Abstract
First-line chemotherapy + bevacizumab (BEV) is one of the standards of care in advanced colorectal cancer (CRC). Contrary to anti-EGFR agents, it is currently not possible to identify the ideal candidate for BEV-based chemotherapy due to the lack of predictors of outcomes. The aim of this study was to perform a systematic review of risk factors for survival after B-based chemotherapy for CRC. We performed a meta-analysis by searching on the databases PubMed, EMBASE, Web of Science and SCOPUS for a published series that focused on prognostic factors for BEV-based therapy in advanced CRC. Pooled hazard ratios (HR) were calculated by using a random-effects model for parameters that could be considered as potential prognostic factors in ≥3 papers. Twenty-nine studies, which included a total of 11,585 patients, were considered in this analysis. Five parameters were associated with survival in ≥3 papers: (1) a longer progression-free interval [PFS: HR 0.87, 95 % confidence interval (CI) 0.78–0.97; P = 0.01]; (2) a single site of metastases (HR 0.63, 95 % CI 0.56–0.71; P < 0.00001); (3) elevated lactate dehydrogenase (LDH: HR 2.08, 95 % CI 1.69–2.57; P < 0.00001); (4) KRAS mutation (HR 1.66, 95 % CI 1.36–2.03; P < 0.00001); and (5) poor performance status (PS: HR 1.99, 95 % CI 1.41–2.82; P < 0.0001). Clinical variables associated with prolonged survival, after first-line treatment with chemotherapy + BEV for metastatic CRC patients, included long PFS, low LDH levels, KRAS wild-type status, good PS and a single site of metastasis. They should be considered when stratifying patients for inclusion in randomized trials. Investigations into new prognostic factors based on tumor biology are needed and of high priority.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Outcomes of patients with advanced colorectal cancer (CRC) have significantly improved with the addition of molecular agents such as anti-EGFR (cetuximab and panitumumab) and anti-angiogenetic monoclonal antibodies [bevacizumab (BEV)] in first-line chemotherapy. Selection of patients for such molecular drugs depends on their performance status (PS) and aim of treatment, the extent of their disease and the clinical or molecular predictors of benefit. It has clearly been demonstrated that anti-EGFR monoclonal antibodies work only if there are no mutations in the RAS protooncogene; in this case, the outcome [overall survival (OS)] is similar or even better than with chemotherapy + BEV [1, 2]. This has been explained by the better deepness of response and early tumor shrinkage associated with anti-EGFR agents [3]. Among patients treated with cetuximab or panitumumab, the development of a skin rash of moderate/severe entity has been associated with a better OS [4]. Conversely, BEV seems to confer a similar magnitude of benefit both in RAS wild-type and in KRAS- or BRAF-mutated tumors [5].
In 2002, Kohne demonstrated, in 19 first-line 5-fluorouracil-based prospective trials, that a clinical risk score based upon four baseline clinical variables such as PS, white blood cells count, alkaline phosphatase and the number of metastatic sites could predict outcome [6]. In four prospective trials, comparing FOLFOX and FOLFIRI, Chibaudel et al. [7] showed that serum lactate dehydrogenase (LDH) level was the main prognostic factor in predicting survival, followed by WHO PS. Similar analysis was not performed for patients treated with targeted therapies, in particular those with first-line BEV-based chemotherapy. Among patients treated with first-line BEV, the predictors of survival have not yet been discovered. However, the development of arterial hypertension has been correlated with increased response rate, progression-free survival and OS in BEV-treated patients [8].
It can be stated that BEV is associated with an increased risk of hypertension, ischemic heart disease, gastrointestinal hemorrhage and/perforation, other than fatal adverse events. So, it is of outstanding importance to discover clinicopathological variables associated with OS in CRC patients exposed to first-line chemotherapy + BEV [9–11]. This could permit decisions for more or less intensive treatments and allow the stratification of patients with different prognoses in randomized clinical trials.
