Abstract
This study was designed to evaluate the expression of C-erbB-2 and p16 in lung cancers using tissue microarray technology and to determine their clinical and pathological significance. Immunohistochemical C-erbB-2 and p16 expressions and their associations with clinical and pathological features were analyzed in two tissue microarrays. The membranous and cytoplasmic expression rates of C-erbB-2 were 40.5 and 66.5% in non-small cell lung cancers (NSCLCs), and 0 and 9.5% in small cell lung cancers (SCLCs), respectively. The nuclear and cytoplasmic expression rates of p16 were 11.5 and 32.2% in NSCLs, and 45 and 80% in SCLCs, respectively. The cytoplasmic expression of both C-erbB-2 and p16 was more frequent than the membranous expression of C-erbB-2 and the nuclear expression of p16. The rates of overexpression of C-erbB-2 and loss of p16 expression were significantly higher in NSCLCs than in SCLCs (P < 0.05). Neither C-erbB-2 nor p16 expression was significantly associated with age, tumor grade or stage, presence of lymph node metastasis or survival duration. The abnormal expressions of p16 and C-erbB-2 may play a role in the progression of lung cancers. The variations in the expression patterns of C-erbB-2 and p16 between NSCLCs and SCLCs may aid the molecular classification of lung cancer. The abnormal expression of p16 may be involved in the development of NSCLCs, and the overexpression of C-erbB-2 in NSCLCs indicates that it can be a candidate target for gene therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lung cancer is one of the most common cancers in China [1], posing a significant health problem. The morbidity and mortality of lung cancer have been significantly increasing in the past two decades [1]. The total survival rate of patients with lung cancer is as low as 10–15% [1]. There are urgent needs for the development of effective approaches to prevention, early detection, diagnosis, and treatment of lung cancer. Although impressive progress has recently been made in molecular and cellular biology studies of lung cancer, the implication in clinical diagnosis and treatment remains uncertain. For instance, the values of C-erbB2 and p16 in clinical diagnosis and prognosis of lung cancer is still controversial.
C-erb-B2, a 185-kDa transmembrane oncoprotein, is the product of the HER-2 (neu) oncogene that is located at chromosome 17q21 and is expressed in various human cancer tissues. In breast cancer, C-erbB-2 has been generally accepted as an independent prognostic marker [2]. However, its expression and clinical relevance in lung cancer are not fully established. The tumor suppressor p16 is encoded by a gene located at 9p21 and plays an important role in regulating the cell cycle; more specifically, p16 can stop the progression from G1 to S phase of the cell cycle by restricting CDK4 expression and function. The p16 mutations increase the risk of developing a variety of cancers. It is generally accepted that p16 is a useful marker for diagnosis and prognosis in cervical adenocarcinoma [3]. However, the value of p16 expression pattern, especially in combination with C-erbB-2, in lung cancer remains unclear.
Therefore, this study was designed to evaluate the membranous and cytoplasmic expressions of C-erbB2 and the cytoplasmic and nuclear expressions of p16 in various forms of lung cancers, and to determine their associations with clinical and pathological changes as well as the use of these markers alone or in combination for diagnosis and prognosis of lung cancers.
Materials and methods
Reagents
Commercially available reagents were used in the present study, including the C-erbB-2 multi-clone antibody (DAKO, USA), the p16 monoclonal antibody (Zhongshan Golden Bridge Biotechnology Co, Ltd, China), the ultrasensitive SP kit (Fuzhou Maixin biotechnology Co, Ltd, China), and the DAB (diaminobenzidine tetrahydrochloride) detection kit (Zhongshan Golden Bridge Biotechnology Co, Ltd, China). The construction of the tissue microarray was made by using a punching manual operation machine (Beecher Instruments arraying device, USA). In brief, the tumor areas were first made from the tissue donor blocks. Each one was 0.6 mm in diameter and was put into a spacing of 1.0 mm in the recipient block. Two lung cancer tissue microarray blocks were constructed, resulting in 435 samples of 321 cases.
