Abstract
The SDF-1/CXCR4 axis is associated with tumor progression and has been reported as a prognostic parameter, although with conflicting data for non-small cell lung cancer (NSCLC). This study examines a large cohort of clinically and pathologically well-characterized NSCLC patients and includes the activated form of CXCR4 (pCXCR4), which has not been studied in this context so far. SDF-1, CXCR4, and pCXCR4 were assessed immunohistochemically in 371 surgically resected NSCLC using a standardized tissue microarray platform. Extensive clinical and pathological data and a postoperative follow-up period of 17 years enabled detailed correlations. CXCR4 and pCXCR4 were frequently expressed on squamous cell carcinoma. Membranous expression of SDF-1 was a marker of poor prognosis and proved to be an independent prognostic parameter for the entire cohort and for patients with adenocarcinoma (ACA) and large cell carcinoma (LCC). Targeted cancer therapies blocking SDF-1/CXCR4 interaction already exist, and our data suggest that expression of SDF-1, especially on poorer prognosis subgroups of LCC and ACA, indicates patients that might benefit more than others. This should be taken into account when assessing the effectiveness of such targeted approaches for NSCLC patients and could lead to important implications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lung cancer is the most common cause of cancer death worldwide [1] and non-small cell lung cancer (NSCLC) is the most frequent type, representing approximately 80 % of all reported cases [2, 3]. Unfortunately, lung cancer is diagnosed at an advanced stage in about two thirds of patients, and thus, curative surgery is generally not an option for this group. In addition, it has been reported that 30–50 % of surgically resected cases have the potential to recur and metastasize to lymph nodes, liver, bones, or brain [2]. Ultimately, only 15 % of NSCLC patients survive longer than 5 years [2, 3]. Therefore, various efforts concerning alternative treatment modalities to surgery and chemotherapy are being conducted [4].
Approximately 50 subtypes of cytokines and/or chemokines have been identified to date. They are divided based on their function (inflammatory chemokines/homeostatic chemokines) or their binding site cysteins, c representing cysteine and x other amino acids (e.g., c, cxc, cx3c, cc) [5]. Cytokines of the CXC chemokine family bind to their chemokine receptors, which are guanine nucleotide-binding protein receptors (G protein receptors) representing integral membrane proteins, each possessing seven areas spanning the membrane. Currently, seven CXC chemokine receptors have been identified numbered CXCR1–CXCR7. Chemokines and their receptors play a role in mediating inflammation and immune reactions and are important in the hematopoietic system [5]. Chemokines chemically attract leukocytes and stem cells, thus determining the migration sites of these cells toward sites with higher concentrations of chemokines [6, 7]. Particularly, the chemokine receptor CXCR4 has also been identified in multiple cancers, including cancers of the lung, skin, breast, ovary, kidney, and thyroid, as well as in leukemia and lymphoma [8–10]. The importance of this receptor has been demonstrated in several studies, showing that inhibition of CXCR4 leads to apoptosis of tumor cells (e.g., hepatoma, ovarian cancer, and chronic lymphocytic leukemia) [11–13]. In NSCLC, immunohistochemical studies have shown that CXCR4 receptors are present on the cell membrane as well as, unexpectedly and a matter of controversy, in the nucleus in all histological subtypes [14–16]. Expression of CXCR4 on the surface of tumor cells has been associated with tumor progression, metastasis, decreased survival, and worse performance status; on the contrary, nuclear expression seems to be a favorable prognostic parameter [14, 16–18]. This somewhat conflicting data requires further clarification, and especially, studies with larger patient groups are lacking. Expression of CXCR4 has also been associated with an increased density of microvasculature structures and microvessel invasion [19]. A causal link between CXCR4 expression and microvessel density would provide one explanation for the reported poor prognosis associated with increased CXCR4 expression. Interestingly, Otsuka et al. have noted a significantly worse overall survival correlating with CXCR4 expression in stage IV female patients suffering from NSCLC, pointing to a possible gender-specific aspect [18, 20].
