Abstract
This study evaluated the efficacy and safety of a new regimen consisting of Topotecan, Ifosfamide, Etoposide, and l-asparaginase (TIEL) in treating aggressive T-cell lymphoma. Twenty-four patients were included in the research, eighteen males and six females. Half of the patients were in stages III and IV, and nearly half of them experienced failure of at least one regimen. Eleven were diagnosed as peripheral T-cell lymphoma (PTCL), five extranodal NK/T-cell lymphoma, non-specific, four angioimmunoblastic, and four anaplastic large-cell lymphoma (2 ALK positive). Patients were given 98 cycles of TIEL altogether. The responsive rate to TIEL was 76.9 % among 13 cases who received the regimen as the first-line treatment. Among 11 cases, TIEL was the second- or more-line treatment, the responsive rate was 63.6 %. The median PFS was 32.0 ± 21.0 (95 % CI 0–73.29) months. Median overall survival (OS) was not reached yet. Approximately 41.3 % of patients showed the third- to fourth-degree hematological side effects. Non-hematological toxicity included nausea, vomiting, diarrhea, and abnormal liver function. Among those patients received l-asparaginase, nine experienced mild abnormal coagulation function after 7 days of initiating chemotherapy, and no pancreatic injury was found. TIEL regimen is effective for aggressive T-cell lymphoma with controllable side effect and can be used for more patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
T-cell lymphomas are a group of heterogeneous malignancies originated from T cell or NK cell, accounting for about 15 % of all non-Hodgkin lymphomas (NHLs) [1]. There are 20 subtypes of mature T-cell and NK-cell neoplasms based on WHO classification. According to the report of “Distribution of Lymphoid Neoplasms in China” by Jian Sun [2], of the 4,638 patients diagnosed with lymphoid neoplasms from 2004 to 2008 in hospitals of Beijing, Shanghai, and Sichuan, mature T-cell and NK-cell lymphoma accounted for 25.5 % of all NHLs, and extranodal NK/T-cell lymphoma, nasal-type (12 %), peripheral T-cell lymphoma (PTCL), NOS (4.3 %), anaplastic large-cell lymphoma (ALCL) (2.6 %), and angioimmunoblastic T-cell lymphoma (ATCL) (1.6 %) are the four most common types.
T-cell lymphomas are aggressive with poor prognosis. Unfortunately, the optimal treatment for T-cell lymphoma has not been determined, although retrospective data from the last decade showed patients benefited from regimens for diffuse large B-cell lymphoma, like CHOP or ESHAP. However, compared to aggressive B-cell lymphoma, more patients of T-cell lymphoma will be resistant to initial therapy and relapse early and rapidly. For PTCL, 5-year overall survival is only 10–30 %. In recent years, some research demonstrated that l-asparaginase might be an effective reagent for extranodal NK/T-cell lymphoma, nasal type [3–7], and it has been suggested as the first-line treatment for this special subtype of T-cell lymphoma in National Comprehensive Cancer Network (NCCN) guideline. But for other subtypes of T-cell lymphoma, clinical trial is still the preferred suggestion.
Topotecan is a semisynthetic camptothecin derivative that inhibits the intracellular enzyme topoisomerase I and induces damage to the cells during DNA replication. It has demonstrated antitumor effects on various malignant diseases in preclinical and clinical studies. In some phase II clinical trial, Topotecan showed promising effect in refractory lymphoma [8–10].
An l-asparaginase- and Topotecan-containing regimen is particularly attractive for the treatment for aggressive T-cell lymphoma. In this study, we combined Topotecan, Ifosfamide, Etoposide, and l-asparaginase (TIEL regimen) as a new approach to treat patients of T-cell lymphoma and achieved improved outcomes. Here, we summarize the treatment’s effect and toxicity.
Patients and methods
Patients’ characteristics
This was a prospective study. Patients included in the study were admitted to PUMCH oncology department between January 2005 and December 2011. Patients were diagnosed with T-cell lymphoma (excluding cutaneous T-cell lymphoma) based on the 2008 WHO classification of lymphoma. All patients were diagnosed from biopsy, and their ECOG score was between 0 and 3 without contraindication for chemotherapy. Patients meeting the conditions were included in the study consecutively except for those who refused. Patients were enrolled after signing an informed consent form that was approved by the Ethics Committee of Peking Union Medical College Hospital. Patient’s consent was obtained.
Clinical staging was decided with Ann Arbor Staging system. The response to the treatment was evaluated according to the 1999 IWG-NHL Response Criteria. Side effects were evaluated according to NCI-CTC 3.0.
