Abstract
Treatment for patients with refractory or relapsed primary CNS lymphoma (PCNSL) remains unsatisfactory. Topotecan is an intravenous topoisomerase I inhibitor with good CSF penetration and documented efficacy in patients with relapsed systemic non-Hodgkin’s lymphoma. In this study 15 patients with refractory or relapsed PCNSL were treated with intravenous topotecan (1.5 mg/m2) for five consecutive days during each 21-day cycle. All 15 patients had measurable, contrast-enhancing tumor on cranial MRI at the time of relapse. Three (20%) patients achieved a complete response after one, three and four cycles, respectively, while three (20%) patients achieved a partial response after two cycles each, for a total response proportion of 40%. Three patients had stable disease at the end of topotecan treatment. Six patients (40%) had progressive disease during treatment. Median overall survival was 981 days (95% CI: 275, NA) and median progression free survival was 60 days (95% CI: 46, 945). Three out of 15 patients had grade 3 thrombocytopenia. Six out of 15 patients had grade 3 neutropenia, while 5/15 patients had grade 4 neutropenia, and 13/15 patients received g-CSF at some point during treatment. There were no deaths directly related to treatment toxicity. Our study shows that topotecan, as a salvage therapy in patients with relapsed or refractory PCNSL, is associated with an overall response proportion of 40% and should be considered in patients who have failed prior methotrexate-based chemotherapy and/or whole brain irradiation. However, progression is frequent and early and most patients required growth factor support due to myelotoxicity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Primary central nervous system lymphoma (PCNSL), a rare subtype of non-Hodgkin’s lymphoma, has increased in incidence over the last three decades and accounted for 3.1% of all primary brain tumors in the United States from 1998 to 2002 [1]. High-dose methotrexate (HD-MTX)-based chemotherapy for newly diagnosed immunocompetent patients with PCNSL has been shown to improve survival when compared with whole brain irradiation (WBI) alone [2]. Moreover, the risk of neurotoxicity is lower for chemotherapy alone approaches to PCNSL. However, nearly all patients with PCNSL relapse at some point and salvage therapy for patients with refractory or relapsed PCNSL remains unsatisfactory. Topotecan, a semi-synthetic derivative of camptothecin and a specific inhibitor of topoisomerase I, crosses the blood brain barrier and has activity against systemic NHL. We report herein the final results of a phase 2 study of 15 patients with refractory or relapsed PCNSL treated with intravenous topotecan at two institutions.
Patients and methods
This prospective phase 2 study consisted of 15 immunocompetent patients with relapsed or refractory PCNSL treated at two institutions. The primary end point was radiographic complete response of measurable intracranial lesions using standardized radiographic criteria. Secondary endpoints were progression-free survival and overall survival. All patients were recorded in an electronic institutional database and information on age, gender, tumor location, sites of involvement, histopathology, treatment, and survival was collected. Supplemental information was also obtained from patient medical records. All patients who enrolled in this Institutional Review Board-approved study signed informed consent prior to any procedures related to the study or any treatment. The dates of enrolment were between August 1998 and March 2002
Inclusion criteria included age ≥18 years; prior history of PCNSL documented by histology (brain or vitreal biopsy; CSF cytopathology), and radiographic evidence of recurrence or progression (new contrast enhancing area or >25% increase in pre-existing contrast-enhancing tumor) after first-line therapy; Karnofsky Performance Score ≥50; adequate bone marrow function (defined as absolute neutrophil count ≥1,500 cells/mm3; platelet count ≥100,000 cells/mm3); adequate renal function (serum creatinine <2 or a 24 h urine collection obtained prior to initiation of therapy with a calculated creatinine clearance ≥50 ml/min) and negative serology for the human immunodeficiency virus (HIV). Concomitant CSF recurrence by positive cytopathology or vitreal recurrence by ophthalmic examination was allowed as long as there was measurable, contrast enhancing intracranial tumor. Exclusion criteria included patients with evidence of lymphoma in any organ other than the eye, brain or leptomeninges; pregnant or breast-feeding women; patients with impending deterioration due to increased intracranial pressure; patients with serious active infections; concurrent treatment with WBI or other intravenous chemotherapies; patients without MRI or CT evidence of measurable (>1 cm in at least one dimension), post-operative, contrast-enhancing residual disease and or inability to provide informed consent.