CXCL12, also known as stromal-derived factor 1 (SDF-1), is the most important ligand of CXCR4. The SDF-1 (CXCL12)/CXCR4 axis plays a notable role in metastasis and invasion, as target tissues (like lung, liver, bones) have high levels of SDF-1 expression. On the other hand, cancer cells may have high expression of CXCR4, allowing targeted migration toward respective organs [21]. SDF-1 expression has been detected in the cytomembranous compartment of more than 80 % of NSCLC, and patients demonstrating a high staining intensity for SDF-1 were more susceptible to disease recurrence than those with weak staining [22]. In pleural fluid, high levels of SDF-1 produced by pleural mesothelial cells have also been reported; thus, cancer cells with high expression of CXCR4 may likely spread to the pleural cavity [23]. It is important to note that upon binding to SDF-1, CXCR4 is activated by phosphorylation (pCXCR4). To date, the activated form of CXCR4 has not been examined regarding its prognostic and clinicopathological role in NSCLC.
This study analyzes a large cohort of clinically and pathologically well-characterized patients with adenocarcinoma (ACA), squamous cell carcinoma (SCC), and large cell carcinoma (LCC) of the lung, for the sake of convenience herein after referred to as non-small cell lung cancer (NSCLC), aiming to resolve the currently controversial role of the SDF-1/CXCR4/pCXCR4 axis. Special emphasis is placed on the activated form of CXCR4 (pCXCR4), which has not been studied in this context so far, but may play a crucial role. The importance of this pathway for patient outcome is underlined by ongoing in vitro and in vivo studies examining the potential of targeted therapeutic approaches.
Materials and methods
Patients and tissue sampling
The archival samples derived from 371 NSCLC patients with radical surgical resection in curative intent between 1992 and 2004 and diagnosed at the Institute of Pathology, Medical University of Innsbruck [24, 25]. Carcinoids were excluded from this analysis. Cases were selected only based on tissue preservation. Hematoxylin and eosin (H&E)-stained slides from all available specimens were reclassified by two pathologists (WS and AT) without knowledge of patient data, according to the current (2015) WHO classification of tumors of the lung [26]. Tumor differentiation was graded as well, moderate, or poor. The clinical information was documented within the TYROL (Twenty Years Retrospective of Lung Cancer) survey, a project aiming to analyze various features of a large number of lung cancer patients [27]. Approval for data acquisition and analysis was obtained from the Ethics Committee of the Medical University of Innsbruck.
Tissue microarray construction
Tumor material consisted of paraffin-embedded tissue after fixation in 10 % neutral-buffered formalin. The tissue microarray (TMA) was constructed as previously described [24]. The first sections were stained by H&E to confirm validity, the following used for immunohistochemistry.
Immunohistochemistry
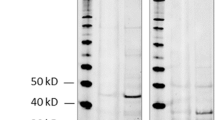
Staining protocols of the primary antibodies CXCR4 and pCXCR4 were performed as recommended by the respective manufacturers, including positive control tissue samples exactly as previously described [28]. For SDF-1, we used a novel anti-C-terminus antibody (Abcam ab135949) that specifically migrates to the predicted 10-kDa band size on Western blotting compared to the older one (Abcam ab80118) [29] with an observed band size of 14 instead of 10 kDa. The ab135949 was diluted 1:20 and incubated on heat-retrieved slides (CC1 conditioning for 8 min) for 16 min. Immunohistochemistry was performed using the automated staining system Benchmark XT (Roche/Ventana Medical Systems, Tucson, USA).
Immunohistochemical evaluation
Only cores containing at least 20 vital tumor cells were evaluated. If all four spots of a case did not meet this criterion, it was excluded; thus, the minimum tumor cell count of evaluated cases was 80 (4 cores with ≥20 tumor cells). Tumor cells were scored independently by S.S. and B.H. and A.T. to study agreement between observers. The percentage of positively stained cells with clearly visible membranous staining was noted for each spot, followed by the calculation of the arithmetic mean value. In addition, cytoplasmic staining as well as staining of stromal cells was noted as either present or absent. The prognostic relevance of respective markers was assessed by means of receiver operating characteristic (ROC) analysis; selecting death as the state variable and optimal cutoff values were calculated using the Youden index (J) for maximum of specificity and sensitivity for variables demonstrating an asymptomatic significance by ROC below 0.2 and an adequately shaped ROC curve; otherwise, the median was selected [30].