Cancer tissue samples
The protocols for the collection and preparation of tissue samples were approved by the institutional review board of our hospital. All patients agreed to provide the resected tissues and related information for this study. The lung tissues were obtained from 321 lung cancer patients, who underwent surgical resection at the Cancer Institute/Hospital, Chinese Academy of Medical Sciences, between 1990 and 1993. Neoadjuvant therapy was not carried out for patients prior to surgery. Based on the criteria of the 1999 WHO [4] Lung and Pleural Membrane Tumor Classification, the 321 tumors were diagnosed as 150 squamous cell carcinomas (SCCs), 127 adenocarcinomas (Ads), 18 adenosquamous carcinomas (AdSqs), and 26 small cell lung cancers (SCLCs). Metastatic lymph nodes were also obtained from 101 of the patients. In addition, normal lung tissues (n = 7) and lymph nodes (n = 6) as controls were consensually obtained from some patients. Tissue microarray (TMA) analyses were performed for all 435 samples.
Immunohistochemical staining
Sections were cut at 5-μm thickness from paraffin-embedded tissues. For every 10 TMA slides, one was stained with hematoxylin and eosin (H&E; Fig. 1). These sections were deparaffinated in xylene and rehydrated in a graded series of ethanol. The sections were heated in a steamer (Fuzhou Maixin biotechnology Co, Ltd, China) for 20 min in the target retrieval solution (Zhongshan Golden Bridge Biotechnology Co, Ltd, China) for antigen retrieval. The activity of endogenous peroxidase was blocked with the peroxidase-blocking reagent (Zhongshan Golden Bridge Biotechnology Co, Ltd, China) for 5 min. After washing the sections with buffer, the sections were incubated with primary antibodies for 30 min and then washed again with buffers.
Negative controls were included by performing the staining with the same procedure, but omitting the primary antibody. External positive controls were included using the tissues, which have been confirmed by positive staining for either C-erbB-2 (breast cancers) or p16 protein (cervical adenocarcinomas). The positive staining for C-erbB-2 or p16 was shown by membranous, cytoplasmic, or nuclear brown granular; three slides were stained for each sample.
The IHC staining for each slide was classified into one of the following four groups according to both intensity and extent: (−), less than 5% of tumor cells with positive staining; (+), 6–25% positive staining; (++), 26–50% positive staining; and (+++), more than 50% positive staining. The evaluation for the immunochemical staining was performed by two independent investigators, with no prior knowledge of the clinical data and grouping information of the samples.
Statistical analysis
The clinical, pathological, and TMA data were processed using the software SPSS10.0 [5]. The comparisons for different protein expression rates were accomplished using χ2-test, and the survival distributions were estimated by the Kaplan–Meier method. Statistical significance was considered as P < 0.05.
Results
Clinical information
The mean age of the patients was 56 (ranging from 29 to 83). The numbers of male and female patients were 243 and 78, respectively with a ratio of 3.1:1 (male/female). The 150 patients with SCCs were further divided into three subgroups: low grade (15 patients), intermediate grade (92 patients), and high grade (43 patients). The 127 patients with Ads were also divided into three subgroups: low grade (30 patients), intermediate grade (49 patients), and high grade (48 patients). According to the 1997 UICC [6] criteria, the staging results for all patients were 157 stage I, 86 stage II, and 78 stage III. Most of the patients were followed up for their prognosis and survival durations; the median follow-up duration was 59 months (range 0–118). Hundred and six patients died of the disease, 114 patients are alive, and 101 patients were lost to follow-up.
Pathological features
Among the samples, 263 tumor specimens (81.9%) were used for C-erbB-2 staining and 253 (78.8%) for p16 staining. Among the 101 patients from whom both primary tumors and metastatic lymph nodes were obtained, 57 samples (56.4%) were analyzed for C-erbB-2 and 45 samples (44.6%) for p16. In our experience, the lack of assessment of some samples was probably due to the IHC technique, especially to the antigen-retrieval step, and the absence of tumor because of an abundant stroma or normal tissue. Moreover, the donor blocks may not be enough thick to obtain long cores.
