Abstract
The objectives of this study were to explore the expression profiles of Raf kinase inhibitor protein (RKIP) in human gastric cancer cell line (SGC-7901) and cisplatin-resistant cell line (SGC-7901/DDP) and investigate the role of RKIP in the sensitivity of human gastric cancer cells to cisplatin and its signaling pathways, with an attempt to identify new approaches and strategies for the management of gastric cancer. The human gastric cancer cell line (SGC-7901) and cisplatin-resistant cell line (SGC-7901/DDP) were separately cultured in vitro. The expression profiles of RKIP in these two cell lines were detected by Western blotting. Forty-eight hours after the transfection of RKIP siRNA in SGC-7901 cells, the change of RKIP expression in the cells was detected using Western blotting, and the change of cell viability after the interference of RKIP expression was determined using 3-(4,5)-dimethylthiahiazo (-z-y1)-3,5-di-phenytetrazoliumromide (MTT) method. The effect of the ectopic expression of RKIP on the cisplatin-induced viability of gastric cancer cell was detected using MTT method. The effect of the ectopic expression of RKIP on the cisplatin-induced apoptosis of gastric cancer cell was detected using flow cytometry after having been double stained with Annexin V/PI. The effect of the ectopic expression of RKIP on the NF-κB and Snail expressions in cisplatin-induced gastric cancer cells was detected using Western blotting. As shown by the Western blotting, the expression of RKIP in SGC-7901/DDP cells significantly decreased when compared with that in SGC-7901 cells (P < 0.05). Compared with the control group, the expression of RKIP in SGC-7901 cells significantly decreased 48 h after the transfection of RKIP siRNA (P < 0.01). After the SGC-7901 cells were transfected with RKIP siRNA, the cell viability was significantly increased (P < 0.05); after the SGC-7901/DDP cells were transfected with RKIP recombinant plasmid, the cell viability was significantly decreased (P < 0.05). After the RKIP expression was suppressed in the cisplatin-treated SGC-7901 cells, the cell viability significantly increased (P < 0.05), and the amount of apoptotic cells significantly decreased (P < 0.05). In contrast, after the RKIP overexpression in the cisplatin-treated SGC-7901/DDP cells, the cell viability significantly decreased (P < 0.05), and the amount of apoptotic cells significantly increased (P < 0.05). The suppression of RKIP expression in SGC-7901 cells could significantly promote the increase of NF-κB expression (P < 0.05); in contrast, the increased expression of RKIP in SGC-7901/DDP cells significantly inhibited the expression of Snail (P < 0.05). The expression of RKIP is downregulated in cisplatin-resistant cell line (SGC-7901/DDP). The overexpression of RKIP can enhance the sensitivity of human gastric cancer cells to cisplatin, which may be achieved via the NF-κB/Snail signaling pathway.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gastric cancer carries a poor prognosis and high mortality. According to the US National Cancer Institute, although the morbidity and mortality of gastric cancer have shown a decreasing trend in the past decades, there was a global annual increase of about 934,000 new cases, accounting for 8.6 % of all tumors [1]. Most stomach cancers are advanced when they are found, and most of the advanced gastric cancer patients will experience relapse and metastasis after radical gastrectomy [2, 3]. Therefore, systemic multidisciplinary treatment of gastric cancer is particularly important. In recent years, adjuvant chemotherapy and neoadjuvant chemotherapy have been adopted by a growing number of medical centers with an attempt to prolong the survival. However, the survivals of patients with advanced gastric cancer remain unsatisfactory [4–6]. Since the success rate of chemotherapy in treating gastric cancer is highly affected by the resistance to chemotherapy, it has been increasingly recognized that the apoptosis of tumor cells is a key indicator for measuring the effectiveness of chemotherapy, and the apoptosis is associated with the chemotherapy drugs in a time- and dose-dependent manner [7, 8].
Raf kinase inhibitor protein (RKIP) belongs to the phosphatidylethanolamine-binding protein (PEBP) family. It was initially a small cytoplasmic protein purified from bovine brain; later, it was diffusely expressed in tissues obtained from monkeys, chickens, rats, and human beings [9, 10]. RKIP belongs to a highly conserved family of proteins, with a relative molecular mass of 2.1–2.3 × 104 Da. Its structure and functions show no similarities with any other known protein. Human RKIP gene is located on chromosome 12q24.23, and its four exons can generate a 1,507-bp mRNA after transcription [11]. RKIP is closely related with cell growth, differentiation, proliferation, and apoptosis. It is involved in the lipid metabolism and the formation of phospholipid membranes, playing a key role in the development of nervous system, spermatogenesis, and maintenance of the normal physiological function of the heart. The abnormal expression of RKIP can cause a variety of diseases including Alzheimer’s disease and diabetic nephropathy [12, 13]. Recent studies have also shown that RKIP can also block the activity of Mitogen-activated protein kinases (MAPK) signaling pathway and inhibit the Raf-1-mediated MEK activation. Thus, RKIP is named as the “Raf kinase inhibitor protein” [10, 14]. In 2013, Martinho et al. reported that RKIP was correlated with chemosensitivity to cisplatin in cervical cancer [15].
