Abstract
Nanog, a key transcription factor in self-renewal and pluripotency of embryonic stem cells, has been proved to play a novel role in solid tumor development. Here, we investigated Nanog protein expression in retrospective clinical samples of 105 patients underwent resective surgery for gastric adenocarcinoma. We found that Nanog protein immunostaining in tumor tissues was stronger than that in their corresponding non-dysplastic tissues. However, no statistical difference of Nanog protein expression between tumor tissues and metastatic lymph nodes was found (P = 0.143). Interestingly, overexpression of Nanog protein was correlated with advanced clinical stage of patients with gastric adenocarcinoma (P = 0.006). And Nanog protein expression was correlated with lymph node status (P = 0.004), infiltrating extent (P = 0.001), and differentiation (P = 0.000) of patients with gastric adenocarcinoma. Survival analysis showed that overexpression of Nanog protein in gastric cancer patients predicted a poorer prognosis (P = 0.000). Our data first demonstrated a potential diagnostic and prognostic role of Nanog for gastric adenocarcinoma.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gastric cancer is the main cause of tumor-associated death in series of cancers [1, 2] with an estimated 934,000 new cases per year in 2002 [3]. In China, the overall 5-year survival rate of patients with gastric cancer is lower than 40%. Most patients with gastric cancer are diagnosed at advanced clinical stages with a high ratio (50–75%) of lymph node metastasis. Therefore, it is very important to find new factors for the early diagnostic and prognostic evaluation of gastric cancer. Because adenocarcinoma accounts for approximately 90% of gastric cancer [4], patients with gastric adenocarcinoma (GAC) were selected in this study.
As far as we know, gastric cancer is a multistep process involving a number of genetic and epigenetic changes. Molecular genetic analysis of gastric cancer has revealed associations of certain genetic changes with pathological features and prognosis of gastric cancer patients. Recent study showed that molecular changes just as c-met, c-erbB2, APC, TP53, and E-cadherin were relevant with gastric carcinogenesis [5]. It suggests that a better understanding of the genetic or epigenetic changes underlying gastric cancer will provide new perspectives for early diagnosis and prognosis.
Nanog, one of the key homeobox transcription factors involved in embryonic stem cells (ESCs) [5, 6], regulates the cell fate of the pluripotent inner cell mass (ICM) by maintaining the pluripotent epiblast and preventing differentiation to primitive endoderm [7, 8]. Similarly, tumor development has long been considered as an abnormal embryogenesis, and cancer cells share a few biological properties with ESCs [9]. And recently, Nanog has been shown to play a role in solid tumor development. Elevated Nanog expressions were found in retinoblastoma, prostate cancer, embryonal carcinoma, metastatic germ cell tumor, ovarian cancer, breast cancer, and colorectal cancer [10–16]. Nevertheless, there is still no report on the role of Nanog in GAC, especially in clinical samples of GAC. Furthermore, the diagnostic and prognostic significance of Nanog in GAC is still unknown.
Here, we aimed to assess the diagnostic and prognostic role of Nanog and the correlation between Nanog protein expression and clinicopathological characteristics in GAC.
Materials and methods
Patients
A cohort of 156 GAC patients who underwent gastric surgery at Nanfang Hospital (Guangzhou, People’s Republic of China) was selected for this study. The study was approved by the Ethics Committee of Southern Medical University. At the beginning of the study, we followed up all 156 GAC patients after the surgery. However, 51 patients were excluded from the study for loss of further follow-up. The characteristics of the 105 patients are summarized in details in Table 1.