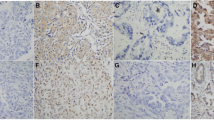
For the C-erbB-2 protein, the cytoplasmic expression was observed more frequently than membranous expression. The cytoplasmic and membranous expression rates of the C-erbB-2 protein were 66.5 and 40.5% in NSCLCs, and 9.5 and 0% in SCLCs, respectively, indicating that C-erbB-2 protein expression in NSCLCs is significantly higher than in SCLCs (P < 0.05). However, neither the membranous nor the cytoplasmic C-erbB-2 expression was significantly associated with age, grading, staging, presence of lymph node metastasis, or survival time (P > 0.05, Tables 1, 2; Figs. 2, 3).
For the p16 protein, the nuclear and cytoplasmic expression rates were 32.2 and 11.5% in NSCLCs and 80 and 45% in SCLCs, respectively. Contrary to the C-erbB-2 expression pattern, the p16 protein expression was significantly less in NSCLC than in SCLC (P < 0.05). Similar to the findings with C-erbB-2 expression, neither cytoplasmic nor nuclear p16 expression was significantly associated with age, grading, staging, presence of lymph node metastasis, or survival duration (Tables 2, 4; Figs. 4, 5). Of note, the expression rate of p16 protein in the tumors from smokers with NSCLCs was less than that of non-smokers with NSCLCs, but no statistical significance was found (P > 0.05, Table 5). There was no significant difference in p16 and C-erbB-2 expression between the primary lung carcinomas and corresponding metastatic lymph nodes (Table 3).
Discussion
Although several studies have been published, there is no definite conclusion on the expression patterns and their values of C-erbB2 and p16 in the clinical diagnosis and prognosis of lung cancer. In this study, it was found that both the membranous and cytoplasmic expressions of C-erbB2 were significantly higher in NSCLCs than in SCLCs, which is consistent with previous studies [4, 5, 7, 8]. However, there was no significant correlation between the C-erbB-2 expression and the prognosis of patients. Although some previous studies have shown a correlation of C-erbB-2 expression with clinical outcomes, other studies show the similar results as this study. Although the exact reasons for this discrepancy are not clear, it may be related to population characteristics, sample size, and disease stage [6–12]. Therefore, the prognostic relevance of the C-erbB-2 expression alone in lung cancer remains to be confirmed.
In the present study, we used the following four categories to classify the intensity and extent of the C-erbB-2 staining: (−), less than 5% of tumor cells with positive staining; (+), 6–25% positive staining; (++), 26–50% positive staining; and (+++), more than 50% positive staining. This assessment system has been validated and used in many clinical marker studies. Although this scoring system is different from the standardized DAKO-scoring scheme used for breast cancer and other solid cancers, it is used extensively in solid tumors. When we did this research, the DAKO-scoring scheme on C-erBb-2 was not routinely applied in China, especially in the area of lung cancer research or clinical practice. To our knowledge, the treatment targeting C-erbB-2 has not yet been widely applied yet in lung cancer. Therefore, we believe that multiple scoring systems for C-erbB-2 immunostaining may be helpful to finally determine the clinical significance of C-erbB-2 expression in lung cancer. Nevertheless, future studies should use the DAKO-scoring scheme and compare the results with our currently used system.
The cytoplasmic expression of C-erbB2 was observed more frequently than the membranous expression, but there was no significant correlation between the patterns of C-erbB-2 either in the cytoplasm or membrane and the clinical outcomes of these patients. Reinmuth et al. [10, 13] demonstrated a similar staining pattern for C-erbB-2 in both the membrane and cytoplasm in lung cancer, while Cheng et al. [11, 14] showed that the cytoplasmic C-erbB2 expression is greater than the membrane expression. Nevertheless, both studies found no significant correlation with the prognosis, which is consistent with the present study. The high number of patients (n = 101) who were lost to follow-up may contribute to weaken the prognostic study.