Cisplatin is one of the most commonly used drug for adjuvant chemotherapy and neoadjuvant chemotherapy, and also a common drug used in the testing for anticancer drug sensitivity performed in in vitro cultured gastric cancer tissues [16–18]. Research has shown that cisplatin can bind to DNA and form cross-links, which can inhibit DNA synthesis and thus suppress cell division and induce apoptosis [19, 20]. However, the presence of drug resistance inside cancer cells will lower the efficacy of cisplatin [21, 22].
It has been found that the decrease or loss of RKIP expression is associated with the occurrence, development, invasion, and metastasis of various tumors. The expression of RKIP is remarkably lower in prostate cancer, melanoma, colorectal cancer, liver cancer, breast cancer, and their corresponding metastatic cancer than those in normal controls [23–27]. In addition, RKIP is also involved in the regulation of many intracellular signaling pathways such as the Raf-l-MAPK, NF-κB, and G protein signaling pathways. The abnormal activation of these signaling pathways is closely associated with tumorigenesis [28, 29]. Our preliminary results have shown that the RKIP expression is downregulated in human gastric cancer cell lines, which can inhibit the proliferation and invasion of gastric cancer cells. Recent studies have shown that RKIP was associated with the sensitivities of cervical cancer and nasopharyngeal cancer to chemotherapy [15, 30]. However, the relationship between RKIP and the sensitivity of gastric cancer cells to cisplatin has not been reported. In our current study, we mainly explored the relationship between RKIP and the sensitivity of gastric cancer cells to cisplatin and its signaling pathways, with an attempt to provide new methods and insights for the management of gastric cancer.
Subjects and methods
Main reagents
Human gastric cancer cell line SGC-7901 was purchased from ATCC, USA, and cisplatin-resistant cell line (SGC-7901/DDP) was purchased from Nanjing KeyGEN Biotech. Co., Ltd. Fetal calf serum (FCS), RPMI 1640 medium, l-glutamine, HEPES, and Lipofectamine 2000 were purchased from Invitrogen, USA.
In accordance with the human RKIP mRNA sequences in GenBank (no. NM_002567.2) and the results of restriction site analysis, a pair of PCR primers was designed at the two sides of the open reading frame (ORF) of RKIP using the Primer Premier 5 software. The primers were synthesized by Invitrogen (Shanghai, China).
The vectors (pcDNA3.1 and pGEM-T), competent cells, and Trizol total RNA extraction kit were purchased from Invitrogen, USA. The restriction endonucleases BamH I and Xho I and DNA Marker were purchased from TaKaRa, Japan. T4 DNA Ligation was purchased from Promega, USA. The Taq DNA polymerase and prestained protein molecular weight marker were purchased from Fermentas, USA. The reverse transcription kit was purchased from Qiagen, Germany. The RKIP siRNA and RKIP scramble were ordered from Santa Cruz, USA.
The 3-(4,5)-dimethylthiahiazo (-z-y1)-3,5-di-phenytetrazoliumromide (MTT), trypsin, and phosphate-buffered saline (PBS) were purchased from Sigma-Aldrich, USA. Cell culture plates or dishes were purchased from Corning, USA. Cisplatin was purchased from Sigma-Aldrich Co. LLC (San Diego, CA, USA). Annexin V and propidium iodide (PI) were purchased from Roche, Germany. Rabbit antihuman RKIP, Snail polyclonal antibody, and mouse antihuman β-actin monoclonal antibody were purchased from Abcam, UK. Rabbit anti-NF-kB p65 (C-20) was purchased from Santa Cruz Biotechnology, USA. Bradford protein assay kit and QuantityOne software were purchased from Bio-Rad (Richmond, CA, USA). PVDF membranes were purchased from Millipore (Bedford, MA, USA). ECL chemiluminescent substrate was purchased from Pierce Company (Rockford, IL, USA).
Culture and treatment of human gastric cancer cell lines
The SGC-7901 cells and the cisplatin-resistant cell line (SGC-7901/DDP) were cultured in RPMI 1640 medium containing 10 % fetal bovine serum under standard conditions (37 °C, 5 % CO2, and saturated humidity). The growth of cells was observed under an inverted microscope. When cells were grown to 70–80 % confluence, they were detached with 0.25 % trypsin. Medium was changed every other day, and the cells were passaged every 4 to 6 days. Cells in the exponential phase were selected for further experiment.
