all values to the untreated group. The bars represent the mean ± SD. Comparisons between groups are indicated by brackets. ***P < 0.001 indicates a significant difference compared to the control. b Total protein extracts were subjected to Western blot analysis using anti-DR4, anti-DR5 and anti-c-myc antibodies. β-actin was used as the internal control. Representative data are shown from one of the three independent experiments with similar findings. c The relative protein expression levels of DR4, DR5 and c-myc were normalized to β-actin. The bars represent the mean ± SD from three separate experiments. NS no significant difference (P > 0.05). ***P < 0.001 indicates a significant difference between the two groups indicated by the brackets
Cisplatin enhanced the release of cyt c and the activation of caspase 3 and caspase 9 in TRAIL-treated MDR cells
Because our data show that the activity of the DNA-PKcs/Akt/GSK-3β pathway was well correlated with the cleaved form of caspase 3 in both SGC7901/VCR and SGC7901/DDP cells, we investigated whether the levels of cyt c and caspase 9 (a downstream target of cyt c) and caspase 3 (a downstream target of caspase 9) are modulated after treatment of SGC7901/VCR and SGC7901/DDP cells with cisplatin with or without TRAIL. SGC7901/VCR and SGC7901/DDP cells were treated with 0–0.5 μg/ml cisplatin, with or without 10 ng/ml TRAIL, for 24 h, and then, the protein levels of cyt c, caspase 9 and caspase 3 were detected using Western blot assays. The expression levels of cyt c and the active forms of caspase 9 and caspase 3 were increased after treatment with 0–0.5 μg/ml cisplatin combined with 10 ng/ml TRAIL for 24 h in both SGC7901/VCR and SGC7901/DDP cells compared with those in cells treated with cisplatin alone (Fig. 7). This result confirmed that cisplatin-mediated cyt c release, which resulted in the activation of caspase 3 and caspase 9, would also contribute to the TRAIL sensitization effect of cisplatin.
Increase in cyt c release from mitochondria with the increase in cisplatin is clear, and cisplatin strengthens the activation of caspase 9 and caspase 3 in TRAIL-treated SGC7901/VCR and SGC7901/DDP cells. a SGC7901/VCR and SGC7901/DDP cells were treated with 0–0.5 μg/ml cisplatin, combined with or without 50 ng/ml TRAIL for 24 h. Total cellular protein extracts were subjected to Western blot analysis using anti-cyt c, anti-caspase 9 and anti-caspase 3 antibodies. β-actin was used as the internal control. Data shown are from one of the three independent experiments with similar findings. b The protein expression levels of cyt c, pro-caspase 9, active caspase 9, pro-caspase 3 and active caspase 3 in cells co-treated with TRAIL (50 ng/ml) and the indicated dose of cisplatin were normalized to β-actin. The bars represent the mean ± SD of triplicate experiments. Each group was compared to the other two using the SNK test; significant differences are indicated
Discussion
Although targeted drugs for oncotherapy have been developed and used in clinical treatment [19], general chemotherapeutic drugs are still widely used for the treatment of gastric cancer, not only because of their low cost but also because they kill cancer cells. However, acquired resistance against anti-cancer drugs is a serious problem in the management of gastric cancer patients. Altered expression of various enzymes and other proteins would be responsible for the drug resistance of multidrug-resistant gastric cancer cells [20–23]. Some studies have shown that TRAIL, a new member of the TNF superfamily, down-regulates the expression of MDR1 and thereby sensitizes MDR cells to anti-cancer drugs [8]. The molecular mechanism by which TRAIL down-regulates MDR1 expression has been characterized as occurring through inhibition of the DNA-PKcs/Akt/GSK-3β pathway and activation of caspase 3 in the multidrug-resistant human lymphoblastic leukemia CCRF-CEM (CEM) line [8]. In the present study, we suggest the same conclusion for multidrug-resistant human gastric cancer cells. In our previous study [17], we found a synergistic effect of TRAIL and cisplatin in reverse multidrug resistance, and we considered that it might correlate well with the expression of the death receptors DR4 and DR5 and the activation of caspase 3 and caspase 9.