Statistical analysis
The degree of agreement between observers was evaluated by interclass correlation coefficients, using reliability Cronbach’s alpha analysis. Correlation analysis of clinicopathological and immunohistochemical parameters was performed using the Spearman test corrected for multiple testing, considering p values ≤0.01 as significant. In addition, for the major histology types (SCC, ACA, and LCC), the mean percentage of positively stained cells was compared by means of ANOVA. Kaplan-Meier curves were calculated for survival estimates and the log-rank statistics used to determine differences between groups; multivariable analysis was performed using the Cox regression model; when not corrected for multiple testing, p values <0.05 were considered as significant. Two-sided tests were used throughout. Statistical calculations were performed using SPSS 22.0 software (SPSS, Chicago, IL).
Results
Histopathology and patient characteristics
Histological subtypes consisted of 215 ACA (17 lepidic, 104 acinary, 4 papillary, 7 micropapillary, 81 solid, and 2 mucinous), 123 SCC, 22 LCC, including 8 neuroendocrine LCC, 8 adenosquamous carcinomas (ASC), 2 sarcomatoid carcinomas (SRC), which were both of the pleomorphic type and 1 mucoepidermoid carcinoma (MEC). Patients’ characteristics are presented in detail in Table 1.
Immunohistochemistry
Cronbach’s alpha for interobserver reproducibility of the immunohistochemical markers was excellent (highest = 0.98 for pCXC4, lowest = 0.77 for SDF-1/CXCL12). Quantitative and qualitative immunohistochemical data are shown in Table 2 and Fig. 1.
Immunohistochemical staining results for CXCR4 in tumor stroma (a), squamous cell carcinoma (b), and adenocarcinoma (c); for pCXCR4 in tumor stroma (d), squamous cell carcinoma (e), and adenocarcinoma (f); and for SDF-1 in tumor stroma (g), squamous cell carcinoma (h), and adenocarcinoma (i). Original magnification A, D, G: ×200; B, C, E, F, H, I: ×320
Three staining patterns were observed for all markers: tumor cell membrane (% of stained tumor cells noted), tumor cell cytoplasms (noted as present or absent), and stromal cell staining (noted as present or absent). The mean and median percentage of stained cells according to the main histologic subtype is shown in Table 2, revealing that positive staining for CXCR4 and pCXCR4 was more frequently found in SCC than ACA. Nuclear staining was not observed.
Correlations between variables
All significant results of the correlation analysis are demonstrated in detail in Table 3. In addition to the presently studied markers, previously assessed molecular parameters were incorporated here as well [24, 25, 31]. Membranous expression of SDF-1 on tumor cells correlated with cytoplasmic SDF-1 expression in tumor cells as well as with SDF-1 expression in stromal cells and was also associated with higher quantities of pCXCR4 (tumor cell membrane and cytoplasm). Cytoplasmic expression of SDF-1 in tumor cells also correlated with pCXCR4 on the tumor cell membrane and cytoplasm and was linked to a higher Ki67 proliferation index. Membranous tumor cell pCXCR4 was also associated with a higher Ki67 index. pCXCR4 correlated with markers of stemness (ABCG5, CD44v6, and CD44). CXCR4 expression on the tumor cell membrane was associated with phosphorylation of CXCR4 (tumor cell membrane and cytoplasm). CXCR4 on stromal cells correlated with phosphorylation of CXCR4 within these cells as well as with increased CXCR4 on tumor cell membranes, and was found more frequently in patients without metastatic disease. pCXCR4 expression on stromal cells also indicated absence of metastasis, whereas SDF-1 expression on tumor cell membrane correlated with metastatic disease.
Survival analysis
Among all presently analyzed markers and expression profiles, SDF-1 expression on the tumor cell membrane was the only significant parameter and indicated inferior overall survival (OS) (Fig. 2a, Table 4). When analyzed according to sex, this was the case for both women and men (Fig. 2b, c, Table 4). SDF-1 expression was also a poor prognostic parameter for patients with early tumor stages (pUICC1 versus pUICC > 1, Fig. 2d; pT1 versus pT > 1, pN0 versus pN > 0; pM0 versus pM1), as opposed to advanced tumor stages (Table 4). Interestingly, SDF-1 expression also indicated decreased OS for patients with recurring disease (Table 4). Analyzed according to the major histological subtypes, SDF-1 expression was associated with inferior OS for ACA (Table 4). When analyzed among the different subtypes of ACA, only the acinary and solid subtypes provided enough cases for reasonable deductions. SDF-1 expression was an indicator of poor prognosis for acinary ACA (median OS not reached versus 46.5 months, p = 0.032 (corrected for multiple testing this is marginal)) but not for solid ACA (median OS 40.6 versus 27.9 months, p = 0.120). When analyzed according to histology combined with tumor stage, only SCC and ACA provided enough cases for reasonable deductions. SDF-1 positive tumors were associated with inferior OS for patients with early stage (pUICC1) ACA compared to the SDF-1 negative counterpart (median OS 112.5 versus 46.5 months, p = 0.005).