Using the lipofection method, we transfected the above constructed eukaryotic expression plasmid pcDNA3.1-RKIP and blank vector into the cisplatin-resistant human gastric cancer cell SGC-7901/DDP with the Lipofectamine 2000. The transfected SGC-7901/DDP cells were further cultured in a medium containing 500 μg/ml G418 for about 3 weeks, which yielded stable anti-G418 cell clones.
Using the Lipofectamine 2000 liposomes, we transfected the RKIP siRNA into the SGC-7901 cells. Meanwhile, a normal control group (normal) and an RKIP siRNA negative control group (RKIP scramble) were set. The expression of RKIP in cells in each group was detected by Western blotting, so as to identify the effect of RNA interference.
After cell treatments, SGC-7901 or SGC-7901/DDP cells were stained with Hoechst 33342 (5 μg/ml). Stained cells were observed under a DMR fluorescence microscope (Leica Microsystems, Bensheim, Germany), and cell numbers were established by counting total population of cell by the aid of an Image Pro Plus 6.0 software (Media Cybernetics Inc., Silver Spring, MD). The cell counting was done from ten random fields in three wells.
Expression of RKIP in human gastric cancer cell lines
The expressions of RKIP in SGC-7901 and SGC-7901/DDP cells were detected by Western blotting or real-time PCR. The in vitro cultured gastric cancer cells were harvested and placed in 6-well cell culture plates. Add 1 ml of RIPA lysis buffer [150 mM NaCl, 1 % NP40, 0.5 % sodium deoxycholate, 0.1 % SDS, 50 mM Tris (pH 7.9), 10 mM NaF, 10 mM PMSF, and 1× protease inhibitors (Complete cocktail tablets, Roche)] in each well. Then, the cell lysates were transferred to a 1.5-ml centrifuge tube and centrifuged at 16,000 rpm for 30 min to collect the supernatants. The protein concentration of the supernatant was determined using bicinchoninic acid (BCA) method. Electrophoretic separation was conducted using 5 % stacking gel and 15 % separating gel, with 50 μg total protein added in each lane. After the protein had been wet transferred onto a PVDF membrane (Bio-Rad, USA), they were blocked in Tris-buffered saline with Tween (TBST) solution containing 5 % skimmed milk powder at room temperature for 1 h, then added with rabbit anti-RKIP polyclonal antibody (1:500 dilution) and rabbit anti-β-actin polyclonal antibody (1:500 dilution), and incubated overnight at 4 °C. Wash three times with fresh TBST for 5 min. Add HRP-labeled goat anti-rabbit IgG secondary antibody and inoculate it at 37 °C for 1 h. Wash three times with fresh TBST for 5 min. Autoradiography was conducted using ECL chemiluminescent substrate. The relative concentration of RKIP was presented RKIP/β-actin ratio, and the change of its relative expression was analyzed using PDQuest software (Bio-Rad, Richmond, CA, USA).
Total RNA was extracted using an RNeasy Mini Kit (Qiagen), according to the manufacturer’s protocol. The cDNA was synthesized using a cDNA Reverse Transcription Kit (Applied Biosystems, Foster, California). Real-time quantitative PCR was performed using the 7300 Real-Time PCR System (Applied Biosystems), according to the manufacturers’ protocols. All reactions were repeated in triplicate. The comparative Ct (DDCt) method was used to analyze the cycle threshold (Ct) values. Levels of mRNAs were normalized by glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as a reference.
Determination of the effect of the ectopic expression of RKIP on the viability of gastric cancer cells
The normally cultured SGC-7901 cells were uniformly seeded in 6-well culture plates at a density of 3 × 105/ml. After the cells reached complete adherence, the transfections of RKIP siRNA and RKIP scramble were performed using Lipofectamine 2000, in accordance with the manufacturer’s instruction. Meanwhile, normal control group (normal) was set. Cells in each group were collected, and the effect of RKIP interference in SGC-7901 cells was detected using Western blotting.
The normally cultured SGC-7901 or SGC-7901/DDP cells were uniformly seeded in 96-well culture plates at a density of 3 × 105/ml. The same transfection method was applied. RKIP siRNA and RKIP scramble were transfected into SGC-7901 cells, and pcDNA3.1 and pcDNA3.1-RKIP were transfected into SGC-7901/DDP cells. Then, 48 h after the transfection, each well was added with 100 μl MTT (0.5 mg/ml), and then, the cells were incubated at 37 °C in a 5 % CO2 incubator for 1 h. After incubation, cell lysis solution (100 μl/well 20 % SDS, 50 % N,N-dimethylformamide) was added to each well, and the plates were placed at 37 °C for 24 h. Record absorbance at 570 nm using a microplate reader (Bio-Tek, USA). Ten duplicate wells were used for each experimental group, and the experiment was repeated three times.
