Abstract
The aim of this study was to determine the incidence and role of pentraxin-3 (PTX-3) overexpression as a predictive/prognostic marker in small-cell lung carcinoma (SCLC). We performed a retrospective study on subjects with a biopsy-proven diagnosis of SCLC. A chart review for demographic and clinical data was performed on patients with SCLC diagnosed between 2005 and 2008. PTX-3 overexpression was evaluated using immunohistochemistry performed on archival paraffin-embedded specimens. 125 patients with SCLC were identified (23 females, 102 males; median age 62.91 ± 8.55 years, range 37–82) all of whom had adequate tissue specimens available for PTX-3 testing. High PTX-3 expression was detected in 25.6 % SCLCs and was significantly associated with male gender and smokers. Moreover, elevated PTX-3 levels were correlated with reduced overall (OS) and disease-free survival, and were an independent negative prognostic factor for OS in SCLC. Our findings suggest that high PTX-3 expression appeared to correlate with aggressive behavior in SCLC, and it may be a useful prognostic marker for SCLC patients and a potential molecular target for SCLC treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lung cancer is one of the leading causes of cancer-related death worldwide. In China, the registered lung cancer mortality rate increased by 464.84 % in the past 3 decades [1]. Small-cell lung cancer (SCLC) is a highly invasive form of lung cancer. Due to the early metastases, patients with SCLC commonly have poor prognosis, with a median survival of 23 months [2, 3]. On the other hand, SCLC is commonly sensitive to radiation and chemotherapy. Therefore, early detection and diagnosis of SCLC may help to improve its prognosis.
So far, a number of molecules, including p53, NES, CEA, Cyfra21-1, and CA19-9, have been proposed as biomarkers for lung cancers. However, these markers have limited clinical value due to lack of specificity [4, 5]. The soluble pattern recognition receptor long pentraxin-3 (PTX-3) is a member of pentraxin family [6]. It has been shown that many cell types including endothelial cells, fibroblasts, mononuclear phagocytes, and dendritic cells were able to produce PTX3 locally in response to inflammatory signals [7]. Thus, PTX3 has been used as a biomarker of inflammation and innate immunity [8]. In addition to inflammation, recent studies have suggested that PTX3 might be used as a serum biomarker for lung cancer, especially non-small-cell lung cancer [9–11]. However, the prognostic value of PTX-3 in SCLC has not been well documented.
In the present study, we analyzed the PTX-3 expression profile in lung tissues in patients with SCLC, and its prognostic value was estimated.
Materials and methods
Patients and specimens
125 patients with newly diagnosed SCLCs who underwent surgery in the Department of Chest Surgery, the Second Hospital of Shandong University, between November 2005 and November 2008 were enrolled, and followed up till October 2013. Histologic type and tumor grade were determined according to the 2004 WHO classification of lung tumors. Tumor, node, metastasis (TNM) classification was performed according to 6th edition of International Union Against Cancer. This study was approved by the Ethics Committee of the Second Hospital of Shandong University.
Immunohistochemistry
Paraffin-embedded SCLC specimens were used for immunohistochemistry analysis. Tumor and surrounding normal tissue were cut into 4 mm sections. Sections were deparaffinized in xylene and then dehydrated with ethanol. For antigen retrieval, the specimens were incubated in a microwave oven (100 °C) in l00 mmol/L citrate buffer (pH 6.0) for 15 min. Endogenous peroxidase activity was blocked with 3 % hydrogen peroxide for 15 min at room temperature. The specimens were incubated with a rabbit polyclonal antibody (1:400, BIOSS, Ltd, China) for 90 min at room temperature and then incubated with secondary antibody (BOSTER, Ltd, China) for 30 min. After three washes in TBS, a reaction product was detected with 3,3′-diaminobenzidine tetra hydrochloride (0.25 mg/ml) and hydrogen peroxide solution (0.01 %). Counter-stained with hematoxylin, the sections were rinsed, dehydrated and covered. Each staining step was included for a negative control except that the primary antibody was omitted.
Immunohistochemical scoring
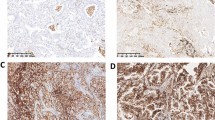
Randomly selected slides were scored by two independent pathologists who were blinded to the clinical status of patients. The intensity of PTX-3 expression was assessed using a scoring system as described previously [12, 13], with minor modifications. In brief, the expression intensity was evaluated using a scale from 0 to 3:0 (negative or trace), 1+ (weak staining), 2+ (moderate staining), 3+ (strong staining). Scores of 0 and 1+ were classified as low expression and scores of 2+ and 3+ were classified as high PTX-3 expression.