Our present study showed that the sensitizing effect of cisplatin on the TRAIL-induced reversal of multidrug resistance of MDR gastric cancer cells can be achieved through two mechanisms. First, in the presence of TRAIL, cisplatin can promote the expression of c-myc, resulting in the up-regulation of the death receptors DR4 and DR5, thereby increasing the sensitivity of MDR gastric cancer cells to TRAIL and enhancing the effect of TRAIL. Second, in the presence of TRAIL, cisplatin can promote the release of cyt c from the mitochondria, greatly increasing the activation of caspase 9 and caspase 3. The activation of caspase 3 played the key role in the down-regulation of the DNA-PKcs/Akt/GSK-3β pathway, thereby down-regulating the expression of MDR1 and MRP1.
We also observed that the tendency of cisplatin-mediated c-myc overexpression and cyt c release can only be seen upon combined treatment with cisplatin and TRAIL, whereas in the absence of TRAIL, cisplatin did not have the same effect. We consider that this may correlate with the expression of ATP-binding cassette (ABC) transporters, which function to pump anti-cancer drugs out of the cytoplasm. In the absence of TRAIL, the MDR gastric cancer cells express high levels of ABC transporters such as MDR1 and MRP1. Their function of pumping anti-cancer drugs out of the cytoplasm makes it impossible to maintain a functional concentration of cisplatin in the cytoplasm. When the efflux activity and the expression of ABC transporters were reversed by TRAIL treatment, the concentration of cisplatin in cytoplasm was greatly improved, thus inducing the expression of c-myc and cyt c.
DNA-PKcs is a member of the PI3 K protein family [24] that is mainly engaged in the repair of double-stranded DNA. Its function of repairing DNA damage [25, 26] is important for maintaining normal cellular function. Studies have shown that DNA-PKcs plays a key role in cancer and aging. It has also been reported that [27, 28] the level of DNA-PKcs was reduced in the early stage of cell malignant transformation and increased after the malignant transformation. This phenomenon has been confirmed in a variety of cancers [28, 29]. When cells become malignant, double-stranded DNA can have irreversible damage such that the repair function of DNA-PKcs promotes cell malignancy. Further, due to the repair function of DNA-PKcs, apoptosis induced by the DNA damage caused by chemotherapy can be avoided, allowing cancer cells with high levels of DNA-PKcs expression to become multidrug resistant. That is likely why the multidrug-resistant cell lines express more DNA-PKcs than their parental cell line.
C-myc is a proto-oncogene that plays an important role in the processes of tumor cell proliferation, migration and apoptosis [30–32]. We found that c-myc can modulate the sensitivity of MDR gastric cancer cells to TRAIL by regulating the expression of the cell-membrane death receptors DR4 and DR5. Dae-Young Kim [5] found similar results in breast cancer cells. Blanc [33] found that c-myc expression was closely correlated with the expression of MDR-related proteins and could promote the expression of MDR-related proteins. Labisso also found that [34] the expression of MCL1 was regulated by c-myc and the suppression of c-myc by siRNA inhibited the expression of MCL1.
Some research has shown that [11, 35–38] MCL1 interferes with the release of cyt c and therefore inhibits the activity of mitochondrial apoptosis pathway, resulting in reducing the susceptibility of MDR cells to TRAIL. This mechanism proved to be the pivotal role in connection with TRAIL resistance and the activation of the STAT3 signaling pathway [39, 40]. It has been reported that chrysin overcomes the TRAIL resistance of cancer cells through the down-regulation of MCL1 by inhibiting STAT3 phosphorylation and that the expression of MCL1 positively correlated with the activity of STAT3 signaling pathway [11]. In our study, cisplatin increased the expression of c-myc in TRAIL-treated MDR cells. Meanwhile, the release of cyt c was not significantly decreased; instead, the expression of cyt c was significantly increased. We hypothesize that the accelerating effect of cisplatin on cyt c release may be greater than the inhibitory effect of MCL1.