Determination of the effect of the ectopic expression of RKIP on the cisplatin-induced viability of gastric cancer cell using MTT method
The normally cultured SGC-7901 or SGC-7901/DDP cells were uniformly seeded in 96-well culture plates at a density of 3 × 105/ml. RKIP siRNA and RKIP scramble were transfected into SGC-7901 cells, and pcDNA3.1 and pcDNA3.1-RKIP were transfected into SGC-7901/DDP cells. After the transfection, cisplatin (DDP) was added at the final concentration of 0.5 μg/ml in the SGC-7901 cells, and cisplatin (DDP) was added at the final concentration of 5 μg/ml in the SGC-7901/DPP cells. Then, 48 h after the transfection, each well was added with 100 μl MTT (0.5 mg/ml), and then, the cells were incubated at 37 °C in a 5 % CO2 incubator for 1 h. After incubation, cell lysis solution (100 μl/well 20 % SDS, 50 % N,N-dimethylformamide) was added to each well, and the plates were placed at 37 °C for 24 h. Record absorbance at 570 nm using a microplate reader (Bio-Tek, USA). Ten duplicate wells were used for each experimental group, and the experiment was repeated three times.
Detection of the effect of the ectopic expression of RKIP on the cisplatin-induced apoptosis of gastric cancer cell using flow cytometry
The normally cultured SGC-7901 or SGC-7901/DDP cells were uniformly seeded in 6-well culture plates at a density of 3 × 105/ml. RKIP siRNA and RKIP scramble were transfected into SGC-7901 cells, and pcDNA3.1 and pcDNA3.1-RKIP were transfected into SGC-7901/DDP cells. After the transfection, cisplatin (DDP) was added at the final concentration of 0.5 μg/ml in the SGC-7901 cells, and cisplatin (DDP) was added at the final concentration of 5 μg/ml in the SGC-7901/DPP cells. Wash the cells one to two times with PBS 48 h after the transfection. Add Annexin V-FITC and PI dye, and then let incubate at room temperature in dark for 15 min. After the mesh filter, the apoptosis was detected using flow cytometry (BD, USA). The cells were counted using the CellQuest software (BD, USA), and the data were analyzed using the Macquit software.
Detection of the effect of the ectopic expression of RKIP on the signaling pathways of the cisplatin-induced sensitivity of gastric cancer cells
The normally cultured SGC-7901 or SGC-7901/DDP cells were uniformly seeded in 6-well culture plates at a density of 3 × 105/ml. RKIP siRNA and RKIP scramble were transfected into SGC-7901 cells, and pcDNA3.1 and pcDNA3.1-RKIP were transfected into SGC-7901/DDP cells. After the transfection, cisplatin (DDP) was added at the final concentration of 0.5 μg/ml in the SGC-7901 cells, and cisplatin (DDP) was added at the final concentration of 5 μg/ml in the SGC-7901/DPP cells. Meanwhile, normal control group (normal) was set. Wash the cells one to two times with PBS 48 h after the transfection. Each well was added with 1 ml of RIPA lysis solution. After cell proteins were harvested in each group, the the protein concentration of the supernatant was determined using BCA method. Electrophoretic separation was performed for each group. After the protein had been wet transferred onto a PVDF membrane (Bio-Rad, USA), they were blocked in TBST solution containing 5 % skimmed milk powder at room temperature for 1 h, then added with rabbit anti-NF-kB p65 (C-20) and anti-Snail primary antibody (1:500 dilution) and mouse antihuman-β-actin monoclonal antibody (1:1,000 dilution), and incubated overnight at 4 °C. Wash three times with fresh TBST for 5 min. Add HRP-labeled goat anti-rabbit IgG secondary antibody and inoculate it at 37 °C for 1 h. Wash three times with fresh TBST for 5 min. Autoradiography was conducted using ECL chemiluminescent substrate. The relative concentration of RKIP was presented as the NF-kB p65/β-actin ratio or Snail/β-actin ratio, and the change of its relative expression was analyzed using PDQuest software (Bio-Rad, Richmond, CA, USA).
The effect of RKIP on the transcriptional activity of NF-κB was detected by NF-κB-driven luciferase reporter assay. The transcriptional activity of NF-κB was tested by NF-κB-dependent reporter gene expression assay. An NF-κB luciferase construct was stably transfected into SGC-7901 cells and a single stable clone and was obtained and used in the present study. In brief, SGC-7901 cells were transfected with RKIP recombinant plasmid or RKIP-siRNA for 48 h, and the NF-κB-luciferase activities were measured using luciferase assay kits from Promega.
Statistical analysis
Data were analyzed using chi square test or t test in Stata 7.0 software. A value of P < 0.05 was considered statistically significant.