Statistical analysis
Statistical analysis was performed using SPSS 17.0 software (SPSS Inc, Chicago, IL). The correlation between PTX3 expression and categorical groups was analyzed by the Chi-square test or Fisher’s exact test. The overall survival (OS) was determined as duration (in months) between the date of diagnosis and the date of death. Disease-free survival (DFS) curves and OS curves were plotted using the Kaplan–Meier method and the log rank test. Multivariate analysis of prognostic factors was done using Cox’s regression model. A value of P < 0.05 was considered statistically significant.
Results
Association between PTX-3 expression and clinicopathological features in SCLC
A summary of the patient characteristics and their correlation coefficients with PTX-3 expression are listed in Table 1. Median patient age was 62.91 ± 8.55 years (range, 37–82 years), 80 % of patients were men. 87 patients had a history of smoking (range, 13–67 years). Tumor staging was available for 125 cases: 63 cases in stage I, 28 cases in stage II, 31 cases in stage III, and 3 cases in stage IV.
Representative results for PTX-3 staining are shown in (Fig. 1). The high Levels of PTX-3 expression were significantly associated with higher in male gender and smokers as compared to female gender (P = 0.030) and non-smokers (P = 0.019). No statistical difference was found between the positive PTX3 expression level was independent of and the characteristics of age (P = 0.788), N stage (P = 0.993), M stage (P = 0.756), and Pathologic stage (P = 0.777).
Relationship between PTX-3 expression and overall survival or disease-free survival
We further analyzed the relationships of PTX-3 expression and other clinic-pathological parameters with OS and DFS in patients with NSCLC (Table 2, Fig. 2a, b).
Survival analysis stratified by the status of PTX-3 expression. a The overall survival (OS) curves according to PTX-3 expression in all patients. b The disease-free survival (DFS) curves according to PTX-3 expression in all patients. The overall survival (OS) curves according to PTX-3 expression in c male d female e smoking f non-smoking patients
Patients with high PTX-3 expression had shorter OS and DFS (17.31 ± 11.13 months, 16.13 ± 10.81 months, respectively) compared to those with low PTX-3 expression (25.59 ± 17.62 months, 23.37 ± 16.83 months, respectively) (P = 0.005, P = 0.010, respectively).
In subgroups, patients with high PTX-3 expression had shorter OS duration than those who had low PTX-3 expression of tumor cells (P = 0.035, P = 0.010 and P = 0.018 respectively; Fig. 2c, e, f), but the difference was not statistically significant in the group of female (P = 0.058; Fig. 2d).
High PTX-3 expression was confirmed as an independent negative prognostic factor affecting OS (P = 0.004, hazard ratio [HR] = 2.492, 95 % confidence interval [95 %CI], 1.612–3.852) using multivariate analyses (Table 3). Other clinical variables such as gender (P = 0.008), smoking history (P = 0.021), age (P = 0.034), Pathologic stage (P < 0.001), T stage (P < 0.001), and N stage (P < 0.001) significantly affected OS, whereas Pathologic stage (P < 0.001), T stage (P < 0.001), and N stage (P < 0.001) were associated with DFS. By multivariate analysis, male gender, smoking history, older age (>60 years), T stage, and N stage were found to be independent prognostic factors of worse OS, whereas T stage and N stage were independent prognostic factors for DFS in SCLC.
Relationship of PTX-3 expression and histological subtypes of SCLC with overall survival
Based on the correlation between SCLC and the prognostic significance of PTX-3 expression, we further examined the prognostic value of PTX-3 expression among SCLC histological subtypes. Patients with oat cell type and mixed oat cell type had shorter OS than patients with intermediate cell type (P < 0.001). However, we found no significant relationship between PTX-3 expression and OS or DFS in the intermediate cell type and mixed oat cell type (P = 0.523 and P = 0.432; P = 0.474 and P = 0.466, respectively) on univariate analyses. The oat cell subtype SCLC patients with high PTX-3 expression tended to have decreased OS and DFS, although the differences were not statistically significant (P = 0.237 and P = 0.468, respectively).
