Abstract
Renal cell carcinoma primarily affects older individuals. Approximately half of all new renal cell carcinoma diagnoses are made in persons 65 years of age or older. Devising a treatment plan for the elderly patient population requires special consideration. Age-related physiological, cognitive, and social characteristics of elderly patients may influence each stage of patient care. Until recently, treatment options were limited for elderly patients with renal cell carcinoma. Sorafenib is the first multikinase inhibitor approved for use in renal cell carcinoma in the United States and Europe. In the phase III Treatment Approaches in Renal Cell Cancer Global Evaluation Trial, sorafenib significantly extended progression-free survival in patients with advanced renal cell carcinoma, regardless of age. Incidence rates of adverse events were not significantly higher in elderly patients receiving sorafenib than in younger patients. Thus, sorafenib represents an important treatment option for elderly patients with renal cell carcinoma. This report describes particular considerations for physicians to be aware of when choosing a treatment regimen for their elderly patients with renal cell carcinoma and offers recommendations on how to integrate specific management strategies into clinical practice that will optimize the use of sorafenib in the elderly. The strategies focus on patient selection, assessment of quality of life, management of adverse events, and appropriate dose modifications. The goal of these recommendations is to maximize the clinical benefit of sorafenib in the elderly patient population through appropriate use.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Age represents the greatest risk factor for the development of cancer [1]. While cancer can occur at any age, the likelihood of developing cancer increases with age. For renal cell carcinoma (RCC), the median age of first diagnosis is ~65 years, and over 25% of newly diagnosed patients are older than 75 years [2]. Considering that the proportion of elderly individuals in the population is growing [3], RCC is expected to pose an increasingly greater health burden in coming years.
The advanced age of many patients with RCC presents specific complications that can affect patient care. Elderly patients are generally more likely to have poorer performance status, lower tolerance to therapy, and more comorbidities than younger patients [4]. Nearly two-thirds of elderly patients with RCC (aged ≥75) concurrently suffer from conditions such as cardiovascular disease or diabetes [4, 5]. On average, elderly patients with cancer (aged >70) will have three comorbidities, in which growing evidence shows can affect survival, cancer progression, and treatment response [6]. In general, elderly patients have poorer prognosis, compared with younger patients. The 5-year overall survival (OS) and progression-free survival (PFS) rates for patients with RCC are 91 and 81% for patients ≤40 years old, and 78 and 71% for patients aged >40, primarily because older patients generally have more advanced disease at diagnosis [7]. Thus, many elderly patients with RCC often require treatment considerations that are distinct from those required by younger patients.
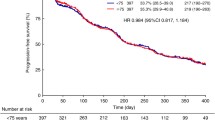
Sorafenib is the first multikinase inhibitor approved for use in RCC. Approved by the United States Food and Drug Administration in 2005 and the European Medicines Agency in 2006, sorafenib is an oral biaryl urea RAF kinase inhibitor that acts against both vascular endothelial growth factor and platelet-derived growth factor receptors, simultaneously targeting both tumor cell proliferation and angiogenesis [8–10]. In the phase III Treatment Approaches in Renal Cancer Global Evaluation Trial (TARGET) evaluating the efficacy and safety of sorafenib in patients with advanced RCC, sorafenib significantly prolonged PFS, compared with placebo (5.5 months vs. 2.8 months; p < 0.01) [11]. In the final analysis of the TARGET trial, sorafenib conferred an OS advantage over placebo, despite the crossover of 48% (n = 216) of placebo-treated patients to the sorafenib arm (17.8 months vs. 15.2 months; p = 0.146) [12]. The OS advantage of sorafenib becomes statistically significant when data from crossover patients are censored (17.8 months vs. 14.3 months; p = 0.029) [12]. Extended access studies of sorafenib in advanced RCC, the Advanced Renal Cell Carcinoma Sorafenib (ARCC) studies in North America (NA-ARCCS) and Europe (EU-ARCCS), report disease control in 84 and 73% of patients, respectively [13, 14]. By significantly prolonging PFS, extending OS, and improving quality of life, sorafenib represents a major advance in the treatment of advanced RCC.
Until sorafenib was approved, treatment options for patients with advanced RCC were limited to cytokine therapy, which is associated with severe toxicity that posed a major barrier to use in elderly patients [4, 15]. By comparison, elderly patients enrolled in the phase III TARGET trial reported rates of adverse events comparable to those reported by younger patients in the trial (Table 1) [16]. These observations were confirmed in expanded access studies of sorafenib in North America (NA-ARCCS) and in Europe (EU-ARCCS), in which elderly and younger patients reported comparable rates of adverse events (Table 2) [13, 17]. More importantly, the elderly patients in both the phase III TARGET trial and the NA-ARCCS and EU-ARCCS trials received sorafenib doses comparable to those received by younger patients and experienced a level of clinical benefit comparable to that of younger patients or the overall patient population (Tables 1, 2). Subset analyses showed that, compared with placebo, sorafenib significantly extended PFS in all patients, regardless of age [16, 17]. In addition, elderly patients receiving sorafenib achieved disease control rates similar to those of the overall population [13, 16, 17].