In conclusion, as depicted in Fig. 8, we have demonstrated that cisplatin-mediated c-myc overexpression and cyt c release resulted in the up-regulation of the death receptors DR4 and DR5 and the activation of caspase 3 and caspase 9, and this activity underlies the TRAIL sensitization effect of cisplatin. Our work suggests the potential of cisplatin for use as a TRAIL sensitizer.
References
Yang L. Incidence and mortality of gastric cancer in China. World J Gastroenterol. 2006;12:17–20.
Anderson WF, Camargo MC, Fraumeni JJ, Correa P, Rosenberg PS, Rabkin CS. Age-specific trends in incidence of noncardia gastric cancer in US adults. JAMA. 2010;303:1723–8.
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90.
Yu PF, Guo JM, Xu Q, Ying JE, Wang XJ, Cheng XD, Wang XB, Yu CD. Significance of multidrug resistance gene-associated proteins in the postoperative adjuvant chemotherapy for gastric carcinoma and the prognosis. Zhonghua Wei Chang Wai Ke Za Zhi. 2010;13:289–93.
Kim DY, Kim MJ, Kim HB, Lee JW, Bae JH, Kim DW, Kang CD, Kim SH. Suppression of multidrug resistance by treatment with TRAIL in human ovarian and breast cancer cells with high level of c-Myc. Biochim Biophys Acta. 2011;1812:796–805.
Zhang KG, Qin CY, Wang HQ, Wang JX, Wang QM. The effect of TRAIL on the expression of multidrug resistant genes MDR1, LRP and GST-pi in drug-resistant gastric cancer cell SGC7901/VCR. Hepatogastroenterology. 2012;59:2672–6.
Shao SL, Cui TT, Zhao W, Zhang WW, Xie ZL, Wang CH, Jia HS, Liu Q. RNAi-based knockdown of multidrug resistance-associated protein 1 is sufficient to reverse multidrug resistance of human lung cells. Asian Pac J Cancer Prev. 2014;15:10597–601.
Seo SB, Hur JG, Kim MJ, Lee JW, Kim HB, Bae JH, Kim DW, Kang CD, Kim SH. TRAIL sensitize MDR cells to MDR-related drugs by down-regulation of P-glycoprotein through inhibition of DNA-PKcs/Akt/GSK-3beta pathway and activation of caspases. Mol Cancer. 2010;9:199.
Walker AI, Hunt T, Jackson RJ, Anderson CW. Double-stranded DNA induces the phosphorylation of several proteins including the 90 000 mol. wt. heat-shock protein in animal cell extracts. EMBO J. 1985;4:139–45.
Beurel E, Jope RS. The paradoxical pro- and anti-apoptotic actions of GSK3 in the intrinsic and extrinsic apoptosis signaling pathways. Prog Neurobiol. 2006;79:173–89.
Lirdprapamongkol K, Sakurai H, Abdelhamed S, Yokoyama S, Athikomkulchai S, Viriyaroj A, Awale S, Ruchirawat S, Svasti J, Saiki I. Chrysin overcomes TRAIL resistance of cancer cells through Mcl-1 downregulation by inhibiting STAT3 phosphorylation. Int J Oncol. 2013;43:329–37.
Wang S. The promise of cancer therapeutics targeting the TNF-related apoptosis-inducing ligand and TRAIL receptor pathway. Oncogene. 2008;27:6207–15.
Shen Y, White E. p53-dependent apoptosis pathways. Adv Cancer Res. 2001;82:55–84.