A systematic review or meta-analysis of predictors of survival with BEV in advanced CRC has not yet been performed. In this study, we systematically analyzed all informative studies related to this topic in order to determine the evidence for clinical, therapeutic, laboratory and genetic predictors of outcomes in stage IV CRC patients with BEV-based chemotherapy.
Methods
This systematic review and meta-analysis are reported in accordance with the preferred reporting items for systematic reviews and meta-analyses (PRISMA) statements (www.prisma-statement.org) [12].
Eligibility criteria
All clinical, therapeutic, laboratory or genetic variables studied in patients with advanced CRC for its possible association with OS were searched for.
Clinical trials or prospective/retrospective cohort series with adult, stage IV, CRC patients studying the relationship between clinical, therapeutic, laboratory or genetic parameters at the time of starting first-line chemotherapy + BEV and survival (OS) published in the English language, without publication date restrictions, were eligible for inclusion. Only the variables reported as significant predictors in at least 3 papers were pooled.
Studies (1) with <10 pts; (2) where the predictors were not determined at first-line therapy; (3) where the described therapy included other biological agents or chemotherapy alone; (3) where predictors were not evaluated as multivariate analysis; and (4) where there was no full text available, were excluded.
Studies were identified by searching PubMed, EMBASE, Web of Science and SCOPUS databases. The search was performed in November 2014. We searched for the terms (CRC or colorectal carcinoma) and (multivariate or multivariable or ‘cox regression’) and BEV and (overall survival) and (hazard ratio or HR).
Two investigators (FP and AC) independently screened all results by reviewing the titles and abstracts. All potentially relevant studies were retrieved as full-text manuscripts. FP and AC evaluated all studies for compliance with the inclusion criteria. In case there was any doubt about their eligibility for inclusion, this was discussed with a third independent senior oncologist (SB). Duplicate reports of studies were excluded by checking authors’ names, affiliations and titles. Duplicate inclusion of patients participating in more than one study was avoided by systematically evaluating patient recruitment periods and participating centers. A patient could only be evaluated in more than one study if a different predictor was analyzed.
Data extraction
Data extraction from manuscripts was performed by two investigators (FP and AC). The following data were extracted from the included studies: first author, year of publication, number of patients, type of study cohort, first-line chemotherapy, prognostic determinants studied as multivariate analysis and significantly associated with OS, hazard ratios (HRs) and their 95 % confidence interval (95 % CI). HRs were extracted from multivariable analyses where available.
Statistical analysis
The primary aim was to determine the independent prognostic value of clinical variables related to OS. All clinical variables studied were recorded, and results were given for prognostic factors found to be significantly associated with OS in multivariate analysis. HRs of OS were used as the primary effect estimate in this meta-analysis. We calculated the pooled HRs and 95 % CI for all predictors presented in at least three papers. To incorporate heterogeneity between studies, we used generic inverse-variance and the random effect model by the implementing the Mantel–Haenszel method [13] and using the Cochrane statistical package in Review Manager 5.3 (RevMan version 5.3; Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014). Heterogeneity was assessed using Cochran Q and I2 statistics. All statistical tests were two-sided, and the statistical significance was defined as P < 0.05. Measures of heterogeneity were calculated and included in all forest plots created with RevMan.
Results
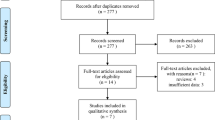
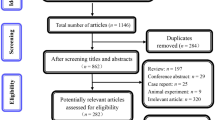
Twenty-nine studies [14–39, 43], among 386 retrieved, which encompassed a total of 11,585 patients, were included. An overview of our search and study selection is shown in the flowchart (Fig. 1). Characteristics of the included studies are shown in Table 1; most were published in 2010 or later. Among them, 21 were prospective or retrospective cohort single or multicenter series and 7 were analyses of the prospective phase II (n = 4) or III (n = 3) studies. Patients ranged from 33 to 3,187.