Discussion
Upregulated expression of PTX-3 has been reported to promote tumor growth, invasion, and metastasis in several types of malignancies (6). However, its prognostic value in patients with SCLC remains unclear. In the present study, the purpose of this study was to determine the prognostic significance of PTX-3 protein expression in SCLCs.
The prototypic long-pentraxin PTX3 [14, 15] is a 45-kDa glycosylated protein produced locally by mononuclear phagocytes, dendritic cells, and endothelial cells in response to primary inflammatory signals [16]. Initially, PTX3 was suggested to be useful only as a marker of lung carcinoma based on studies performed on lung cancer cell lines [9] but more recent studies have determined that PTX3 could be used as a serum biomarker for the diagnosis and prognosis of lung carcinoma. Its suitability is overwhelming due to its ability to differentiate between cancer patients and non cancer patients who are at higher risk of developing lung cancer [10].
Owing to the key role of PTX-3 in invasion and metastasis, upregulated expression of PTX-3 may play a significant role in tumor progression. High PTX-3 levels maybe associated with aggressive tumour behavior, including high pTNM stage, high nuclear grade, large tumor size, and nodal metastasis in various cancers [17, 18].
In the present investigation, we showed that high PTX-3 expression was prevalent in males, smokers, non-smokers, and the overexpression of PTX-3 was significantly correlated with lymph node metastasis of SCLC patients. The OS of patients with PTX-3 high was significantly lower than those in PTX-3 low group. Multivariate analysis revealed PTX-3 was an independent prognostic factor in patients’ OS. Moreover, PTX-3 overexpression appears to be related to aggressive tumor behavior, and ultimately influences patients’ clinical outcomes. In addition, PTX-3 overexpression has been shown to be a negative prognostic factor for the survival of patients with cancers of various organs including the breast [18], pancreas [19], esophagus [20], and lung [11]. However, the clinical and prognostic significance of PTX-3 expression in lung cancer especially SCLC has not been previously reported. In this study, we demonstrated that elevated PTX-3 level was associated with decreased OS in 125 patients with SCLC, and was an independent prognostic factor in SCLC patients. Since PTX-3 expression can be easily assessed by immunohistochemistry, it could act as a promising tumour marker for prognosis in SCLC patients.
The identification of correlations between histological subtypes and clinical parameters in lung cancer has important clinical significance [21]. However, there were no statistically significant associations between PTX-3 expression and survival in any SCLC subtype in this study. Only for the oat cell type subtype, patients with high PTX-3 expression tended to have shorter OS and DFS compared to those with low PTX-3 expression. Further studies are needed to better understand the precise relationship between PTX-3 expression and each histological SCLC subtype.
Several researches has suggested that targeting PTX-3 cancer therapy could be a promising therapeutic strategy [22, 23]. PTX-3 inhibition significantly impaired the invasion and metastasis of tumor cells [16, 17]. In our study, high PTX-3 expression predicted aggressive tumor behavior and acted as an independent prognostic factor for SCLC patients. Besides, anti-PTX-3 monoclonal antibody treatment has the potential to decrease cell invasion and metastasis in high PTX-3-expression tumors. Therefore, the up-regulation expression of PTX-3 in SCLC would facilitate the development of targeted cancer therapy.
References
She J, Yang P, Hong Q, Bai C. Lung cancer in china: challenges and interventions. Chest. 2013;143:1117–26.
Elias AD. Small cell lung cancer state-of-the-art therapy in 1996. Chest. 1997;112:251S–8S.
Zikos E, Ghislain I, Coens C, Ediebah DE, Sloan E, Quinten C, et al. Health-related quality of life in small-cell lung cancer: a systematic review on reporting of methods and clinical issues in randomised controlled trials. Lancet Oncol. 2014;15:e78–89.
Brundage MD, Davies D, Mackillop WJ. Prognostic factors in non-small cell lung cancer: a decade of progress. Chest. 2002;122:1037–57.
Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–99.
Ronca R, Alessi P, Coltrini D, Di Salle E, Giacomini A, Leali D. Long pentraxin-3 as an epithelial–stromal fibroblast growth factor-targeting inhibitor in prostate cancer. J Pathol. 2013;230:228–38.
Lin Q, Fu F, Shen L, Zhu B. Pentraxin 3 in the assessment of ventilator-associated pneumonia: an early marker of severity. Heart Lung. 2013;42:139–45.