Regarding the loss of p16 protein expression, it was found that NSCLCs had significantly less expression of p16 than SCLCs, suggesting that the p16 protein may have a stronger tumor suppressing role in NSCLCs than in SCLCs. Previous studies have indicated that, as the inactivation of RB tumor suppressor gene stimulates the development of SCLCs, the inactivation of p16 expression is the main cause of cell losing growth control in NSCLCs [12, 13, 15, 16]. The results may support this notion, as it was determined that tissues from smokers with NSCLCs often had the loss of p16 expression when compared with their non-smoking counterparts. This suggests that smoking may cause a suppression in p16 expression, which in turn increases the initiation of NSCLCs as proposed by others [14, 15, 17, 18].
In the present study, no significant correlation was found between p16 protein expression and the prognosis. Nakata et al. [16, 19] proposed that p16 gene might have a correlation with the development of lung cancer, but did not have any relevance with the prognosis. In contrast, both Singhal et al. [17, 20] and Abdulkader et al. [18, 21] found a correlation of the loss of p16 expression with worse prognoses of NSCLC patients. The variations for the p16 expression and clinical relevance in lung cancer in different populations should be further investigated.
The cytoplasmic expression of p16 occured more frequently than nuclear expression in this study. Evangelou et al. [19, 22] demonstrated that p16INK4A cytoplasmic staining is specific and suggested that it represents a mechanism of p16INK4A inactivation similar to that observed with other tumor suppressor genes. Several other studies with breast and colon carcinomas suggest that cytoplasmic immunoreactivity should not be ignored, but considered as an abnormal staining pattern [20, 21, 23, 24].
In summary, the expression patterns of C-erbB2 and p16 protein may be useful in molecular classification of NSCLCs and SCLCs. In addition, there was no association between C-erbB2 and p16 expression. The overexpression of C-erbB2 protein in NSCLC indicates that C-erbB-2 can be a candidate target gene for lung cancer therapy. There was no significant difference in clinical relevance between cytoplasmic and membranous expressions of C-erbB2 or p16 protein in the lung cancers. However, the expression of C-erbB2 and p16 protein may have no prognostic significance in lung cancers. It should be pointed out that due to various factors such as race, sample size, and methodology compared with other studies, the above conclusions should be considered tentative, and more research is needed in the future.
References
Li L, Lu F, Zhang S. Analyses of variation trend and short-term detection of Chinese malignant tumor mortality during twenty years. Zhonghua Zhong Liu Za Zhi. 1997;19:3–9.
Ferretti G, Felici A, Papaldo P, Fabi A, Cognetti F. HER2/neu role in breast cancer: from a prognostic foe to a predictive friend. Curr Opin Obstet Gynecol. 2007;19:56–62.
Liang J, Mittal KR, Wei JJ, Yee H, Chiriboga L, Shukla P. Utility of p16INK4a, CEA, Ki67, P53 and ER/PR in the differential diagnosis of benign, premalignant, and malignant glandular lesions of the uterine cervix and their relationship with Silverberg scoring system for endocervical glandular lesions. Int J Gynecol Pathol. 2007;26:71–5.
Travis WD, Colby TV, Corrin B, et al. World health organization international histological classification of tumours: histological typing of lung and pleural tumours. 3rd ed. Berlin, Germany: Springer; 1999.
SPSS Inc. SPSS Base10.0 Applications Guide. Chicago, IL: SPSS Inc.; 1999.
Mountain CF. Revisions in the international system for staging lung cancer. Chest. 1997;111:1711–7.
Hirsch FR, Langer CJ. The role of HER2/neu expression and trastuzumab in non-small cell lung cancer. Semin Oncol. 2004;31:75–82.
Ugocsai K, Mándoky L, Tiszlavicz L, Molnár J. Investigation of HER2 overexpression in non-small cell lung cancer. Anticancer Res. 2005;25:3061–6.
Calikusu Z, Yildirim Y, Akcali Z, Sakalli H, Bal N, Ozyilkan O. Prognostic significance of the C-erbB-2 expression in turkish non-small cell lung cancer patients. Asian Pac J Cancer Prev. 2009;10:479–82.
Seidenfeld J, Samsom DJ, Rothenberg BM, Bonnell CJ, Ziegler KM. Aronson N HER2 testing to manage patients with breast cancer or other solid tumors. Evid Rep Technol Assess (Full Rep). 2008;172:1–362.