LDH levels
Three studies contributed to the analysis (Fig. 2). No heterogeneity across the studies was detected (I 2 = 0; P = 0.37). An elevated LDH level was associated with an increased risk of death (HR 2.08, 95 % CI 1.69–2.57; P < 0.00001).
Number of metastatic sites
Six studies contributed to the pooled analysis (Fig. 3). No heterogeneity across the studies was detected (I 2 = 0; P = 0.54). A single metastatic site was associated with a reduced risk of death compared to multiple sites of disease (HR 0.63, 95 % CI 0.56–0.71; P < 0.00001).
Performance status
In five studies with data available (Fig. 4), a poor PS was associated with an increased risk of death (HR 1.99, 95 % CI 1.41–2.82; P < 0.00001). Significant heterogeneity was observed (I 2 = 84 %; P < 0.0001).
Progression-free survival/time to progression
Three studies contributed to this analysis. A longer time to progression or PFS was moderately associated with a reduced risk of death (HR 0.87, 95 % CI 0.78–0.97; P = 0.01) (Fig. 5). High heterogeneity across the studies was detected (I 2 = 98; P < 0.00001).
KRAS status
Three studies contributed to this analysis (Fig. 6). KRAS-mutated status was associated with an increased risk of death (HR 1.66, 95 % CI = 1.36–2.03; P < 0.00001). No heterogeneity across the studies was detected (I 2 = 0; P = 0.8).
Discussion
The data presented here provide robust prognostic information regarding more than 11,000 patients with CCR who underwent first-line chemotherapy including BEV. Three clinicopathological parameters clearly emerged as significant predictors of poor outcomes for patients treated with BEV: high LDH level, poor PS and KRAS mutation. The presence of each of these three variables increases the probability of death by about a factor of two. Conversely, a prolonged time to progression during first-line therapy and a single metastatic site were associated with an improved outcome. To our knowledge, this systematic multivariate analysis represents the largest meta-analysis that identifies the clinicopathological prognostic factors for patients with metastatic CCR treated with BEV-based chemotherapy. It derives from the inclusion of 29 studies published in the last decade, when BEV was approved for use in advanced CRC. BEV targets the angiogenesis pathway through circulating VEGF but actually lacks any predictive factors, so an evaluation of predictors of outcome could permit oncologists to select the best candidate for the combination of chemotherapy plus BEV.
An examination of some of these parameters usually belongs to the clinical routine practice before a patient starts any chemotherapy regimens for stage IV CRC. PS and an evaluation of the extent of disease, other than serum chemical analysis including LDH levels, are usually checked at baseline evaluation. KRAS mutation status may also exclude patients from anti- to EGFR therapy, as commonly stated in the clinical guidelines, and it identifies metastatic CRC patients with a poor prognosis, even those treated with BEV [42].
The definition of these prognostic factors has several implications in clinical practice. The PS other than the sites of metastases permits the validation of the scope of cure (palliative or curative) in a metastatic setting. A liver-confined disease in a fit and young patient should induce the start of a neoadjuvant course of chemotherapy (plus a biological agent?) with the aim of the resection of hepatic metastases. Conversely, in unfit patients with widespread metastatic disease and KRAS-mutated status, the intensity of cure can be attenuated with sequential mono- or polychemotherapy alone, which could represent the preferable choice. In a CRC setting, LDH has assumed rising importance in defining the prognosis of patients treated with anti-VEGF(R) agents. Recently, Silvestris and colleagues [43] showed a statistically significant association between high pretreatment LDH levels and progressive disease compared to low basal LDH patients. Furthermore, the median PFS was 7.3 versus 10.8 months for high and low LDH levels, respectively. High LDH levels have been correlated with intratumoral gene expression of VEGFA and VEGFR1, thus supporting the hypothesis that serum LDH levels may serve as a surrogate marker for activation of the hypoxia-inducible factor-related genes in the tumor [44]. Finally, progression-free survival or time to progression have already been validated as surrogate endpoints in advanced CRC with targeted therapies [45] and are also common predictors of better outcomes in patients treated with first-line therapy in many other solid tumors.