Immunohistochemistry
Briefly, 4-μm, formalin-fixed, paraffin-embedded specimens of the tissue sections from patients with GAC were available for immunohistochemical analysis. Slides were deparaffinized and rehydrated following standard methods. A microwave antigen retrieval procedure was carried out for 20 min in citrate buffer (pH 6.0). Hydrogen peroxide was used to block non-specific peroxidase reaction. After washing with phosphate-buffered saline (PBS, pH 7.4), the sections were incubated with Rabbit anti-human Nanog polyclonal antibody (Biosynthesis Biotechnology Co., LTD, Beijing, China, 1:200) for 12 h at 4°C. After washing three times with PBS, sections were incubated at 37°C with HRP Goat anti-rabbit IgG Conjugated (ZYMED, San Francisco, USA) for 20 min. The visualization was achieved by incubation with diaminobenzidine for 10 min, and the slides were counterstained with Mayer hematoxylin. After hydrated in graded alcohol and cleared with xylene, the slides were mounted with neutral gum. Seminomas, which have been proved to overexpress Nanog [17], were chosen as appropriate positive control, and negative controls were performed by omitting the primary antibody.
Evaluation of Nanog protein expression
In this study, positive cells were scored based on nucleus staining of Nanog protein. The number of positive immunostained cells out of 100 in 10 random high-power fields (X400-Zeiss microscope) was scored. The immunoassaying frequency scores and stain intensity scores were ranged from 0–4 and 0–3, respectively. An overall protein expression score (0–12) was calculated by multiplying two scores above. For statistical analysis, a final score of ≥6 was considered as a strong expression of Nanog protein, and a score of 2, or less, was presumed as a low expression, and scores between them were regarded as a moderate expression. Slides were independently reviewed by two pathologists blinded to the clinical data, and consensus agreements were reached.
Statistical analysis
All statistical analyses were performed by SPSS13.0 software package for Windows. The associations of Nanog expression with different clinical parameters were performed by non-parametric tests. In detail, chi-square test was used for categorical variables and Mann–Whitney U test for continuous variables. Chi-square test was also performed to compare the expression of Nanog between tumor tissues and adjacent benign tissues, as well as metastasis of lymph nodes. Survival analysis was performed by Kaplan–Meier method and compared by log-rank test. Multivariate analysis by Cox proportional hazard regression model was performed to find the potential independent prognosis factors in GAC. P < 0.05 was regarded statistically significant.
Results
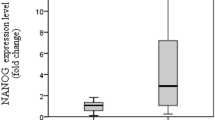
We compared 105 samples of GAC tissues with 70 samples of non-dysplastic tissues, which had enough normal counterparts adjacent to cancer tissues from the 105 cases, GAC tissues. Nanog protein was mainly localized in the nucleus of the tumor cell of GAC. Negative staining could be found in normal counterpart non-dysplastic tissue. High and moderate staining could be found in the tumor cells of GAC. Specially, negative expression of Nanog was found in most of the tumor cells in the gastric signet-ring cell carcinoma (GSRC), a special type of GAC (Fig. 1). Moderate and strong expression of Nanog was found in the majority of GAC tissues (76/105), and strong expression of Nanog was observed in 38 cases of them. In contrast, low expression of Nanog was found in most of non-dysplastic tissues (58/70), and strong expression of Nanog was only observed in 4 samples (5.71%) of them (Fig. 2a). However, when we randomly selected 10 samples from 34 patients with metastasis in lymph nodes to compare Nanog staining in the primary cancers and its corresponding metastasis in lymph nodes, no statistical difference was detected in Nanog expression between the cancer tissues and the metastatic lymph nodes (P = 0.143) (Fig. 2b).
Expression of Nanog protein in GAC patients. a Low expression of Nanog in normal counterpart non-dysplastic tissue (×400). b Low expression of Nanog protein in GAC tissues (×400). c Low expression of Nanog protein in signet-ring cell carcinoma of gastric cancer (×400). d Moderate expression of Nanog protein in GAC tissue (×400). e and f High expression of Nanog protein in GAC tissue (×100, ×400). g and h High expression of Nanog protein in metastatic lymph nodes (×100, ×400)
a Population pyramid of Nanog protein expression in 105 cases of GAC patients (blue color) and 70 cases of adjacent benign tissues (green color) from GAC patients (P = 0.000). b Population pyramid of Nanog protein expression in tumor tissues (105 samples) and metastatic lymph nodes (10 samples) of GAC patients (P = 0.143)
In order to know the clinical role of Nanog in GAC, we further analyzed the associations between protein expression levels of Nanog and clinicopathological characteristics such as gender, age, location, invasion, tumor differentiation, lymph nodes status, and TNM stage of patients with GAC. No significant associations were found between Nanog expression and age, gender, tumor location (P > 0.05) of GAC patients. Interestingly, Nanog expression was positively correlated with tumor invasion (P = 0.001), lymph node status (P = 0.000), and TNM staging (P < 0.001) of GAC patients (Table 2).