Results
Expression of RKIP in human gastric cancer cell lines
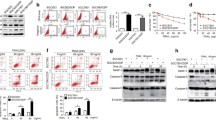
As shown by Western blotting, the expression of RKIP protein was significantly lower in SGC-7901/DDP cells than that in SGC-7901 cells (P < 0.05) (Fig. 1), suggesting that RKIP has lower expression in cisplatin-resistant gastric cancer cells.
Effect of the ectopic expression of RKIP on the viability of gastric cancer cells
The RKIP expression was interfered by transfecting RKIP siRNA into SGC-7901 cells. Its interference effectiveness was measureed by Western blotting, which showed that the expression of RKIP protein in cells significantly decreased 48 h after the transfection of siRNA (P < 0.05) (Fig. 2). Thus, the transfection of RKIP siRNA can effectively suppress the RKIP expression in cells.
Effect of the ectopic expression of RKIP on the viability of gastric cancer cells
As shown by MTT, the viability of the SGC-7901 cells transfected with RKIP was significantly higher than that of negative control group (RKIP scramble) and normal control group (P < 0.05). In contrast, the viability of the SGC-7901/DDP cells transfected with pcDNA3.1-RKIP was significantly lower than that of negative control group (pcDNA3.1) and normal control group (P < 0.05) (Fig. 3a). The result of growth curve was just like MTT (Fig. 3b). The cell numbers of SGC-7901 cells transfected with RKIP was significantly higher than that of negative control group (RKIP scramble) and normal control group (P < 0.01).
Effect of the ectopic expression of RKIP on the viability of gastric cancer cells. a MTT was used to analyze the effect of the ectopic expression of RKIP on the viability of gastric cancer cells. b Growth curve assay was used to analyze the effect of the ectopic expression of RKIP on the viability of gastric cancer cells. *P < 0.05 and **P < 0.01
Effect of the ectopic expression of RKIP on the cisplatin-induced viability of gastric cancer cell
The effect of the ectopic expression of RKIP on the cisplatin-induced viability of gastric cancer cell was detected using MTT method. As shown by MTT, the viability of the SGC-7901 cells that had been transfected with RKIP siRNA and treated by 0.5 μg/ml DDP for 48 h was significantly higher than that of negative control group (RKIP scramble) (P < 0.05). In contrast, the the viability of the SGC-7901/DDP cells that had been transfected with pcDNA3.1-RKIP and treated by 0.5 μg/ml DDP for 48 h was significantly lower than that of negative control group (pcDNA3.1) (P < 0.05) (Fig. 4a). The result of growth curve assay is shown in Fig. 4b. The results of MTT and growth curve all proved that overexpression of RKIP enhanced the sensitivity of SGC-7901/DDP cells to cisplatin.
Effect of the ectopic expression of RKIP on the cisplatin-induced viability of gastric cancer cell. a MTT was used to analyze the effect of the ectopic expression of RKIP on the cisplatin-induced viability of gastric cancer cell. b Growth curve assay was used to analyze the effect of the ectopic expression of RKIP on the cisplatin-induced viability of gastric cancer cell. *P < 0.05 and **P < 0.01
Effect of the ectopic expression of RKIP on the cisplatin-induced apoptosis of gastric cancer cells
As shown by flow cytometry, the amount of the apoptotic SGC-7901 cells that had been transfected with RKIP siRNA and treated by 0.5 μg/ml DDP for 48 h was significantly smaller than that of negative control group (RKIP scramble) (P < 0.05). In contrast, the amount of the apoptotic SGC-7901/DDP cells that had been transfected with pcDNA3.1-RKIP and treated by 5 μg/ml DDP for 48 h was significantly larger than that of negative control group (pcDNA3.1) (P < 0.05) (Fig. 5). Therefore, the sensitivity of SGC-7901/DDP cells to cisplatin decreases after the RKIP expression was suppressed.
Detection of the effect of the ectopic expression of RKIP on the signaling pathways of the cisplatin-induced sensitivity of gastric cancer cells
As shown by Western blotting, the SGC-7901 cells that had been transfected with RKIP siRNA and treated by 0.5 μg/ml DDP for 48 h had significantly higher NF-κB/β-actin and Snail/β-actin ratios when comapred with the negative control group (RKIP scramble) and normal control group (P < 0.01). In contrast, the SGC-7901/DDP cells that had been transfected with pcDNA3.1-RKIP and treated by 5 μg/ml DDP for 48 h had significantly lower NF-κB/β-actin and Snail/β-actin ratios when comapred with the negative control group (pcDNA3.1) (P < 0.01) (Fig. 6a, b). Therefere, the suppresion of the RKIP expression in SGC-7901 cells can promote the expressions of NF-κB and Snail.