There are several limitations to the present study, and our results should be interpreted cautiously because there is a possibility of bias regarding selection criteria. The study, also, should not be taken as advocacy for excluding some patients from BEV therapy. Rather, it highlights the importance of selection criteria for intensive therapy including chemotherapy + BEV for advanced disease. In addition, there is likely to be substantial variation among the included series in terms of the timing of disease evaluation and median follow-up. We observed significant heterogeneity for two out of five parameters: first, PS meta-analysis includes comparisons of PS 0–1 versus PS ≥ 2 or PS 1 versus PS > 1. Second, significant heterogeneity was present for the PFS analysis. This finding might be explained by the fact that imaging studies could have been performed differently in clinical studies versus cohort studies. Finally, the results of this meta-analysis do not provide a predictive significance for patients treated with BEV, and the aim of this study was restricted to the observation of prognostic risk factors for survival.
Other prognostic variables have been investigated in patients exposed to BEV. Among them, the development of hypertension seems a reliable prognostic parameter with anti-angiogenetic agents. A meta-analysis previously published confirmed the favorable prognostic significance of hypertension development in CCR studies [8]. The occurrence of BEV-induced hypertension in patients was highly associated with improvements in PFS (HR 0.57, 95 % CI 0.46–0.72; P < 0.001), OS (HR 0.50; 95 % CI 0.37–0.68; P < 0.001) and response rate (relative risk = 1.57, 95 % CI 1.07–2.30, P < 0.05), as compared to patients without hypertension. Monitoring hypertension during treatment is of medical and clinical importance in preventing fatal events and is likely to reassure patients as a result of its prognostic significance. This clinical parameter, however, needs to be prospectively investigated. Inflammation parameters have also acquired prognostic importance. The level of neutrophils and the ratio between neutrophils and lymphocytes have been correlated with poor prognosis in CRC according to a meta-analysis of cohort studies [46]. In our meta-analysis, few trials reported a significant association of hypertension and neutrophils count or neutrophil/lymphocyte ratio and outcome, and as such were not included in the final analysis.
This meta-analysis can be of paramount importance for medical oncologists treating CRC patients with first-line chemotherapy. It defines, in fact, a portrait of an ideal candidate for first-line treatment with chemotherapy and BEV for advanced CRC in clinical practice. Fit patients, with a low burden of disease and normal LDH levels, probably obtain the greater benefit from first-line BEV. However, the need for rapid tumor shrinkage with a neoadjuvant treatment, or for a symptomatic palliation of symptoms in the presence of a high burden of disease, could lead to the preference of anti-EGFR agents.
In conclusion, we found five reliable predictors of survival for (first-line) BEV-treated CRC patients. These parameters should be considered when selecting first-line therapy and stratifying patients for inclusion in future randomized trials including BEV for managing stage IV CRC patients.
References
Heinemann V, von Weikersthal LF, Decker T, et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (FIRE-3): a randomised, open-label, phase 3 trial. Lancet Oncol. 2014;15(10):1065–75.
Venook AP, Niedzwiecki D, Lenz H-J, et al. CALGB/SWOG 80405: phase III trial of irinotecan/5-FU/leucovorin (FOLFIRI) or oxaliplatin/5-FU/leucovorin (mFOLFOX6) with bevacizumab (BV) or cetuximab (CET) for patients (pts) with KRAS wild-type (wt) untreated metastatic adenocarcinoma of the colon or rectum (MCRC). J Clin Oncol. 2014;32:5 s (suppl; abstr LBA3).
Heinemann V, Modest D, von Weikersthal LF, et al. Independent radiological evaluation of objective response early tumor shrinkage, and depth of response in FIRE-3 (AIO KRK-0306). Ann Oncol. 2014;25:ii117.
Petrelli F, Borgonovo K, Barni S. The predictive role of skin rash with cetuximab and panitumumab in colorectal cancer patients: a systematic review and meta-analysis of published trials. Target Oncol. 2013;8(3):173–81.