When all significant parameters (including previous publications [24, 25, 31]) by univariable analysis (pUICC stage, age, Ki-67, and SDF-1) were tested by multivariable analysis, expression of SDF-1 on the tumor cell membrane was found to be an independent prognostic marker for the entire cohort (Table 5). When stratified according to histological subtype, this was also the case for LCC (p = 0.013, hazard ratio 6.363, 95 % confidence interval 1.478–27.390) and ACA (p = 0.026, hazard ratio 1.554, 95 % confidence interval 1.054–2.291).
Discussion
Our data verifies the significance of the SDF-1/CXCR4 axis for NSCLC and, importantly, brings evidence that expression of SDF-1 is an independent parameter of poor prognosis, especially for patients with LCC. We considerably extend and bring clarification to some existing but highly controversial data on that issue in NSCLC applying reliably working antibodies.
We only partly support what has been speculated or suggested by these mentioned contradictory reports. Various studies have shown that the expression of both CXCR4 and SDF-1 are frequently found in NSCLC, suggesting an autocrine mechanism of activation of the SDF-1/CXCR4 axis in NSCLC; however, the activated (phosphorylated) form of CXCR4 (pCXCR4) has not been studied to date [18, 20]. We included this important parameter in our study and found that membranous expression of SDF-1 on tumor cells does in fact correlate with membranous expression of pCXCR4, making an autocrine activation likely. In tumor cells, we found expression of CXCR4 and pCXCR4 significantly more often on SCC as compared to other histologic types. The prognostic importance of pCXCR4 for NSCLC has not yet been described and our data also does not reveal an association between expression of pCXCR4 and OS. There are however several reports indicating that CXCR4 is a poor prognostic factor for NSCLC. Overexpression of CXCR4 has been associated with significantly worse survival in stage IV NSCLC patients, distant metastasis, and decreased disease-free survival [16, 18]. Interestingly, nuclear expression of CXCR4 is often found to be a positive prognostic marker, although conflicting data show nuclear CXCR4 immunohistochemical expression correlating with increasing lymph node metastasis [14, 16, 17]. Nuclear CXCR4 expression was not detected in our cohort, and in previously published reports, expression of CXCR4 was also not present in the nucleus of tumor cells [28, 32]. Also considering CXCR4’s function, a nuclear sub-compartmentalization is hardly imaginable. Nuclear expression of CXCR4 may therefore represent an unspecific finding, i.e., staining artifact of poorly working/poorly tested antibodies. In a previous study using such an antibody (yet, the only available at that time), which did not properly migrate to the predicted band size on Western blot, we found SDF-1 expression to correlate with better prognosis; however, it was only positive in a very small amount of cases (6.7 %) and we therefore did not ascribe much importance to this finding [29].
In our cohort, expression of CXCR4 was not prognostically significant. On the other hand, membranous expression of SDF-1 proved to be a parameter indicating poor prognosis. Although expression of SDF-1 has been correlated to recurring disease, advanced disease, and nodal metastasis, its role as a prognostic parameter is contradictory [16, 33]. Our analyses showed that SDF-1 indicates poor OS in early tumor stages, and for patients with ACA (acinary subtype) or LCC. Importantly, SDF-1 was an independent parameter of decreased survival for the entire cohort and for patients with ACA and LCC when analyzed according to histology. Underlining the association of aggressiveness with SDF-1 expression and underscoring the robustness of our observations, our data show a correlation of SDF-1 with metastatic disease and increasing Ki-67 proliferation index. In accordance with this finding and further pointing toward an autocrine activation of CXCR4, expression of pCXCR4 was also associated with increasing Ki-67 values. SDF-1 has been shown to promote epithelial-to-mesenchymal transition in primary tumors and in vitro studies have demonstrated that SDF-1 increases CXCR4-mediated motility [34, 35]. In contrast, various studies have reported a tumor-suppressive role of SDF-1 in human malignancy, showing decreased ability to metastasize and poor prognosis with loss of SDF-1 expression [36, 37]. On the other hand, Suzuki et al. found SDF-1 expression to be present in significantly more cases with nodal metastasis, with advanced disease stages, and to correlate with poor prognosis in ACA [38], and NSCLC patients demonstrating a high staining intensity for SDF-1 were more susceptible to disease recurrence and displayed therefore poorer outcomes than those with weak staining [21], as in our study.