Presta M, Camozzi M, Salvatori G, Rusnati M. Role of the soluble pattern recognition receptor PTX3 in vascular biology. J Cell Mol Med. 2007;11:723–38.
Planque C, Kulasingam V, Smith CR, Reckamp K, Goodglick L, Diamandis EP. Identification of five candidate lung cancer biomarkers by proteomics analysis of conditioned media of four lung cancer cell lines. Mol Cell Proteomics. 2009;8:2746–58.
Diamandis EP, Goodglick L, Planque C, Thornquist MD. Pentraxin-3 is a novel biomarker of lung carcinoma. Clin Cancer Res. 2011;17:2395–9.
Zhang D, Ren WH, Gao Y, Wang NY, Wu WJ. Clinical significance and prognostic value of pentraxin-3 as serologic biomarker for lung cancer. Asian Pac J Cancer Prev. 2013;14:4215–21.
Chang MH, Lee J, Han J, et al. Prognostic role of insulin-like growth factor receptor-1 expression in small cell lung cancer. Apmis. 2009;117:861–9.
Suzuki S, Miyazaki T, Tanaka N, Park YH, Ahn JS, Park K, et al. Prognostic significance of CD151 expression in esophageal squamous cell carcinoma with aggressive cell proliferation and invasiveness. Ann Surg Oncol. 2011;18:888–93.
Breviario F, d’Aniello EM, Golay J, Peri G, Bottazzi B, Bairoch A, et al. Interleukin-1-inducible genes in endothelial cells. Cloning of a new gene related to C-reactive protein and serum amyloid P component. J Biol Chem. 1992;267:22190–7.
Lee GW, Lee TH, Vilcek J. TSG-14, a tumor necrosis factor- and IL-1-inducible protein, is a novel member of the pentaxin family of acute phase proteins. J Immunol. 1993;150:1804–12.
Basile A, Sica A, d’Aniello E, Breviario F, Garrido G, Castellano M, et al. Characterization of the promoter for the human long pentraxin PTX3. Role of NF-kappaB in tumor necrosis factor-alpha and interleukin-1beta regulation. J Biol Chem. 1997;272:8172–8.
Leali D, Alessi P, Coltrini D, Ronca R, Corsini M, Nardo G, et al. Long pentraxin-3 inhibits FGF8b-dependent angiogenesis and growth of steroid hormone–regulated tumors. Mol Cancer Ther. 2011;10:1600–10.
Margheri F, Serratì S, Lapucci A, et al. Systemic sclerosis-endothelial cell antiangiogenic pentraxin 3 and matrix metalloprotease 12 control human breast cancer tumor vascularization and development in mice. Neoplasia. 2009;11:1106.
Sardana G, Jung K, Stephan C, Anastasia C, Giusti B, Pucci M, et al. Proteomic analysis of conditioned media from the PC3, LNCaP, and 22Rv1 prostate cancer cell lines: discovery and validation of candidate prostate cancer biomarkers. J Proteome Res. 2008;7:3329–38.
Wang JX, He YL, Zhu ST, Yang S, Zhang ST. Aberrant methylation of the 3q25 tumor suppressor gene PTX3 in human esophageal squamous cell carcinoma. World J Gastroenterol. 2011;17:4225–30.
Suh JH. Current readings: pathology, prognosis, and lung cancer. Semin Thorac Cardiovasc Surg. 2013;25:14–21.
Kinuya S, Yokoyama K, Konishi S, Li XF, Watanabe N, Shuke N, et al. Improved response of colon cancer xenografts to radioimmunotherapy with pentoxifylline treatment. Eur J Nucl Med. 2001;28:750–5.
Liu H, Qu XK, Yuan F, Zhang M, Fang WY, et al. A RTK-based functional RNAi screen reveals determinants of PTX-3 expression. Int J clin Exp Pathol. 2013;6:660.
Acknowledgments
This study was financially supported by the Shandong Provincial Natural and Scientific Foundation (Grant No. 2011GSF11813). The authors are grateful for the immunohistochemical technical support of Miss Yao. We would also like to thank the many physicians who cared for the patients at the Departments of Chest Surgery of Second Hospital of Shandong University.
Conflict of interest
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, C., Yao, Y. & Wang, W. Pentraxin-3 as a prognostic marker in patients with small-cell lung cancer. Med Oncol 31, 207 (2014). https://doi.org/10.1007/s12032-014-0207-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-014-0207-1