Ince WL, Jubb AM, Holden SN, Holmgren EB, Tobin P, Sridhar M, Hurwitz HI, Kabbinavar F, Novotny WF, Hillan KJ, Koeppen H. Association of k-ras, b-raf, and p53 status with the treatment effect of bevacizumab. J Natl Cancer Inst. 2005;97(13):981–9.
Köhne CH, Cunningham D, Di Costanzo F, Glimelius B, Blijham G, Aranda E, Scheithauer W, Rougier P, Palmer M, Wils J, Baron B, Pignatti F, Schöffski P, Micheel S, Hecker H. Clinical determinants of survival in patients with 5-fluorouracil-based treatment for metastatic colorectal cancer: results of a multivariate analysis of 3825 patients. Ann Oncol. 2002;13(2):308–17.
Chibaudel B, Bonnetain F, Tournigand C, Bengrine-Lefevre L, Teixeira L, Artru P, Desramé J, Larsen AK, André T, Louvet C, de Gramont A. Simplified prognostic model in patients with oxaliplatin-based or irinotecan-based first-line chemotherapy for metastatic colorectal cancer: a GERCOR study. Oncologist. 2011;16(9):1228–38.
Cai J, Ma H, Huang F, Zhu D, Bi J, Ke Y, Zhang T. Correlation of bevacizumab-induced hypertension and outcomes of metastatic colorectal cancer patients treated with bevacizumab: a systematic review and meta-analysis. World J Surg Oncol. 2013;28(11):306.
Dai F, Shu L, Bian Y, Wang Z, Yang Z, Chu W, Gao S. Safety of bevacizumab in treating metastatic colorectal cancer: a systematic review and meta-analysis of all randomized clinical trials. Clin Drug Investig. 2013;33(11):779–88.
Chen XL, Lei YH, Liu CF, Yang QF, Zuo PY, Liu CY, Chen CZ, Liu YW. Angiogenesis inhibitor bevacizumab increases the risk of ischemic heart disease associated with chemotherapy: a meta-analysis. PLoS One. 2013;8(6):e66721.
Ranpura V, Hapani S, Wu S. Treatment-related mortality with bevacizumab in cancer patients: a meta-analysis. JAMA. 2011;305(5):487–94.
Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Br Med J. 2009;339:b2535.
Cochrane handbook for systematic reviews of interventions. http://www.cochrane.org/training/cochrane-handbook. Accessed November 15th, 2013.
Aoyagi Y, Iinuma H, Horiuchi A, Shimada R, Watanabe T. Association of plasma VEGF-A, soluble VEGFR-1 and VEGFR-2 levels and clinical response and survival in advanced colorectal cancer patients receiving bevacizumab with modified FOLFOX6. Oncol Lett. 2010;1(2):253–9.
Boisen MK, Dehlendorff C, Linnemann D, Nielsen BS, Larsen JS, Osterlind K, Nielsen SE, Tarpgaard LS, Qvortrup C, Pfeiffer P, Holländer NH, Keldsen N, Hansen TF, Jensen BB, Høgdall EV, Jensen BV, Johansen JS. Tissue MicroRNAs as predictors of outcome in patients with metastatic colorectal cancer treated with first line capecitabine and oxaliplatin with or without bevacizumab. PLoS One. 2014;9(10):e109430.
Boisen MK, Johansen JS, Dehlendorff C, Larsen JS, Osterlind K, Hansen J, Nielsen SE, Pfeiffer P, Tarpgaard LS, Holländer NH, Keldsen N, Hansen TF, Jensen BB, Jensen BV. Primary tumor location and bevacizumab effectiveness in patients with metastatic colorectal cancer. Ann Oncol. 2013;24(10):2554–9.
Buchler T, Pavlik T, Melichar B, Bortlicek Z, Usiakova Z, Dusek L, Kiss I, Kohoutek M, Benesova V, Vyzula R, Abrahamova J, Obermannova R. Bevacizumab with 5-fluorouracil, leucovorin, and oxaliplatin versus bevacizumab with capecitabine and oxaliplatin for metastatic colorectal carcinoma: results of a large registry-based cohort analysis. BMC Cancer. 2014;7(14):323.