Cheng EH, Wei MC, Weiler S, Flavell RA, Mak TW, Lindsten T, Korsmeyer SJ. BCL-2, BCL-X(L) sequester BH3 domain-only molecules preventing BAX- and BAK-mediated mitochondrial apoptosis. Mol Cell. 2001;8:705–11.
Wei MC, Zong WX, Cheng EH, Lindsten T, Panoutsakopoulou V, Ross AJ, Roth KA, MacGregor GR, Thompson CB, Korsmeyer SJ. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science. 2001;292:727–30.
Zou H, Henzel WJ, Liu X, Lutschg A, Wang X. Apaf-1, a human protein homologous to C. elegans CED-4, participates in cytochrome c-dependent activation of caspase-3. Cell. 1997;90:405–13.
Cui YF, Yu LS, Wang HQ, Gou YW, Wang QM, Zhang KG. Effect of TRAIL in combination with DDP on the expression of MDR1 gene in gastric cancer cells. Prz Gastroenterol. 2014;9:214–9.
Gatti L, Cossa G, Tinelli S, Carenini N, Arrighetti N, Pennati M, Cominetti D, De Cesare M, Zunino F, Zaffaroni N, Perego P. Improved apoptotic cell death in drug-resistant non-small-cell lung cancer cells by tumor necrosis factor-related apoptosis-inducing ligand-based treatment. J Pharmacol Exp Ther. 2014;348:360–71.
Tsuruo T, Naito M, Tomida A, Fujita N, Mashima T, Sakamoto H, Haga N. Molecular targeting therapy of cancer: drug resistance, apoptosis and survival signal. Cancer Sci. 2003;94:15–21.
Trock BJ, Leonessa F, Clarke R. Multidrug resistance in breast cancer: a meta-analysis of MDR1/gp170 expression and its possible functional significance. J Natl Cancer Inst. 1997;89:917–31.
Norgaard JM, Bukh A, Langkjer ST, Clausen N, Palshof T, Hokland P. MDR1 gene expression and drug resistance of AML cells. Br J Haematol. 1998;100:534–40.
Steinbach D, Legrand O. ABC transporters and drug resistance in leukemia: was P-gp nothing but the first head of the Hydra? Leukemia. 2007;21:1172–6.
Ivy SP, Olshefski RS. Correlation of P-glycoprotein expression and function in childhood acute leukemia: a children’s cancer group study. Blood. 1996;88:309–18.
Rivera-Calzada A, Maman JD, Spagnolo L, Pearl LH, Llorca O. Three-dimensional structure and regulation of the DNA-dependent protein kinase catalytic subunit (DNA-PKcs). Structure. 2005;13:243–55.
Cook AJ, Oganesian L, Harumal P, Basten A, Brink R, Jolly CJ. Reduced switching in SCID B cells is associated with altered somatic mutation of recombined S regions. J Immunol. 2003;171:6556–64.
Falck J, Coates J, Jackson SP. Conserved modes of recruitment of ATM, ATR and DNA-PKcs to sites of DNA damage. Nature. 2005;434:605–11.
Sun JF, Sui JL, Zhou PK, Geng Y, Hu YC, Cao ZS, Ge SL, Lou TZ, Wu DC. Decreased efficiency of gamma-ray-induced DNA double-strand break rejoining in malignant transformants of human bronchial epithelial cells generated by alpha-particle exposure. Int J Radiat Biol. 2002;78:773–80.
Ochiai M, Ubagai T, Kawamori T, Imai H, Sugimura T, Nakagama H. High susceptibility of Scid mice to colon carcinogenesis induced by azoxymethane indicates a possible caretaker role for DNA-dependent protein kinase. Carcinogenesis. 2001;22:1551–5.
Mandal M, Adam L, Kumar R. Redistribution of activated caspase-3 to the nucleus during butyric acid-induced apoptosis. Biochem Biophys Res Commun. 1999;260:775–80.