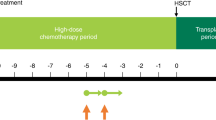
Topotecan was administered as an intravenous infusion over 30 min on days 1–5 of each 21-day cycle. Pre-chemotherapy anti-emetic therapy consisted of ondansetron. Routine, prophylactic granulocyte-colony stimulating factor (g-CSF) was not planned as part of this treatment protocol. Treatment with topotecan continued until objective evidence of disease progression (brain, leptomeningeal, ocular, epidural or systemic progression); occurrence of treatment-related, dose-limiting toxicity; patient request to discontinue treatment for any reason or a maximum of 10 cycles of therapy. Radiographic response was determined using standard criteria [3]. Overall survival (OS) was measured from the date of the first topotecan treatment to the date of last follow-up evaluation or death, and progression-free survival (PFS) was measured from the date of the first topotecan treatment to the date of progression documented in brain, CSF, eye or in another organ.
This phase II study was designed to accrue 15 refractory or relapsed PCNSL patients with assessment at the end of accrual. The chance that the therapy would be declared inactive (i.e., five or fewer responses) was 94% if the true response rate was at most 20%, but only 15% if the true response rate was at least 50%. If six or more patients responded (C.R.), then the therapy would be declared active and considered worth of further study (type I error of 0.06 and power of 85%). The overall event rates (hazard rates) were estimated with 95% confidence intervals.
Results
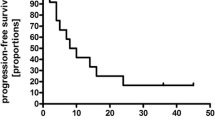
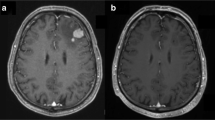
Fifteen patients (nine males and six females) were enrolled into this study. The median age of the study participants was 56 years (range 34–74). Prior treatment of the 15 patients enrolled in this study consisted of methotrexate-based chemotherapy. Eleven patients had received methotrexate, 8 g/m2, alone. Two patients had received methotrexate and Ara-C. One patient had received methotrexate, cyclosphosphamide, and vincristine. One patient had received methotrexate and WBI. At the time of enrollment into the topotecan study ocular involvement was present in two out of 15 patients (13.33%), while positive CSF cytology was documented in one out of eight patients tested (12.5%). All patients had measurable, contrast-enhancing lesions in the brain parenchyma. Multiple lesions on MRI occurred in 4/15 (26.66%) subjects. Treatment results are summarized in Table 1. Six out of 15 patients achieved radiographic responses. Three patients (20%) achieved a complete response (CR) after one, three and four cycles, respectively, while three additional patients (20%) achieved a partial response (PR) after two cycles each, for a total response proportion of 40% (6/15). Three patients achieved stable disease (SD) but did not complete the planned 10 cycles due to grade 3 or 4 toxicity. Six patients (40%) had progressive disease during treatment with topotecan. Median overall survival was 981 days (95% CI: 275, NA) and median progression-free survival was 60 days (95% CI: 46, 945).
Three out of 15 (20%) patients experienced grade 3 thrombocytopenia; 6/15 (40%) had grade 3 neutropenia; and 5/15 (33.33%) patients had grade 4 neutropenia. Four out of 15 patients discontinued treatment after one dose due to toxicity. Out of the remaining 11 patients, four had dose reductions, one received one dose of the second cycle but discontinued due to neutropenic fever, and five patients had dose delays. The remaining five patients did not have dose reductions or delays. None of the 15 patients treated experienced grade 4 thrombocytopenia, and there were no deaths directly related to treatment toxicity. Thirteen out of 15 patients received g-CSF at some point during treatment.