Budai B, Komlósi V, Adleff V, Pap É, Réti A, Nagy T, Kralovánszky J, Láng I, Hitre E. Impact of SHMT1 polymorphism on the clinical outcome of patients with metastatic colorectal cancer treated with first-line FOLFIRI + bevacizumab. Pharmacogenet Genomics. 2012;22(1):69–72.
Cartwright TH, Yim YM, Yu E, Chung H, Halm M, Forsyth M. Survival outcomes of bevacizumab beyond progression in metastatic colorectal cancer patients treated in US community oncology. Clin Colorectal Cancer. 2012;11(4):238–46.
Cetin B, Kaplan MA, Berk V, Ozturk SC, Benekli M, Isıkdogan A, Ozkan M, Coskun U, Buyukberber S. Prognostic factors for overall survival in patients with metastatic colorectal carcinoma treated with vascular endothelial growth factor-targeting agents. Asian Pac J Cancer Prev. 2012;13(3):1059–63.
Crea F, Fornaro L, Paolicchi E, Masi G, Frumento P, Loupakis F, Salvatore L, Cremolini C, Schirripa M, Graziano F, Ronzoni M, Ricci V, Farrar WL, Falcone A, Danesi R. An EZH2 polymorphism is associated with clinical outcome in metastatic colorectal cancer patients. Ann Oncol. 2012;23(5):1207–13.
Díaz-Rubio E, Gómez-España A, Massutí B, Sastre J, Reboredo M, Manzano JL, Rivera F, Safont MJ, Montagut C, González E, Benavides M, Marcuello E, Cervantes A, Martínez de Prado P, C Fernández-Martos, Arrivi A, Bando I, Aranda E. Spanish Cooperative Group for the treatment of digestive Tumors (TTD). Role of Kras status in patients with metastatic colorectal cancer receiving first-line chemotherapy plus bevacizumab: a TTD group cooperative study. PLoS One. 2012;7(10):e47345.
Formica V, Luccchetti J, Cunningham D, Smyth EC, Ferroni P, Nardecchia A, Tesauro M, Cereda V, Guadagni F, Roselli M. Systemic inflammation, as measured by the neutrophil/lymphocyte ratio, may have differential prognostic impact before and during treatment with fluorouracil, irinotecan and bevacizumab in metastatic colorectal cancer patients. Med Oncol. 2014;31(9):166.
Gerger A, El-Khoueiry A, Zhang W, Yang D, Singh H, Bohanes P, Ning Y, Winder T, Labonte MJ, Wilson PM, Benhaim L, Paez D, El-Khoueiry R, Absenger G, Lenz HJ. Pharmacogenetic angiogenesis profiling for first-line Bevacizumab plus oxaliplatin-based chemotherapy in patients with metastatic colorectal cancer. Clin Cancer Res. 2011;17(17):5783–92.
Grothey A, Sugrue MM, Purdie DM, Dong W, Sargent D, Hedrick E, Kozloff M. Bevacizumab beyond first progression is associated with prolonged overall survival in metastatic colorectal cancer: results from a large observational cohort study (BRiTE). J Clin Oncol. 2008;26(33):5326–34.
Guiu B, Petit JM, Bonnetain F, Ladoire S, Guiu S, Cercueil JP, Krausé D, Hillon P, Borg C, Chauffert B, Ghiringhelli F. Visceral fat area is an independent predictive biomarker of outcome after first-line bevacizumab-based treatment in metastatic colorectal cancer. Gut. 2010;59(3):341–7.
Kim S, Dobi E, Jary M, Monnien F, Curtit E, Nguyen T, Lakkis Z, Heyd B, Fratte S, Cléau D, Lamfichekh N, Nerich V, Guiu B, Demarchi M, Borg C. Bifractionated CPT-11 with LV5FU2 infusion (FOLFIRI-3) in combination with bevacizumab: clinical outcomes in first-line metastatic colorectal cancers according to plasma angiopoietin-2 levels. BMC Cancer. 2013;27(13):611.