Survival analysis using Kaplan–Meier analysis method showed that clinical stage, serosal invasion, lymph node metastasis, and differentiation (Table 3), as well as Nanog protein expression, could predict the prognosis of GAC patients. Overexpression of Nanog protein correlated with poorer overall 5-year survival in patients with GAC. The Cox proportional hazard regression model indicated that Nanog protein expression (P = 0.000) (Fig. 3a) and clinical stage (P = 0.000) (Fig. 3b) were the two potential independent prognostic factors in our study (Table 4). According to the relative risk, Nanog, as well as clinical stage, serosal invasion, lymph nodes metastasis, and differentiation was positively related to the risk of death from GAC (Table 4).
Discussion
Nanog, a new transcription factor reported by Chambers and Mistsui [18], is one of the four factors to reprogram adult cells into germ-line-competent-induced pluripotent stem cells [19–21]. Moreover, Nanog plays a critical role in maintaining self-renewal and pluripotent of ESCs by regulating cell fate of pluripotent ICM [22–24]. Interestingly, the elevated expression of Nanog protein in several human cancers was recently reported, suggesting that Nanog may be implicated in tumor genesis and progression [25]. A systematic study using animal model and in vitro cell systems has supplied many evidences about the key role of Nanog in human tumor development [26]. Recent study showed TGFβ pathway was involved in the regulation of Nanog gene expression via binding with the Nanog proximal promoter [27]. As we know, TGFβ pathway is one of the most important signaling pathways which are frequently altered in human gastric cancer [28, 29]. Interestingly, our previous study showed that Nanog played as both an inducer and a receipt of epithelial–mesenchymal transition (EMT)-related signal of colorectal cancer [16]. However, the role of Nanog in human gastric cancer is still unclear.
Here, we aimed to find the clinical role of Nanog in GAC. Overexpression of Nanog protein was found in 105 cases of GAC clinical samples using immunohistochemical methods, indicating a potential diagnostic value of Nanog protein. In our study, strong expression of Nanog protein was found in 36% samples of cancer tissue and 60% samples of lymph nodes with metastasis. As mentioned before, several groups have investigated the overexpression of Nanog protein in several types of carcinomas. Here, we report that Nanog protein was overexpressed in GAC. As far as we know, molecular-targeted therapy has been a hot field for cancer therapy, and our study gives a potential clue for clinical trials of Nanog-targeted drug which may work in the management of GAC patients.
The results about the association between Nanog and pathological features showed that Nanog protein expression predicted TNM stage, serosal invasion, lymph nodes metastasis, and tumor differentiation of GAC patients. As we know, the TNM stage system lymph node status are two prognostic indexes widely used in clinic for GAC [30, 31], and poorly differentiated cancer cells of gastric cancer often show stronger aggressive and metastatic ability [32]. The relevance between Nanog expression and the above clinicopathological characteristics indicates that Nanog could be used as a potential factor to predict tumor progression and poor prognosis in GAC.
Our further results indicated that there was a strong correlation between expressions of Nanog protein and a 5-year survival of the 105 GAC patients. Kaplan–Meier analysis in this study showed that the main significant factors that impacted prognosis of patients with GAC were Nanog, TNM stage, invasive depth, lymph nodes metastasis, and differentiation. Cox regression analysis showed that Nanog and clinical stage were the two potential independent factors in GAC. Therefore, Nanog protein, as well as TNM stage, could be used as a potential prognosis factor for GAC. Interestingly, negative staining of Nanog protein was found in 11 of 19 cases of GSRC in this study. Our data showed that expression of Nanog protein was not relevant to 5-year survival of GSRC patients (P = 0.168), and in contrast, TNM was the significant prognostic factor of GSRC (P = 0.003) (Table 5). The reason for this outcome needs further study, and it indicates that Nanog may not be a suitable prognostic index for GSRC, a special type of GAC.