Patterns of failure to topotecan included six cases of local brain progression without ever achieving a response or stable disease to topotecan; three cases of local brain progression after achieving SD to topotecan; three cases of local brain progression after PR to topotecan; one case of systemic relapse only after CR to topotecan; one case of systemic and CSF relapse after CR to topotecan and one case of local brain relapse after CR to topotecan.
Salvage therapy after topotecan included WBI alone in six patients; WBI and temozolomide in two patients; WBI, temozolomide, rituximab and radiosurgery in one patient; WBI, rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone in one patient; radiosurgery alone in two patients; rituximab and temozolomide in one patient; Ara-C in one patient. No salvage therapy information was available for one patient. Detailed response data for salvage therapy subsequent to topotecan was not available.
Discussion
Primary central nervous system lymphoma, an extra-nodal form of non-Hodgkin’s lymphoma, is a rare brain neoplasm although the incidence of this cancer has increased in immunocompetent persons over the past few decades. Whole brain irradiation as a single treatment modality results in survival of only 11.6 months [4]. The addition of methotrexate-based chemotherapy to WBI more than triples survival compared to radiation alone, but radiation-containing regimens may result in significant neurotoxicity, especially in patients over the age of 60 [5]. Nearly all patients with PCNSL require salvage therapy for disease refractory to methotrexate-based chemotherapy and radiation or at the time of relapse after successful initial treatment. Treatment options in such patients include WBI; reinduction with methotrexate in patients who previously responded to methotrexate; alternative chemotherapy agents including temozolomide, rituximab, the procarbazine, CCNU, vincristine (PCV) regimen; or high dose chemotherapy followed by stem cell rescue (Table 2).
Whole brain irradiation as a salvage therapy after chemotherapy failure is associated with a radiographic response proportion of 74%, and a median survival of 10.9 months [6]. In a multicenter study [7] of 22 PCNSL patients who relapsed after initially achieving a CR to methotrexate, re-induction with methotrexate at the same dose and schedule resulted in an overall response rate of 91% (20 of 22 patients); a median progression-free survival of 61.9 months and an overall survival of 91.9 months in this selected group of patients. However, HD-MTX should generally be avoided in patients who have received prior WBI, and in patients with significant renal insufficiency due to the risks of neurotoxicity and nephrotoxicity, respectively. A trial of intensive chemotherapy followed by hematopoietic stem cell rescue was conducted in 22 patients with relapsed and refractory PCNSL or intraocular lymphoma in a prospective multicenter study. Twenty patients received the intensive chemotherapy and hematopoietic cell rescue. Seven patients had neurological adverse events during the treatment course. An update on that report [12] with a median follow up of 6.2 years, revealed a median overall survival of 91 months. Thirty-seven relapsed PCNSL patients, most who had failed prior methotrexate-based chemotherapy, were treated with intraarterial (i.a.) carboplatin-based chemotherapy with blood barrier disruption (BBBD) [13]. Nine patients had received prior WBI. The median overall survival from first i.a. carboplatin/BBBD treatment was 6.8 months. Seven out of 37 patients survived ≥27 months. The median time to failure for patients with CR and PR was 9.1 months. PCV was administered at the time of relapse to seven patients with PCNSL [8]. Four patients achieved a CR and two achieved a PR for a response proportion of 86%. The median overall survival was 16+ months. Leukoencephalopathy was noted in two of the patients after treatment. The