A number of studies have demonstrated that SDF-1 derived from cancer-associated fibroblasts can stimulate cancer cell growth through the CXCR4 receptor displayed on tumor cells, as well as recruit endothelial progenitor cells and promote neoangiogenesis [39]. Regarding stromal cells in NSCLC, we found a correlation between CXCR4 and its activated form pCXCR4, which were both associated with lack of metastasis. We also found a correlation between SDF-1, CXCR4, and pCXCR4 in stromal cells, pointing to an autocrine stimulation as proposed for tumor cells. In addition, SDF-1 on stromal cells was associated with SDF1 expression by tumor cells (membranous and cytoplasmic) as well as with pCXCR4 on tumor and stroma cells, underlining the above described interaction between stromal and tumor cells, although no association with disease progression or OS could be linked to the expression profile of stromal cells. According to the histological patterns of ACA, only the acinary type had enough cases for sufficient correlation analysis (results not shown) and SDF-1 expression on stromal cells again correlated with membranous expression of SDF-1 on tumor cells as well as with pCXCR4 on stromal cells. Membranous SDF-1 expression on tumor cells also correlated with cytoplasmic SDF-1 expression as well as with membranous tumor cell expression of pCXCR4, thus being well representative of the entire cohort.
Hypoxia has been shown to regulate expression of SDF-1 and CXCR4 at the transcriptional level [40]. With this in mind, we incorporated various data from our previously published study on the microvasculature in NSCLC, though without relevant results (not shown) [41]. We did however find a correlation between pCXCR4 on tumor cells and alleged markers of stemness [25]. This is in line with data by Bertolini et al., who reported that NSCLC cells with high self-renewal capacity and with increased chemotherapeutic resistance (traits of cancer stem cells) expressed high levels of CXCR4 relative to NSCLC that lack these characteristics [42].
An essential issue of the SDF-1/CXCR4 axis is the possibility of targeted cancer therapies, thus inhibiting metastatic and tumorigenic potential. It has been shown that anti-SDF-1 neutralizing antibodies to immunodeficient mice harboring human NSCLC tumors abrogated organ-specific metastasis [15]. Furthermore, drugs such as AMD3100 and BKT140, which specifically block the SDF-1/CXCR4 axis, can inhibit NSCLC growth in vitro and in vivo [33, 43, 44]. By blocking SDF-1/CXCR4 interaction and thus inhibiting recruitment of bone marrow-derived cells eminent for vasculogenesis, recurrence of glioblastoma after radiation has been reduced in mice [45]. A factor that must be considered here and may prevent successful clinical use of such targeted anticancer therapy is a potential side effect on the stem cell compartment in normal tissues.
Our data suggest that expression of SDF-1, especially on LCC and ACA, could point to a group of patients that might benefit more from targeting the SDF-1/CXCR4 axis than others. This should be taken into account by future studies assessing the effectiveness of such targeted approaches for NSCLC patients and could lead to important implications.