Papila C, Uzun H, Balci H, Zerdali H, Sezgin C, Can G, et al. Clinical significance and prognostic value of serum sHER-2/neu levels in patients with solid tumors. Med Oncol. 2009;26:151–6.
Feng XL, Zhang XB, Wan YX, Ma JH, Sun YT. Expressions of C-erbB-2 protein in tissues and cell lines of renal cell carcinoma. Chin J Pathol. 2002;31:158–9.
Reinmuth N, Brandt B, Kunze WP, Junker K, Thomas M, Achatzy R, et al. Ploidy, expression of erbB1, erbB-2, P53 and amplification of erbB1, erbB2, and erbB3 in non-small cell lung cancer. Eur Respir J. 2000;16:991–6.
Cheng CM, Tsuneyama K, Matsui K, Takahashi H, Ishizawa S, Takano Y. Cytoplasmic expression of c-erbB2 in non-small cell lung cancers. Virchows Arch. 2005;446:596–603.
Wikenheiser-Brokamp KA. Retinoblastoma regulatory pathway in lung cancer. Curr Mol Med. 2006;6:783–93.
Esposito V, Baldi A, De Luca A, Tonini G, Vincenzi B, Santini D, et al. Cell cycle related proteins as prognostic parameters in radically resected non-small cell lung cancer. J Clin Pathol. 2005;58:734–9.
Sanz-Ortega J, Roig F, Al-Mousa MM, Saez MC, Muñoz A, Sanz-Esponera J, et al. 17p13 (p53 locus), 5q21 (APC locus) and 9p21 (p16 locus) allelic deletions are frequently found in oral exfoliative cytology cells from smoker patients with non-small-cell lung cancer. Histol Histopathol. 2007;22:541–5.
Ho WL, Chang JW, Tseng RC, Chen JT, Chen CY, Jou YS, et al. Loss of heterozygosity at loci of candidate tumor suppressor genes in microdissected primary non-small cell lung cancer. Cancer Detect Prev. 2002;26:343–9.
Nakata S, Sugio K, Uramoto H, Oyama T, Hanagiri T, Morita M, et al. The methylation status and protein expression of CDH1, p16 (INK4A), and fragile histidine triad in non-small cell lung carcinoma: epigenetic silencing, clinical features, and prognostic significance. Cancer. 2006;106:2190–9.
Singhal S, Vachani A, Antin-Ozerkis D, Kaiser LR, Albelda SM. Prognostic implications of cell cycle, apoptosis, and angiogenesis biomarkers in non-small cell lung cancer: a review. Clin Cancer Res. 2005;11:3974–86.
Abdulkader I, Sánchez L, Cameselle-Teijeiro J, Gude F, Chávez JE, López-López R, et al. Cell-cycle-associated markers and clinical outcome in human epithelial cancers: a tissue microarray study. Oncol Rep. 2005;14:1527–31.
Evangelou K, Bramis J, Peros I, Zacharatos P, Dasiou-Plakida D, Kalogeropoulos N, et al. Electron microscopy evidence that cytoplasmic localization of the p16(INK4A) “nuclear” cyclin-dependent kinase inhibitor (CKI) in tumor cells is specific and not an artifact. A study in non-small cell lung carcinomas. Biotech Histochem. 2004;79:5–10.
Milde-Langosch K, Bamberger AM, Rieck G, Kelp B, Loning T. Overexpression of the p16 cell cycle inhibitor in breast cancer is associated with a more malignant phenotype. Breast Cancer Res Treat. 2001;67:61–70.
Zhao P, Hu YC, Talbot IC. Expressing patterns of p16 and CDk4 correlated to prognosis in colorectal carcinoma. World J Gastroenterol. 2003;9:2202–6.
Acknowledgments
This study was supported by the Chinese National Nature Science Foundation (No. 30270582).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Feng, Xl., Li, L., Gao, Yn. et al. Overexpression of c-erbB-2 and loss of p16 have molecular diagnostic relevance but no prognostic value in lung cancer. Med Oncol 28, 336–341 (2011). https://doi.org/10.1007/s12032-010-9452-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12032-010-9452-0