Detection of the effect of the ectopic expression of RKIP on the signaling pathways of the cisplatin-induced sensitivity of gastric cancer cells. a Results of Western blotting of the expressions of NF-κB, Snail, and β-actin proteins in cells in each group. b Relative expressions of NF-κB and β-actin proteins in cells in each group. c Changes of the relative expressions of Snail and β-actin proteins in cells in each group. d The effect of RKIP on the transcriptional activity of NF-κB. 1 Normal control group; 2 SGC-7901 cells transfected with RKIP scramble; 3 SGC-7901 cells transfected with RKIP siRNA; 4 SGC-7901/DDP cells transfected with pcDNA3.1; and 5 SGC-7901/DDP cells transfected with pcDNA3.1-RKIP. ** P < 0.01
We used an NF-κB-luciferase reporter gene expression assay to determine the effect of RKIP on transcriptional activity of NF-κB. Overexpression of RKIP (SGC-7901/DDP cells transfected with pcDNA3.1) resulted in strong decreases in NF-κB transcriptional activity (P < 0.01). However, the inhibition of RKIP expression (SGC-7901 cells transfected with RKIP siRNA) improved the NF-κB transcriptional activity (P < 0.01).
Discussion
Studies have shown that resistance is a major cause of treatment failure in chemotherapeutic drugs. Chemotherapy resistance in cancer treatment can be caused by a variety of factors including intracellular drug isolation/efflux, metabolic detoxication, change of molecular drug targets, enhanced DNA repair capacity, and regulation of apoptosis [31, 32]. Some resistance-related genes (e.g., P-gp, MRP, GST-p, and Topo II) have been identified after extensive gene and protein studies on the mechanisms behind resistance to chemotherapy [33]. However, cancer cell resistance to chemotherapy has not clinically improved after the interference of the above genes and their upstream and downstream targets [34]. Thus, in-depth studies on more resistance-related factors from a wider range of perspectives will be of particular importance.
RKIP can regulate some key intracellular signaling pathways including MAPK, NF-κB, and G protein-coupled receptors (GPCRs) and thus plays a key role in cell division, growth, proliferation, and apoptosis. Our preliminary results have shown that the RKIP expression is downregulated in human gastric cancer cell lines, which can inhibit the proliferation and invasion of gastric cancer cells. In our current study, we explored the relationship between the RKIP and the sensitivity of gastric cancer cells to the chemotherapeutic drug cisplatin in cisplatin-resistant gastric cancer cell line SGC-7901/DDP and normal gastric cancer cell line SGC-7901. By transfecting RKIP siRNA in SGC-7901 cells and transfecting pcDNA3.1-RKIP in SGC-7901/DDP cells, we studied the effect of the ectopic expression of RKIP on the cisplatin-induced sensitivity of gastric cancer cells.
As shown by MTT and flow cytometry, the viability of the SGC-7901 cells that had been transfected with RKIP siRNA and treated by DDP for 48 h was significantly higher than that of negative control group (RKIP scramble) (P < 0.05), and the amount of the apoptotic SGC-7901 cells was significantly smaller (P < 0.05). In contrast, the SGC-7901/DDP cells that had been transfected with pcDNA3.1-RKIP and treated by 5 μg/ml DDP for 48 h had significantly lower viability when comapred with the negative control group (pcDNA3.1) (P < 0.05), and the amount of the apoptotic SGC-7901 cells was significantly larger (P < 0.05). Therefore, the sensitivity of SGC-7901 cells to cisplatin decreases after the RKIP expression is suppressed, whereas the overexpression of RKIP enhances the sensitivity of SGC-7901/DDP cells to cisplatin. Recent studies have shown that RKIP was associated with the sensitivities of cervical cancer and nasopharyngeal cancer to chemotherapy [15, 30]. Our current study further demonstrated the correlation between RKIP and chemosensitivity in human gastric cancer cell lines.
NF-κB, an important transcription-regulating factor, is found to be associated with immunological stress, cell differentiation, cell cycle regulation, apoptosis, and malignancies [35–37]. Abnormal NF-κB expression has also been found in some drug-resistant tumor cell lines [38]. On one hand, chemotherapy can cause DNA damage, which leads the apoptosis of tumor cells; on the other hand, the chemotherapy-associated DNA damage can trigger the activation of NF-κB and further block apoptosis. The suppressed NF-κB expression can promote the apoptosis of tumor cells and enhances chemosensitivity [39]. The Snail is a zinc finger transcription factors. It can mediate the epithelial-mesenchymal transition and inhibit the expression of E-cadherin at the transcriptional level [40]. It is known that the NF-kB complex upregulates downstream antiapoptotic genes, including the metastasis inducer and antiapoptotic Snail gene [41]. As shown in our current study, the inhibition of the RKIP expression in SGC-7901 cells could promote the expressions of NF-κB and Snail, whereas the overexpression of RKIP in SGC-7901/DDP cells remarkably inhibited the expressions of NF-κB and Snail. Also, the overexpression of RKIP can enhance the sensitivity of human gastric cancer cells to cisplatin, which may be achieved via the NF-κB/Snail signaling pathway.