References
Lovly CM, Carbone DP (2011) Lung cancer in 2010: one size does not fit all. Nat Rev Clin Oncol 8:68–70
Siegel R, DeSantis C, Virgo K, Stein K et al (2012) Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin 62:220–241
Pallis AG, Serfass L, Dziadziusko R et al (2009) Targeted therapies in the treatment of advanced/metastatic NSCLC. Eur J Cancer 45:2473–2487
Dempke WC, Suto TM, Reck M (2010) Targeted therapies for non-small cell lung cancer. Lung Cancer 67:257–274
Raman D, Sobolik-Delmaire T, Richmond A (2011) Chemokines in health and disease. Exp Cell Res 317:575–589
Zlotnik A, Yoshie O (2000) Chemokines: a new classification system and their role in immunity. Immunity 12:121–127
Murphy PM, Baggiolini M, Charo IF et al (2000) International union of pharmacology. XXII. Nomenclature for chemokine receptors. Pharmacol Rev 52:145–176
Kijima T, Maulik G, Ma PC et al (2002) Regulation of cellular proliferation, cytoskeletal function, and signal transduction through CXCR4 and c-Kit in small cell lung cancer cells. Cancer Res 62:6304–6311
Balkwill F (2004) Cancer and the chemokine network. Nat Rev Cancer 4:540–550
Barretina J, Juncà J, Llano A et al (2003) CXCR4 and SDF-1 expression in B-cell chronic lymphocytic leukemia and stage of the disease. Ann Hematol 82:500–505
Nomiyama H, Osada N, Yoshie O (2010) The evolution of mammalian chemokine genes. Cytokine Growth Factor Rev 21:253–262
Messmer D, Fecteau JF, O’Hayre M et al (2011) Chronic lymphocytic leukemia cells receive RAF-dependent survival signals in response to CXCL12 that are sensitive to inhibition by sorafenib. Blood 117:882–889
Bertran E, Caja L, Navarro E et al (2009) Role of CXCR4/SDF-1 alpha in the migratory phenotype of hepatoma cells that have undergone epithelial-mesenchymal transition in response to the transforming growth factor-beta. Cell Signal 21:1595–1606
Spano JP, Andre F, Morat L et al (2004) Chemokine receptor CXCR4 and early-stage non-small cell lung cancer: pattern of expression and correlation with outcome. Ann Oncol 15:613–617
Phillips RJ, Burdick MD, Lutz M et al (2003) The stromal derived factor-1/CXCL12-CXC chemokine receptor 4 biological axis in non-small cell lung cancer metastases. Am J Respir Crit Care Med 167:1676–1686
Wagner PL, Hyjek E, Vazquez MF et al (2009) CXCL12 and CXCR4 in adenocarcinoma of the lung: association with metastasis and survival. J Thorac Cardiovasc Surg 137:615–621
Na IK, Scheibenbogen C, Adam C et al (2008) Nuclear expression of CXCR4 in tumor cells of non-small cell lung cancer is correlated with lymph node metastasis. Hum Pathol 39:1751–1755
Otsuka S, Klimowicz AC, Kopciuk K et al (2011) CXCR4 overexpression is associated with poor outcome in females diagnosed with stage IV non-small cell lung cancer. J Thorac Oncol 6:1169–1178
Franco R, Pirozzi G, Scala S et al (2012) CXCL12-binding receptors expression in non-small cell lung cancer relates to tumoral microvascular density and CXCR4 positive circulating tumoral cells in lung draining venous blood. Eur J Cardiothorac Surg 41:368–375
Wald O, Shapira OM, Izhar U (2013) CXCR4/CXCL12 axis in non small cell lung cancer (NSCLC) pathologic roles and therapeutic potential. Theranostics 3:26–33
Su L, Zhang J, Xu H et al (2005) Differential expression of CXCR4 is associated with the metastatic potential of human non-small cell lung cancer cells. Clin Cancer Res 11:8273–8280
Wald O, Izhar U, Amir G, Avniel S et al (2006) CD4+CXCR4 high CD69+ T cells accumulate in lung adenocarcinoma. J Immunol 177:6983–6990
Oonakahara K, Matsuyama W, Higashimoto I et al (2004) Stromal-derived factor-1alpha/CXCL12-CXCR 4 axis is involved in the dissemination of NSCLC cells into pleural space. Am J Respir Cell Mol Biol 30:671–677
Sterlacci W, Fiegl M, Hilbe W et al (2009) Clinical relevance of neuroendocrine differentiation in non-small cell lung cancer assessed by immunohistochemistry: a retrospective study on 405 surgically resected cases. Virchows Arch 455:125–132
Sterlacci W, Savic S, Fiegl M et al (2014) Putative stem cell markers in non-small-cell lung cancer: a clinicopathologic characterization. J Thorac Oncol 9:41–49