In this study, we found that Nanog protein was mainly located in cellular nucleus. So positive cells were scored based on nucleus staining of Nanog protein, and cytoplastic staining in a small fraction of cancer cells was considered as non-specific expression. Both cytoplasmic Nanog and nuclear Nanog have been observed in some cultured tumor cells [26]. In malignant cervical epithelial cells, Nanog was found only in cytoplasm [33]. Only nuclear Nanog was found in both cell line and clinical samples of oral squamous cell carcinoma [34]. Interestingly, a study using the same antibody in our group found Nanog was localized in both cytoplasm and nucleus in colorectal cancer [16]. The significance of distribution of Nanog protein is still unknown.
In conclusion, our study shows that overexpression of Nanog protein correlates with TNM stage, serosal invasion, lymph nodes metastasis, and tumor differentiation of GAC patients. Our data first demonstrate that Nanog has a potential diagnostic and prognostic role for GAC.
References
Echem R. Gastric cancer is a major cause of cancer death. Niger J Med. 2003;12:175–6.
Gruvberger SK, Ringner M, Eden P, Borg A, Ferno M, et al. Expression profiling to predict outcome in breast cancer: the influence of sample selection. Breast Cancer Res. 2003;5:23–6.
Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108.
Gruvberger-Saal SK, Cunliffe HE, Carr KM, Hedenfalk IA. Microarrays in breast cancer research and clinical practice–the future lies ahead. Endocr Relat Cancer. 2006;13:1017–31.
Smith MG, Hold GL, Tahara E, El-Omar EM. Cellular and molecular aspects of gastric cancer. World J Gastroenterol. 2006;12:2979–90.
Boyer LA, Lee TI, Cole MF, Johnstone SE, Levine SS, et al. Core transcriptional regulatory circuitry in human embryonic stem cells. Cell. 2005;122:947–56.
Chambers I, Colby D, Robertson M, Nichols J, Lee S, et al. Functional expression cloning of Nanog, a pluripotency sustaining factor in embryonic stem cells. Cell. 2003;113:643–55.
Jerevall PL, Brommesson S, Strand C, Gruvberger-Saal S, Malmstrom P, et al. Exploring the two-gene ratio in breast cancer–independent roles for HOXB13 and IL17BR in prediction of clinical outcome. Breast Cancer Res Treat. 2008;107:225–34.
Linderholm BK, Gruvberger-Saal S, Ferno M, Bendahl PO, Malmstrom P. Vascular endothelial growth factor is a strong predictor of early distant recurrences in a prospective study of premenopausal women with lymph-node negative breast cancer. Breast. 2008;17:484–91.
Seigel GM, Hackam AS, Ganguly A, Mandell LM, Gonzalez-Fernandez F. Human embryonic and neuronal stem cell markers in retinoblastoma. Mol Vis. 2007;13:823–32.
Santagata S, Ligon KL, Hornick JL. Embryonic stem cell transcription factor signatures in the diagnosis of primary and metastatic germ cell tumors. Am J Surg Pathol. 2007;31:836–45.
Gu G, Yuan J, Wills M, Kasper S. Prostate cancer cells with stem cell characteristics reconstitute the original human tumor in vivo. Cancer Res. 2007;67:4807–15.
Freberg CT, Dahl JA, Timoskainen S, Collas P. Epigenetic reprogramming of OCT4 and NANOG regulatory regions by embryonal carcinoma cell extract. Mol Biol Cell. 2007;18:1543–53.
Hoei-Hansen CE, Kraggerud SM, Abeler VM, Kaern J, Rajpert-De Meyts E. Ovarian dysgerminomas are characterised by frequent KIT mutations and abundant expression of pluripotency markers. Mol Cancer. 2007;6:12.
Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA. 2003;100:3983–8.
Meng HM, Zheng P, Wang XY, Liu C, Sui HM, et al. Overexpression of nanog predicts tumor progression and poor prognosis in colorectal cancer. Cancer Biol Ther. 2010;9:295–302.
Hart AH, Hartley L, Parker K, Ibrahim M, Looijenga LH, et al. The pluripotency homeobox gene NANOG is expressed in human germ cell tumors. Cancer. 2005;104:2092–8.
Mitsui K, Tokuzawa Y, Itoh H, Segawa K, Murakami M, et al. The homeoprotein Nanog is required for maintenance of pluripotency in mouse epiblast and ES cells. Cell. 2003;113:631–42.
Okita K, Ichisaka T, Yamanaka S. Generation of germline-competent induced pluripotent stem cells. Nature. 2007;448:313–7.
Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–20.
Park IH, Zhao R, West JA, Yabuuchi A, Huo H, et al. Reprogramming of human somatic cells to pluripotency with defined factors. Nature. 2008;451:141–6.
Pan G, Thomson JA. Nanog and transcriptional networks in embryonic stem cell pluripotency. Cell Res. 2007;17:42–9.
Pereira L, Yi F, Merrill BJ. Repression of Nanog gene transcription by Tcf3 limits embryonic stem cell self-renewal. Mol Cell Biol. 2006;26:7479–91.
Suzuki A, Raya A, Kawakami Y, Morita M, Matsui T, et al. Maintenance of embryonic stem cell pluripotency by Nanog-mediated reversal of mesoderm specification. Nat Clin Pract Cardiovasc Med. 2006;3:114–22.
Ezeh UI, Turek PJ, Reijo RA, Clark AT. Human embryonic stem cell genes OCT4, NANOG, STELLAR, and GDF3 are expressed in both seminoma and breast carcinoma. Cancer. 2005;104:2255–65.
Jeter CR, Badeaux M, Choy G, Chandra D, Patrawala L, et al. Functional evidence that the self-renewal gene NANOG regulates human tumor development. Stem Cells. 2009;27:993–1005.
Xu RH, Sampsell-Barron TL, Gu F, Root S, Peck RM, Pan G, et al. NANOG is a direct target of TGFbeta/activin-mediated SMAD signaling in human ESCs. Cell Stem Cell. 2008;3:196–206.
Stock M, Otto F. Gene deregulation in gastric cancer. Gene. 2005;360:1–19.
Shinto O, Yashiro M, Kawajiri H, Shimizu K, Shimizu T, et al. Inhibitory effect of a TGF beta receptor type-I inhibitor, Ki26894, on invasiveness of scirrhous gastric cancer cells. Br J Cancer. 2010;102:844–51.
Wang Z, Xu H, Wang S, Chen J. Relationship between new TNM classification and the prognosis and biological behavior of gastric cancer. Zhonghua Wai Ke Za Zhi. 2000;38:493–5.
Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70.
Strosberg JR, Nasir A, Hodul P, Kvols L. Biology and treatment of metastatic gastrointestinal neuroendocrine tumors. Gastrointest Cancer Res. 2008;2:113–25.
Ye F, Zhou C, Cheng Q, Shen J, Chen H. Stem-cell-abundant proteins Nanog, Nucleostemin and Musashi1 are highly expressed in malignant cervical epithelial cells. BMC Cancer. 2008;8:108.
Chiou SH, Yu CC, Huang CY, Lin SC, Liu CJ, et al. Positive correlations of Oct-4 and Nanog in oral cancer stem-like cells and high-grade oral squamous cell carcinoma. Clin Cancer Res. 2008;14:4085–95.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lin, T., Ding, YQ. & Li, JM. Overexpression of Nanog protein is associated with poor prognosis in gastric adenocarcinoma. Med Oncol 29, 878–885 (2012). https://doi.org/10.1007/s12032-011-9860-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12032-011-9860-9