In summary, the expression of RKIP is downregulated in cisplatin-resistant cell line (SGC-7901/DDP). The overexpression of RKIP can enhance the sensitivity of human gastric cancer cells to cisplatin, which may be achieved via the NF-κB/Snail signaling pathway.
References
Chen W, Zheng R, Zhang S, Zhao P, Li G, Wu L, et al. Report of incidence and mortality in China cancer registries, 2009. Chin J Cancer Res. 2013;25:10–21.
Hu B, El Hajj N, Sittler S, Lammert N, Barnes R, Meloni-Ehrig A. Gastric cancer: classification, histology and application of molecular pathology. J Gastrointest Oncol. 2012;3:251–61.
Ryu KW, SENORITA Study Group. Future perspective of laparoscopic surgery for gastric cancer: sentinel node navigation function-preserving surgery for early gastric cancer. Transl Gastrointest Cancer. 2013;2:160–3.
Ling TC, Kang JI, Slater JD, Yang GY. Proton therapy for gastrointestinal cancers. Transl Cancer Res. 2012;1:150–8.
Wang YX, Wang DW, Zhu XM, Zhao F, Leung KC. Carbon coated superparamagnetic iron oxide nanoparticles for sentinel lymph nodes mapping. Quant Imaging Med Surg. 2012;2:53–6.
Al Mushref M, Srinivasan S. Effect of high fat-diet and obesity on gastrointestinal motility. Ann Transl Med. 2013;1:14.
Yamada Y. Molecular therapy for gastric cancer. Chin Clin Oncol. 2013;2:5.
Kang B, Sun XH. Regulation of cancer stem cells by RING finger ubiquitin ligases. Stem Cell Investig. 2014;1:5.
Yeung K, Seitz T, Li S, Janosch P, McFerran B, Kaiser C, et al. Suppression of Raf-1 kinase activity and MAP kinase signalling by RKIP. Nature. 1999;401:173–7.
Zeng L, Imamoto A, Rosner MR. Raf kinase inhibitory protein (RKIP): a physiological regulator and future therapeutic target. Expert Opin Ther Targets. 2008;12:1275–87.
Granovsky AE, Rosner MR. Raf kinase inhibitory protein: a signal transduction modulator and metastasis suppressor. Cell Res. 2008;18:452–7.
Khamis ZI, Iczkowski KA, Sang QX. Metastasis suppressors in human benign prostate, intraepithelial neoplasia, and invasive cancer: their prospects as therapeutic agents. Med Res Rev. 2012;32:1026–77.
Morecroft I, Doyle B, Nilsen M, Kolch W, Mair K, Maclean MR. Mice lacking the Raf-1 kinase inhibitor protein exhibit exaggerated hypoxia-induced pulmonary hypertension. Br J Pharmacol. 2011;163:948–63.
Beshir AB, Ren G, Magpusao AN, Barone LM, Yeung KC, Fenteany G. Raf kinase inhibitor protein suppresses nuclear factor-kappaB-dependent cancer cell invasion through negative regulation of matrix metalloproteinase expression. Cancer Lett. 2010;299:137–49.
Martinho O, Pinto F, Granja S, Miranda-Goncalves V, Moreira MA, Ribeiro LF, et al. RKIP inhibition in cervical cancer is associated with higher tumor aggressive behavior and resistance to cisplatin therapy. PLoS ONE. 2013;8:e59104.
Lorusso D, Petrelli F, Coinu A, Raspagliesi F, Barni S. A systematic review comparing cisplatin and carboplatin plus paclitaxel-based chemotherapy for recurrent or metastatic cervical cancer. Gynecol Oncol. 2014;33:117–23.
Marcu LG. Improving therapeutic ratio in head and neck cancer with adjuvant and cisplatin-based treatments. BioMed Res Int. 2013;2013:817279.
Ciarimboli G. Membrane transporters as mediators of cisplatin side-effects. Anticancer Res. 2014;34:547–50.
Peres LA, Cunha Junior AD. Acute nephrotoxicity of cisplatin: molecular mechanisms. J Bras Nefrol Orgao Oficial Soc Bras Latino-Am Nefrol. 2013;35:332–40.
Smith HS, Smith JM, Seidner P. Opioidinduced nausea and vomiting. Ann Palliat Med. 2012;1:121–9.
Peters GJ, Avan A, Ruiz MG, Orsini V, Avan A, Giovannetti E, et al. Predictive role of repair enzymes in the efficacy of cisplatin combinations in pancreatic and lung cancer. Anticancer Res. 2014;34:435–42.