cell surface antigen CD20 is expressed on most PCNSL tumor specimens as 90% of PCNSL cases are B-cell lymphomas. This provides a potential target for therapy [14]. However, after intravenous administration, rituximab CSF levels are approximately 0.1% of the serum levels associated with therapeutic activity in patients with systemic NHL. Despite these findings intravenous rituximab has been administered to patients with relapsed or refractory PCNSL and anecdotal radiographic responses have been reported [15]. A phase 2 trial of temozolomide 150 mg/m2/day, for 5 days every 28 days was conducted in 23 immunocompetent PCNSL patients who had failed prior methotrexate-based chemotherapy and/or WBI [9]. In this study there were 5/23 CR (median duration of response 6+ months, range 2–36 months); 1/23 PR, 4/23 SD and 13/23 PD. In this study temozolomide yielded a 26% objective response proportion without any major toxicity. Immunochemotherapy with rituximab and temozolomide was administered to 15 patients with recurrent or refractory PCNSL. [10]. Patients were treated in 28-day cycles with rituximab at 750 mg/m2 on days 1, 8, 15, 22 and temozolomide at 100–200 mg/m2 on days 1–7 and 15–21. The maximum number of rituximab cycles was two. Following 1–2 cycles of this combination, patients with an objective response and an acceptable level of toxicity continued treatment with temozolomide (days 1–5, in 28-day cycles). There was a 53% radiographic response proportion with a median progression free survival of 7.7 months and a median overall survival of 14 months. Toxicity was modest and consisted of grade 3 thrombocytopenia in four patients, grade 3 anemia in one, and grade 3 leukopenia in one.
Topotecan is a semi-synthetic derivative of camptothecin that inhibits topoisomerase I. It has been observed that topoisomerase I is overexpressed in crude tumor extracts from patients with systemic NHL. The cytotoxicity of topotecan is thought to be due to double-strand DNA damage produced during DNA synthesis. Topotecan crosses the blood-brain barrier [16] and the CSF to plasma ratio is 0.3. Topotecan is associated with a 50% response proportion as a salvage agent in relapsed systemic NHL [17]. Topotecan also has activity against brain metastases from solid tumors [18]. Unlike methotrexate, topotecan is not contraindicated in patients with renal or cardiac insufficiency or in patients with ascites. Topotecan has been studied as a salvage therapy for immunocompetent patients with refractory or relapsed PCNSL [11, 19]. In one study, 27 patients were treated with topotecan at 1.5 mg/m2/day, administered i.v. over 30 min on days 1–5 of a 21-day cycle. In this study 14 patients were refractory to their last therapy and 13 had relapsed since their last therapy. Prior therapy included up to four chemotherapy regimens in 26 patients and WBI in one patient. Five patients achieved CR and 4 achieved PR. The median event-free survival (EFS) was 2 months and the median overall survival was 8.4 months in this study. In the nine patients who achieved a radiographic response the median EFS was 9.1 months. In the five patients who achieved a CR the median overall survival ranged from 9 to 28 months. In this study grade 3 or 4 leukopenia occurred in 26% of patients and grade 3 or 4 thrombocytopenia in 11% of patients. [11].
In our phase 2 study of 15 patients with relapsed or refractory PCNSL who had failed prior methotrexate-based chemotherapy, topotecan was associated with a 20% complete response proportion, acceptable toxicity and no treatment-related deaths. Although we failed to reach our target endpoint with respect to complete responses, and most patients relapsed or progressed after topotecan administration, the drug may contribute to durable survival in a subset of patients. The addition of other CSF-penetrating, anti-lymphoma agents to topotecan may improve the response proportion and duration of tumor control.