The chemotherapy regimen was as the following: Topotecan hydrochloride (Hanfang Pharmaceutical) or hycamtin (GlaxoSmithKline), IV, 1.5 mg/m2, d1–3; Ifosfamide, IV, 1.3 g/m2, d1–3. The total dosage of Mesna was equivalent to 20 % of Ifosfamide dosage, given three times in infusion at 0, 4, and 8 h after initiating Ifosfamide; Etoposide, IV, 65 mg/m2, d4–6; l-asparaginase (Kyowa Hakko Kirin Co., Ltd), IV 10,000 u/d, d15–24.
Patient follow-up
All patients were followed up until August 1, 2012. Time to progression (TTP) was measured from the starting date of chemotherapy to time of cancer progression. Survival was measured from the starting date of chemotherapy to either death due to any cause or lost to follow-up.
Statistical analysis
Statistical analysis was done using SPSS20.0. Kaplan–Meier estimator was used to estimate the survival function. Overall survival rate was calculated with life table.
Result
Patients’ characteristics
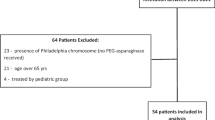
In total, 24 patients were included in the study. The mean age was 44.4 years with a range from 15 to 77 years (Table 1). Nine patients were in stages III and IV, and 45.8 % of all patients experienced failure of at least one regimen. For pathology, 11 were diagnosed as PTCL, five extranodal NK/T-cell lymphoma, four ATCL, and four ALCL (two ALK positive). Two patients had bone metastasis, two patients had liver metastasis, and one patient had ocular metastasis. None of the patients had intracranial metastasis.
Regimen
Twenty-four cases accepted 98 cycles of chemotherapy altogether (Table 2). Only one 75-year-old patient with ECOG 3 heavily treated before was given half dose, others were all given 80–100 % dose density. There were 8 patients treated without l-asparaginase, because of skin test (+). Eight patients received radiotherapy following chemotherapy, and one patient received autologous stem cell transplantation. Two patients with NK/T-cell lymphoma were given MTX 10 mg/Ara-c50 mg intrathecal injection for brain metastasis prevention.
Response evaluation
Short-term analysis: of all the 24 patients, 13 received TIEL as the first-line treatment, and all were evaluable. CR/Cru 61.5 % (8/13), PR 15.4 % (2/13), SD 7.7 % (1/13), RR 76.9 % (10/13). For CR/PR/SD patients, 3 PD later, and TTP is 2.5, 5, and 11 months, respectively. The other 8 patients are all disease-free survival (DFS). Eleven patients were second- or more-line treatment (9 failures from pre-chemotherapy and 2 recurrences),and all were evaluable, CR 3, PR4 (2 nearly CR), RR 63.6 % (7/11). TTP was 4–27 months. For patients treated without l-asparaginase, four were the first-line chemotherapy, with CR/PR/SD/PD 2/1/1/0 and four were the second- or more-line treatment, 1 CR; 2SD; 1PD.
Long-term analysis: Follow-up from 3 to 65 months (mean 24.27 m), only one was lost during follow-up, ten died from T-cell lymphoma, 9 survived without lymphoma, and four were still on maintenance. The median PFS was 32.0 ± 21.0 (95 % CI 0–73.29) months, median OS is not reached (Fig. 1). 1-, 2-, 3-, and 5-year overall survivals are 73, 58, 50, 50 %, respectively.
Safety evaluation
Third- to fourth-degree hematological toxicity happened in 41.3 % of patients. Non-hematological toxicities include nausea and vomiting, diarrhea, and liver dysfunction. Third- to fourth degree of these toxicities was about 8 %. For patients received the l-asparaginase, nine showed mild abnormal in coagulation function but without bleeding; none of them developed pancreatic dysfunction.
Discussion
T-cell lymphoma accounts for 15 % of non-Hodgkin lymphoma. It is aggressive and resistant to traditional chemotherapy. Currently, there is still no uniformed treatment regimen for this disease, and the related clinical research data are very limited. NCCN guideline highly recommends clinical research to improve treatment in exploring optimizing regimen. CHOP is the most commonly used treatment in the past studies. Literature reported that CHOP regimen as the first-line treatment in T-cell lymphoma has a response rate of 50–70 % [11], PFS 12–14 months, and 5-year DFS as 30 % [12]. In one study of 135 patients, HyperCVAD chemoregimen was compared with CHOP, without significant advantages, but with more severe side effects [13]. In 2012, Iriyama et al. [14] reported dose-intensified regimen followed by autologous stem cell transplantation (ASCT) or high-dose methotrexate (HDMTX) treating peripheral T-cell lymphoma, achieving CR 68 %, and 3- or 5-year overall survival rates 68 and 63 %, but not for relapsed or refractory patients.