Koutras AK, Antonacopoulou AG, Eleftheraki AG, Dimitrakopoulos FI, Koumarianou A, Varthalitis I, Fostira F, Sgouros J, Briasoulis E, Bournakis E, Bafaloukos D, Bompolaki I, Galani E, Kalogeras KT, Pectasides D, Fountzilas G, Kalofonos HP. Vascular endothelial growth factor polymorphisms and clinical outcome in colorectal cancer patients treated with irinotecan-based chemotherapy and bevacizumab. Pharmacogenomics J. 2012;12(6):468–75.
Lastoria S, Piccirillo MC, Caracò C, Nasti G, Aloj L, Arrichiello C, Lutio di Castelguidone E, Tatangelo F, Ottaiano A, Iaffaioli RV, Izzo F, Romano G, Giordano P, Signoriello S, Gallo C, Perrone F. Early PET/CT scan is more effective than RECIST in predicting outcome of patients with liver metastases from colorectal cancer treated with preoperative chemotherapy plus bevacizumab. J Nucl Med. 2013;54(12):2062–9.
Loupakis F, Cremolini C, Yang D, Salvatore L, Zhang W, Wakatsuki T, Bohanes P, Schirripa M, Benhaim L, Lonardi S, Antoniotti C, Aprile G, Graziano F, Ruzzo A, Lucchesi S, Ronzoni M, De Vita F, Tonini G, Falcone A, Lenz HJ. Prospective validation of candidate SNPs of VEGF/VEGFR pathway in metastatic colorectal cancer patients treated with first-line FOLFIRI plus bevacizumab. PLoS One. 2013;8(7):e66774.
Loupakis F, Ruzzo A, Salvatore L, Cremolini C, Masi G, Frumento P, Schirripa M, Catalano V, Galluccio N, Canestrari E, Vincenzi B, Santini D, Bencardino K, Ricci V, Manzoni M, Danova M, Tonini G, Magnani M, Falcone A, Graziano F. Retrospective exploratory analysis of VEGF polymorphisms in the prediction of benefit from first-line FOLFIRI plus bevacizumab in metastatic colorectal cancer. BMC Cancer. 2011;14(11):247.
Maillet M, Dréanic J, Dhooge M, Mir O, Brezault C, Goldwasser F, Chaussade S, Coriat R. The predictive and prognostic value of the Glasgow Prognostic Score in metastatic colorectal carcinoma patients receiving bevacizumab. Anti-cancer Drugs. 2014;25(10):1215–9.
Malka D, Boige V, Jacques N, Vimond N, Adenis A, Boucher E, Pierga JY, Conroy T, Chauffert B, François E, Guichard P, Galais MP, Cvitkovic F, Ducreux M, Farace F. Clinical value of circulating endothelial cell levels in metastatic colorectal cancer patients treated with first-line chemotherapy and bevacizumab. Ann Oncol. 2012;23(4):919–27.
Pectasides D, Papaxoinis G, Kalogeras KT, Eleftheraki AG, Xanthakis I, Makatsoris T, Samantas E, Varthalitis I, Papakostas P, Nikitas N, Papandreou CN, Pentheroudakis G, Timotheadou E, Koutras A, Sgouros J, Bafaloukos D, Klouvas G, Economopoulos T, Syrigos KN, Fountzilas G. XELIRI-bevacizumab versus FOLFIRI-bevacizumab as first-line treatment in patients with metastatic colorectal cancer: a Hellenic Cooperative Oncology Group phase III trial with collateral biomarker analysis. BMC Cancer. 2012;12:271.
Ryanne WuR, Lindenberg PA, Slack R, Noone AM, Marshall JL, He AR. Evaluation of hypertension as a marker of bevacizumab efficacy. J Gastrointest Cancer. 2009;40(3–4):101–8.