Lombardi G, Pambuku A, Bellu L, Della Puppa A, Rumano L, Gardiman MP, et al. Cisplatin and temozolomide combination in the treatment of supratentorial anaplastic ependymoma. Chemotherapy. 2013;59:176–80.
Fu Z, Smith PC, Zhang L, Rubin MA, Dunn RL, Yao Z, et al. Effects of raf kinase inhibitor protein expression on suppression of prostate cancer metastasis. J Natl Cancer Inst. 2003;95:878–89.
Xu YF, Yi Y, Qiu SJ, Gao Q, Li YW, Dai CX, et al. PEBP1 downregulation is associated to poor prognosis in HCC related to hepatitis B infection. J Hepatol. 2010;53:872–9.
Lin K, Baritaki S, Militello L, Malaponte G, Bevelacqua Y, Bonavida B. The role of B-RAF mutations in melanoma and the induction of EMT via dysregulation of the NF-kappaB/Snail/RKIP/PTEN circuit. Genes Cancer. 2010;1:409–20.
Al-Mulla F, Hagan S, Al-Ali W, Jacob SP, Behbehani AI, Bitar MS, et al. Raf kinase inhibitor protein: mechanism of loss of expression and association with genomic instability. J Clin Pathol. 2008;61:524–9.
Li HZ, Gao Y, Zhao XL, Liu YX, Sun BC, Yang J, et al. Effects of raf kinase inhibitor protein expression on metastasis and progression of human breast cancer. Mol Cancer Res. 2009;7:832–40.
Minn AJ, Bevilacqua E, Yun J, Rosner MR. Identification of novel metastasis suppressor signaling pathways for breast cancer. Cell Cycle. 2012;11:2452–7.
Bonavida B, Baritaki S. The novel role of Yin Yang 1 in the regulation of epithelial to mesenchymal transition in cancer via the dysregulated NF-kappaB/Snail/YY1/RKIP/PTEN Circuitry. Crit Rev Oncog. 2011;16:211–26.
Li SW, Wang H, Liu ML, Zhang HB, Xiang YQ, Lv X, et al. Positive effect of high RKIP expression on reduced distant metastasis by chemotherapy when combined with radiotherapy in locoregionally advanced nasopharyngeal carcinoma: a prospective study. Med Oncol. 2013;30:322.
Jagadeesh D, Smith MR. Novel targeted therapies in peripheral T cell lymphoma. Discov Med. 2013;15:367–78.
Morotti M, Becker C, Menada MV, Ferrero S. Targeting tyrosine-kinase in ovarian cancer. Expert Opin Investig Drugs. 2013;22:1265–79.
Lubin J, Markowska A, Knapp P. Factors affecting response of chemotherapy in women with ovarian cancer. Eur J Gynaecol Oncol. 2012;33:644–7.
Breier A, Gibalova L, Seres M, Barancik M, Sulova Z. New insight into p-glycoprotein as a drug target. Anticancer Agents Med Chem. 2013;13:159–70.
Al-Mulla F, Bitar MS, Taqi Z, Yeung KC. RKIP: much more than Raf kinase inhibitory protein. J Cell Physiol. 2013;228:1688–702.
Escara-Wilke J, Yeung K, Keller ET. Raf kinase inhibitor protein (RKIP) in cancer. Cancer Metastasis Rev. 2012;31:615–20.
Altura BM, Shah NC, Shah GJ, Zhang A, Li W, Zheng T, et al. Short-term Mg deficiency upregulates protein kinase C isoforms in cardiovascular tissues and cells; relation to NF-kB, cytokines, ceramide salvage sphingolipid pathway and PKC-zeta: hypothesis and review. Int J Clin Exp Med. 2014;7:1–21.
Hoesel B, Schmid JA. The complexity of NF-kappaB signaling in inflammation and cancer. Mol Cancer. 2013;12:86.
Fuchs O. Targeting of NF-kappaB signaling pathway, other signaling pathways and epigenetics in therapy of multiple myeloma. Cardiovasc Hematol Disord Drug Targets. 2013;13:16–34.
Bonavida B, Jazirehi A, Vega MI, Huerta-Yepez S, Baritaki S. Roles each of Snail, Yin Yang 1 and RKIP in the regulation of tumor cells chemo-immuno-resistance to apoptosis. Forum Immunopathol Dis Ther. 2013;4.
Rapozzi V, Umezawa K, Xodo LE. Role of NF-kappaB/Snail/RKIP loop in the response of tumor cells to photodynamic therapy. Lasers Surg Med. 2011;43:575–85.
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, H., Li, P., Li, B. et al. RKIP promotes cisplatin-induced gastric cancer cell death through NF-κB/Snail pathway. Tumor Biol. 36, 1445–1453 (2015). https://doi.org/10.1007/s13277-014-2496-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-014-2496-6