Topotecan as topoisomeraseI showed its effect on the inhibition of DNA repair in several human cell lines and xenografts [15, 16]. In the recent years, several literatures reported that Topotecan alone [8, 9] or in combination [10, 17, 18] showed some efficacy in treating relapsed lymphoma, even in refractory primary central nervous system lymphoma [19]. Kraut et al. [8] reported Topotecan alone treated 32 cases of relapsed lymphoma with 15.7 % response rate (Topotecan is administered as 1.25 mg/m2, d1-d5 given every 3 weeks). The medium remission is 2 months, and 90 % of patients showed third- to fourth-degree hematotoxicity. In a phase II study [16], twenty-six patients with relapsed or refractory aggressive non-Hodgkin’s lymphomas received doxorubicin 25 mg/m2 intravenous (IV) on day 1 and Topotecan 1.75 mg/m2/day IV on days 3–5, every 21 days. 20 % response rate (CR 8 % and PR 12 %) was achieved, which was similar to the result of previous research.
l-asparaginase (l-ASP) has promising efficacy in treating aggressive lymphoma and acute leukemia as reported in recent researches. l-ASP is an enzymatic antitumor drug from E. coli. It is hypothesized that T cells are less sensitive to chemotherapy due to overexpression of P-glycoprotein. The hydrolysis of asparagine by l-ASP is not affected by P-glycoprotein, making l-ASP an effective agent [20]. Peking University Cancer Hospital [5] treated 32 NK/T-cell lymphoma (n = 22) and lymphoblastic lymphoma (n = 10) with pegylated l-asparaginase-based chemotherapy. The overall response rate was 71.9 % (CR 40.6 %, PR 31.3 %). Myelosuppression was the most common adverse event at an incidence of 81.2 %. Data from the Asia Lymphoma Study Group [3] and China [4, 5, 7] also showed the similar results. There were also reports of new drug-based regimen, such as Denileukin Diftitox and Bendamustine, with RR to be 50–60 %, PFS to be 12 months or less [21, 22].
We designed a new regimen, Topotecan plus Etoposide, Ifosfamide, and l-asparaginase (TIEL regimen), to treat aggressive T-cell lymphoma. Among the 24 patients treated, PTCL accounted for 45 %, extranodal NK/T-cell lymphoma, angioimmunoblastic T-cell lymphoma, and ALK-negative ALCL accounted for 46 %. TIEL was used as either first- or second-line treatment, and the response rate was 76.9 and 63.6 %, respectively. The outcomes were remarkable, although l-asparaginase was not used by all the patients, which suggested that Topotecan plus Etoposide and Ifosfamide might be an effective regimen itself. The 24 patients in our study had median PFS of 32 months. The median overall survival, which needed longer follow-up, was not obtained. All these results were better than those reported before.
With regard to side effects, the toxicities occurred in this trial were similar to others, mainly of hematological toxicities. Patients did recover after chemotherapy was stopped and no chemo-related death. Allergic reaction (skin test positive) was common in this trial, which resulted in less l-asparaginase treatment and might be compromised the effect of the TIEL regimen.
In the last decade, little improvements have been made in treatment of aggressive T-cell lymphoma. Although l-asparaginase has been successful in the treatment for extranodal NK/T-cell lymphoma, there are little data of this drug in the treatment for other aggressive subtypes of T-cell lymphoma. We used TIEL regimen and got the satisfactory results with controllable side effects. So, the combined chemotherapy of Topotecan with Ifosfamide, Etoposide, and l-asparaginase (TIEL regimen) in treating aggressive T-cell lymphoma has a relatively high response rate and is quite tolerable. The efficacy of TIEL regimen should be further evaluated through larger-scale clinical trial in the future.
References
The Non-Hodgkin’s Lymphoma Classification Project. A clinical evaluation of the international lymphoma study group classification of non-Hodgkin’s lymphoma. Blood. 1997;89(11):3909–18.
Sun J, Yang Q, Lu Z, et al. Distribution of lymphoid neoplasms in China: analysis of 4,638 cases according to the World Health Organization classification. Am J Clin Pathol. 2012;138(3):429–34.
Kwong YL, Kim WS, Lim ST, et al. SMILE for natural killer/T-cell lymphoma: analysis of safety and efficacy from the Asia Lymphoma Study Group. Blood. 2012;120(15):2973–80.
Yang L, Liu H, Xu XH, et al. Retrospective study of modified SMILE chemotherapy for advanced-stage, relapsed, or refractory extranodal natural killer (NK)/T cell lymphoma, nasal type. Med Oncol. 2013;30(4):720–6.
Ping LY, Zheng W, Wang XP. Safety and adverse event profiling of pegylated L-asparaginase combined chemotherapy in the treatment of lymphoma. Zhong Hua Yi Xue Za Zhi. 2012;92(46):3257–60.