Li Y, Choi PS, Casey SC, Dill DL, Felsher DW. MYC through miR-17-92 suppresses specific target genes to maintain survival, autonomous proliferation, and a neoplastic state. Cancer Cell. 2014;26:262–72.
Sabo A, Kress TR, Pelizzola M, de Pretis S, Gorski MM, Tesi A, Morelli MJ, Bora P, Doni M, Verrecchia A, Tonelli C, Faga G, Bianchi V, Ronchi A, Low D, Muller H, Guccione E, Campaner S, Amati B. Selective transcriptional regulation by Myc in cellular growth control and lymphomagenesis. Nature. 2014;511:488–92.
Ehninger A, Boch T, Uckelmann H, Essers MA, Mudder K, Sleckman BP, Trumpp A. Posttranscriptional regulation of c-Myc expression in adult murine HSCs during homeostasis and interferon-alpha-induced stress response. Blood. 2014;123:3909–13.
Blanc E, Goldschneider D, Ferrandis E, Barrois M, Le Roux G, Leonce S, Douc-Rasy S, Benard J, Raguenez G. MYCN enhances P-gp/MDR1 gene expression in the human metastatic neuroblastoma IGR-N-91 model. Am J Pathol. 2003;163:321–31.
Labisso WL, Wirth M, Stojanovic N, Stauber RH, Schnieke A, Schmid RM, Kramer OH, Saur D, Schneider G. MYC directs transcription of MCL1 and eIF4E genes to control sensitivity of gastric cancer cells toward HDAC inhibitors. Cell Cycle. 2012;11:1593–602.
Henson ES, Gibson EM, Villanueva J, Bristow NA, Haney N, Gibson SB. Increased expression of Mcl-1 is responsible for the blockage of TRAIL-induced apoptosis mediated by EGF/ErbB1 signaling pathway. J Cell Biochem. 2003;89:1177–92.
Clohessy JG, Zhuang J, de Boer J, Gil-Gomez G, Brady HJ. Mcl-1 interacts with truncated Bid and inhibits its induction of cytochrome c release and its role in receptor-mediated apoptosis. J Biol Chem. 2006;281:5750–9.
Sun JG, Li H, Li X, Zeng X, Wu P, Fung KP, Liu FY. Clitocine targets Mcl-1 to induce drug-resistant human cancer cell apoptosis in vitro and tumor growth inhibition in vivo. Apoptosis. 2014;19:871–82.
Nakazato T, Sagawa M, Kizaki M. Triptolide induces apoptotic cell death of multiple myeloma cells via transcriptional repression of Mcl-1. Int J Oncol. 2014;44:1131–8.
Chen KF, Chen HL, Liu CY, Tai WT, Ichikawa K, Chen PJ, Cheng AL. Dovitinib sensitizes hepatocellular carcinoma cells to TRAIL and tigatuzumab, a novel anti-DR5 antibody, through SHP-1-dependent inhibition of STAT3. Biochem Pharmacol. 2012;83:769–77.
Abdulghani J, Allen JE, Dicker DT, Liu YY, Goldenberg D, Smith CD, Humphreys R, El-Deiry WS. Sorafenib sensitizes solid tumors to Apo2L/TRAIL and Apo2L/TRAIL receptor agonist antibodies by the Jak2-Stat3-Mcl1 axis. PLoS One. 2013;8:e75414.
Acknowledgments
This work was supported by a grant from the Natural Science Foundation of Anhui Province, Grant Number 1308085MH167.
Conflict of interest
None.
Ethical standards
Our research not involving Human Participants or Animals, so we do not need informed consent.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhu, X., Zhang, K., Wang, Q. et al. Cisplatin-mediated c-myc overexpression and cytochrome c (cyt c) release result in the up-regulation of the death receptors DR4 and DR5 and the activation of caspase 3 and caspase 9, likely responsible for the TRAIL-sensitizing effect of cisplatin. Med Oncol 32, 133 (2015). https://doi.org/10.1007/s12032-015-0588-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-015-0588-9