References
CBTRUS, Central Brain Tumor Registry of the United States (2005–2006) Primary brain tumors in the United States. Statistical Report 1998–2002
Batchelor T, Carson K, O’Neill A et al (2003) Treatment of primary CNS lymphoma with methotrexate and deferred radiotherapy: a report of NABTT 96-07. J Clin Oncol 21:1044–1049
Macdonald DR, Cascino TL et al (1990) Response criteria of phase II studies for supratentorial malignant glioma. J Clin Oncol 8(7):1277–1280
Nelson DF, Martz KL, Bonner H, Nelson JS, Newall J, Kerman HD, Thomson LW, Murray KJ (1992) Non-Hodgkin’s lymphoma of the brain: can high dose, large volume radiation therapy improve survival? Report on prospective trial by the Radiation Therapy Oncology Group (RTOG): RTOG 8315. Int J Radiat Onc Biol Phys 23:9–17
Abrey LE, Yahalom J, DeAngelis LM (1997) Relapse and late neurotoxicity in primary central nervous system lymphoma. Neurology 48:A18
Nguyen PL, Chakravarti A, Finkelstein DM, Hochberg FH, Batchelor TT, Loeffler JS (2005) Results of whole-brain radiation as salvage of methotrexate failure for immunocompetent patients with primary CNS lymphoma. J Clin Oncol 23(7):1507–1513
Plotkin SR, Betensky RA, Hochberg FH, Grossman SA, Lesser GJ, Nabors LB, Chon B, Batchelor TT (2004) Treatment of relapsed central nervous system lymphoma with high-dose methotrexate. Clin Cancer Res 10(17):5643–5646
Herrlinger U, Brugger W, Bamberg M et al (2000) PCV salvage therapy for recurrent primary CNS lymphoma. Neurology 54:1707–1708
Reni M, Mason W, Zaja F et al (2004) Salvage chemotherapy with temozolomide in primary CNS lymphomas: preliminary results of a phase II trial. Eur J Cancer 40:1682–1688
Enting RH, Demopoulos A, DeAngelis LM, Abrey LE (2004) Salvage therapy for primary CNS lymphoma with a combination of temozolomide and rituximab. Neurology 63:901–903
Fischer L et al (2006) Prospective trial on topotecan salvage therapy in primary CNS lymphoma. Ann Oncol 17:1141–1145
Soussain C, Hoang-Xuan K, Levy V (2004) Results of intensive chemotherapy followed by hematopoietic stem-cell rescue in 22 patients with refractory or recurrent primary CNS lymphoma or intraocular lymphoma. Bull Cancer 91(2):189–192
Tyson RM, Siegal T, Doolittle ND, Lacy C, Kraemer DF, Neutwelt EA (2003) Current status and future of relapsed primary central nervous system lymphoma (PCNSL). Leuk Lymphoma 44(4):627–633
Bashir R, Freedman A, Harris N, Bain K, Nadler L, Hochberg F (1989) Immunophenotypic profile of CNS lymphoma: a review of eighteen cases. J Neurooncol 7(3):249–254
Raizer JJ, DeAngelis L, Zelenetz A, Abrey L (2000) Activity of rituximab in primary central nervous system lymphoma. Proc Am Soc Clin Oncol 19:166a
Sung C, Blaney SM, Cole DE et al (1994) A pharmacokinetic model of topotecan clearance from plasma and cerebrospinal fluid. Cancer Res 54:5118–5122
Preti HA, Plunkett W, Sarris AH, Younes A, Hagemeister F, Rodriguez MA, Romaguera J, McLaughlin P, Bachier C, Cabanillas F (1995) Preliminary results of a phase II trial of topotecan in patients with relapsing lymphoma. Blood 86(10, Suppl 1):820A
Wong ET, Berkenblit A (2004) The role of topotecan in the treatment of brain metastases. Oncologist 9(1):68–79
Voloschin A, Wen P, Hochberg F, Batchelor T (2004) Topotecan as salvage therapy for refractory or relapsed primary central nervous system lymphoma: final report. Neurology 62:A478
Acknowledgments
This study was supported by the Richard and Nancy Simches Fund for Brain Tumor Research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Voloschin, A.D., Betensky, R., Wen, P.Y. et al. Topotecan as salvage therapy for relapsed or refractory primary central nervous system lymphoma. J Neurooncol 86, 211–215 (2008). https://doi.org/10.1007/s11060-007-9464-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-007-9464-6