Ramya LN, Doble M, Rekha VP. L-asparaginase as potent anti-leukemic agent and its significance of having reduced glutaminase side activity for better treatment of acute lymphoblastic leukaemia. Appl Biochem Biotechnol. 2012;167(8):2144–59.
Yong W, Zheng W, Zhang Y, et al. L-asparaginase-based regimen in the treatment of refractory midline nasal/nasal-type T/NK-cell lymphoma. Int J Hematol. 2003;78(2):163–7.
Kraut EH, Balcerzak SP, Young D, et al. A phase II study of topotecan in non-Hodgkin’s lymphoma: an Ohio State University phase II research consortium study. Cancer Invest. 2002;20(2):174–9.
Wiernik PH, Li H, Weller E, et al. Activity of topotecan 21-day infusion in patients with previously treated large cell lymphoma: long-term follow-up of an Eastern Cooperative Oncology Group study (E5493). Leuk Lymphoma. 2012;53(6):1137–42.
Crump M, Couban S, Meyer R, et al. Phase II study of sequential topotecan and etoposide in patients with intermediate grade non-Hodgkin’s lymphoma: a National Cancer Institute of Canada Clinical Trials Group study. Leuk Lymphoma. 2002;43(8):1581–7.
Schmitz N, Trümper L, Ziepert M, et al. Treatment and prognosis of mature T-cell and NK-cell lymphoma: an analysis of patients with T-cell lymphoma treated in studies of the German High-Grade Non-Hodgkin Lymphoma Study Group. Blood. 2010;116(18):3418–25.
Vose J, Armitage J, Weisenburger D, et al. International peripheral T-cell and natural killer/T-cell lymphoma study: pathology findings and clinical outcomes. J Clin Oncol. 2008;26(25):4124–30.
Escalon MP, Liu NS, Yang Y, et al. Prognostic factors and treatment of patients with T-cell non-Hodgkin lymphoma: the M.D. Anderson Cancer Center experience. Cancer. 2005;103(10):2091–8.
Iriyama N, Takahashi H, Hatta Y, et al. Efficacy of a dose-intensified CHOP (Double-CHOP) regimen for peripheral T-cell lymphomas. Oncol Rep. 2012;29(2):805–11.
Cheng MF, Chatterjee S, Berger NA. Schedule-dependent cytotoxicity of topotecan alone and in combination chemotherapy regimens. Oncol Res. 1994;6:269–79.
Kaufmann SH, Peereboom D, Buckwalter CA, et al. Cytotoxic effects of topotecan combined with various anticancer agents in human cancer cell lines. J Natl Cancer Inst. 1996;88(11):734–41.
Smith SM, Johnson JL, Niedzwiecki D, et al. Sequential doxorubicin and topotecan in relapsed/refractory aggressive non-Hodgkin’s lymphoma: results of CALGB 59906. Leuk Lymphoma. 2006;47(8):1511–7.
Perkins JB, Goldstein SC, Dawson JL, et al. Phase I Study of Topotecan, Ifosfamide, and Etoposide (TIME) with autologous stem cell transplant in refractory cancer: pharmacokinetic and pharmacodynamics correlates. Clin Cancer Res. 2011;17(24):7743–53.
Voloschin AD, Betensky R, Wen PY, et al. Topotecan as salvage therapy for relapsed or refractory primary central nervous system lymphoma. J Neuro Oncol. 2008;86(2):211–5.
Verma N, Kumar K, Kaur G, et al. L-asparaginase: a promising chemotherapeutic agent. Crit Rev Biotechnol. 2007;27(1):45–62.
Foss FM, Sjak-Shie N, Goy A, et al. A multicenter phase II trial to determine the safety and efficacy of combination therapy with denileukin diftitox and cyclophosphamide, doxorubicin, vincristine and prednisone in untreated peripheral T-cell lymphoma: the CONCEPT study. Leuk Lymphoma. 2013;54(7):1373–9.
Damaj G, Gressin R, Bouabdallah K, et al. Results from a prospective, open-label, phase II trial of bendamustine in refractory or relapsed T-cell lymphomas: the BENTLY trial. J Clin Oncol. 2013;31(1):104–10.
Acknowledgments
We thank Miss Sherry Bai and HuiJun Han M.D for part of the translating work, Prof. ChunMei Bai is very helpful in making it carried out smoothly. We thank all the doctors and nurses in our department for doing the trial responsibly and carefully.
Conflict of interest
The authors confirm that there is no conflict of interest and no fund.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shao, Y., Guan, M., Chen, S. et al. Topotecan combined with Ifosfamide, Etoposide, and l-asparaginase (TIEL) regimen improves outcomes in aggressive T-cell lymphoma. Med Oncol 32, 402 (2015). https://doi.org/10.1007/s12032-014-0402-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-014-0402-0