Sastre J, Vidaurreta M, Gómez A, Rivera F, Massutí B, López MR, Abad A, Gallen M, Benavides M, Aranda E, Rubio ED. Spanish cooperative group for the treatment of digestive tumors. prognostic value of the combination of circulating tumor cells plus KRAS in patients with metastatic colorectal cancer treated with chemotherapy plus bevacizumab. Clin Colorectal Cancer. 2013;12(4):280–6.
Slavicek L, Pavlik T, Tomasek J, Bortlicek Z, Buchler T, Melichar B, Vyzula R, Prausova J, Finek J, Majek O, Dusek L. Efficacy and safety of bevacizumab in elderly patients with metastatic colorectal cancer: results from the Czech population-based registry. BMC Gastroenterol. 2014;25(14):53.
Vauthey JN, Zimmitti G, Kopetz SE, Shindoh J, Chen SS, Andreou A, Curley SA, Aloia TA, Maru DM. RAS mutation status predicts survival and patterns of recurrence in patients undergoing hepatectomy for colorectal liver metastases. Ann Surg. 2013;258(4):619–626; discussion 626–627.
Vincenzi B, Zoccoli A, Schiavon G, Iuliani M, Pantano F, Dell’aquila E, Ratta R, Muda AO, Perrone G, Brunelli C, Correale P, Riva E, Russo A, Loupakis F, Falcone A, Santini D, Tonini G. Dicer and Drosha expression and response to bevacizumab-based therapy in advanced colorectal cancer patients. Eur J Cancer. 2013;49(6):1501–8.
Moscetti L, Nelli F, Fabbri MA, Sperduti I, Alesini D, Cortesi E, Gemma D, Gamucci T, Grande R, Pavese I, Franco D, Ruggeri EM. Maintenance single-agent bevacizumab or observation after first-line chemotherapy in patients with metastatic colorectal cancer: a multicenter retrospective study. Invest New Drugs. 2013;31(4):1035–43.
Matsusaka S, Suenaga M, Mishima Y, Takagi K, Terui Y, Mizunuma N, Hatake K. Circulating endothelial cells predict for response to bevacizumab-based chemotherapy in metastatic colorectal cancer. Cancer Chemother Pharmacol. 2011;68(3):763–8.
Petrelli F, Coinu A, Cabiddu M, Ghilardi M, Barni S. KRAS as prognostic biomarker in metastatic colorectal cancer patients treated with bevacizumab: a pooled analysis of 12 published trials. Med Oncol. 2013;30(3):650.
Silvestris N, Scartozzi M, Graziano G, et al. Basal and bevacizumab-based therapy-induced changes of lactate dehydrogenases and fibrinogen levels and clinical outcome of previously untreated metastatic colorectal cancer patients: a multicentric retrospective analysis. Expert Opin Biol Ther. 2014;19:1–8.
Azuma M, Shi M, Danenberg KD, et al. Serum lactate dehydrogenase levels and glycolysis significantly correlate with tumor VEGFA and VEGFR expression in metastatic CRC patients. Pharmacogenomics. 2007;8:1705–13.
Petrelli F, Barni S. Correlation of progression-free and post-progression survival with overall survival in advanced colorectal cancer. Ann Oncol. 2013;24(1):186–92.
Li MX, Liu XM, Zhang XF, Zhang JF, Wang WL, Zhu Y, Dong J, Cheng JW, Liu ZW, Ma L, Lv Y. Prognostic role of neutrophil-to-lymphocyte ratio in colorectal cancer: a systematic review and meta-analysis. Int J Cancer. 2014;134(10):2403–13.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Petrelli, F., Coinu, A., Cabiddu, M. et al. Prognostic factors for survival with bevacizumab-based therapy in colorectal cancer patients: a systematic review and pooled analysis of 11,585 patients. Med Oncol 32, 15 (2015). https://doi.org/10.1007/s12032-014-0456-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-014-0456-z