Travis W, Brambilla E, Burke A et al (2015) WHO classification of tumours of the lung, pleura, thymus and heart. IARC Press, Lyon
Kocher F, Hilbe W, Seeber A et al (2015) Longitudinal analysis of 2293 NSCLC patients: a comprehensive study from the TYROL registry. Lung Cancer 87:193–200
Brault L, Rovó A, Decker S et al (2014) CXCR4-SERINE339 regulates cellular adhesion, retention and mobilization, and is a marker for poor prognosis in acute myeloid leukemia. Leukemia 28:566–576
Sterlacci W, Wolf D, Savic S et al (2012) High transforming growth factor β expression represents an important prognostic parameter for surgically resected non-small cell lung cancer. Hum Pathol 43:339–349
Tzankov A, Zlobec I, Went P et al (2010) Prognostic immunophenotypic biomarker studies in diffuse large B cell lymphoma with special emphasis on rational determination of cut-off scores. Leuk Lymphoma 51:199–212
Sterlacci W, Tzankov A, Veits L et al (2011) The prognostic impact of sex on surgically resected non-small cell lung cancer depends on clinicopathologic characteristics. Am J Clin Pathol 135:611–618
Brault L, Menter T, Obermann EC et al (2012) PIM kinases are progression markers and emerging therapeutic targets in diffuse large B-cell lymphoma. Br J Cancer 24 107:491–500
Wald O, Izhar U, Amir G et al (2011) Interaction between neoplastic cells and cancer-associated fibroblasts through the CXCL12/CXCR4 axis: role in non-small cell lung cancer tumor proliferation. J Thorac Cardio vasc Surg 141:1503–1512
Jung Y, Kim JK, Shiozawa Y et al (2013) Recruitment of mesenchymal stem cells into prostate tumours promotes metastasis. Nat Commun 4:1795
Huang YC, Hsiao YC, Chen YJ et al (2007) Stromal cell-derived factor-1 enhances motility and integrin up-regulation through CXCR4, ERK and NF-kappaB-dependent pathway in human lung cancer cells. Biochem Pharmacol 74:1702–1712
Wendt MK, Johanesen PA, Kang-Decker N et al (2006) Silencing of epithelial CXCL12 expression by DNA hypermethylation promotes colonic carcinoma metastasis. Oncogene 25:4986–4997
Kobayashi T, Tsuda H, Moriya T et al (2010) Expression pattern of stromal cell-derived factor-1 chemokine in invasive breast cancer is correlated with estrogen receptor status and patient prognosis. Breast Cancer Res Treat 123:733–745
Suzuki M, Mohamed S, Nakajima T et al (2008) Aberrant methylation of CXCL12 in non-small cell lung cancer is associated with an unfavorable prognosis. Int J Oncol 33:113–119
Xu J, Clark RA (1996) Extracellular matrix alters PDGF regulation of fibroblast integrins. J Cell Biol 132:239–249
Cojoc M, Peitzsch C, Trautmann F et al (2013) Emerging targets in cancer management: role of the CXCL12/CXCR4 axis. Onco Targets Ther 6:1347–1361
Pomme G, Augustin F, Fiegl M et al (2015) Detailed assessment of microvasculature markers in non-small cell lung cancer reveals potentially clinically relevant characteristics. Virchows Arch 467:55–66
Bertolini G, Roz L, Perego P et al (2009) Highly tumorigenic lung cancer CD133+ cells display stem-like features and are spared by cisplatin treatment. Proc Natl Acad Sci U S A 106:16281–16286
Fujii N, Nakashima H, Tamamura H (2003) The therapeutic potential of CXCR4 antagonists in the treatment of HIV. Expert Opin Investig Drugs 12:185–195
Fahham D, Weiss ID, Abraham M et al (2012) In vitro and in vivo therapeutic efficacy of CXCR4 antagonist BKT140 against human non-small cell lung cancer. J Thorac Cardio vasc Surg 144:1167–1175
Kioi M, Vogel H, Schultz G et al (2010) Inhibition of vasculogenesis, but not angiogenesis, prevents the recurrence of glioblastoma after irradiation in mice. J Clin Invest 120:694–705
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Sterlacci, W., Saker, S., Huber, B. et al. Expression of the CXCR4 ligand SDF-1/CXCL12 is prognostically important for adenocarcinoma and large cell carcinoma of the lung. Virchows Arch 468, 463–471 (2016). https://doi.org/10.1007/s00428-015-1900-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-015-1900-y