At present, clinical practice guidelines in RCC do not specify recommendations for management decisions in the elderly population [18], although general recommendations have been put forth by the International Society of Geriatric Oncology [4]. This article captures the discussion of an expert panel of physicians that was convened on February 26, 2009, to review management strategies for elderly patients with RCC receiving sorafenib.
Elderly patient selection for sorafenib therapy
In the TARGET trial, elderly patients (aged ≥70) made up 13% of the study population [16]. In the NA-ARCCS and EU-ARCCS studies, elderly patients (aged ≥70) made up 28 and 23% of the patient population, respectively [13, 17]. In our clinical experience, about 20–30% of patients with RCC are elderly.
Because factors such as cognitive capacity, physical health, and goals of therapy can vary widely in the elderly patient population, geriatric assessment is an important component of treatment individualization [19]. This involves evaluating patients for comorbidities, dependency, nutritional status, and mental status [19]. A number of factors should be considered when deciding to prescribe sorafenib for an elderly patient with RCC (Table 3).
Chronological versus physiological age
Chronological age refers to an individual’s actual age. Physical and environmental factors, including lifestyle (e.g., smoking history and diet), socioeconomic status (e.g., employment grade), markers of overall physical health (e.g., serum cholesterol levels), fitness level (e.g., exercise regimen), independence level, and markers of age (e.g., muscle mass loss) can influence an individual’s physiological age, or how young or old an individual feels [20]. For some patients, their chronological age differs from their physiological age, and patients may be physiologically “younger” or “older” than their actual age in years. Controversy over the age threshold separating elderly from non-elderly patients continues to raise debate. While some guidelines define elderly as aged ≥65, clinicians understand that the age threshold is not fixed and depends on the physiological age of each patient [21]. In our opinion, an estimated chronological age threshold for classifying the elderly is ~70 years. A growing body of evidence shows that prognostic factors in elderly patients include measures of functional performance, independence in activities of daily life, cognitive strength, and comorbidities but not chronological age [22, 23]. Despite this evidence, elderly patients are often denied standard treatment based on their age [24], even though such treatment may be beneficial. Physiological age should be considered when deciding to start sorafenib therapy. Following initial physical exam and interview about medical and social histories, consider making an estimation of physiological age and devising an appropriate treatment plan that is best suited for each individual patient.
Comorbidities
Specific guidelines are not available that outline exclusion criteria for sorafenib based on comorbidities, so such a determination should be made on a case-by-case basis, depending on the judgment of the treatment physician. Indices have been developed to measure the burden of comorbidities in the elderly, including the Charlson index [25]. In our clinical experience, sorafenib therapy may not be generally suitable for elderly patients with serious cardiac conditions including unstable angina, coronary artery disease, or recent stroke or myocardial infarction. Patients with left-ventricular ejection fraction (LVEF) ≤40% may not tolerate sorafenib well [26]. Although extensive safety data are not available to allow a general recommendation, we believe that individual patients with LVEF >40–50% may be considered for sorafenib therapy if they are asymptomatic and have no other cardiac risk factors. Other comorbidities, such as hypertension, may be exacerbated during treatment [11], although this condition is not necessarily a contraindication for sorafenib, since medical management is usually successful in controlling high blood pressure. In our clinical experience, hypertension as a comorbidity should not necessarily preclude a patient from sorafenib therapy. A thorough understanding of all preexisting conditions is important in determining whether a patient is suitable for sorafenib treatment.
Sorafenib administration and the elderly patient
Before starting an elderly patient on sorafenib, inquire about the patient’s support system. Determine whether or not a family member or friend can offer assistance with drug administration and/or at-home management of adverse effects, if necessary. If no support system is available, then have a nurse make frequent contact with the patient to monitor adherence to therapy, the status of adverse effects, and overall well-being. Patients should be educated about the importance of drug administration and adherence and how to recognize and handle adverse events when they develop.
When prescribing therapies to elderly patients, physicians must consider any age-related physiological characteristics that may impact clinical pharmacology, adherence, and the patients’ ability to self-administer medications [27]. Elderly patients with comorbidities requiring treatment face the added challenge of coordinating multiple drug regimens without omitting doses. Varied pill sizes and colors, dosing schedules, and dosing requirements may confuse patients whose eyesight and memory may be weakening. For elderly patients receiving sorafenib, it is important to emphasize the importance of taking the drug on an empty stomach at least 1 h before or 2 h after eating [28]. This may confound those patients who are accustomed to or need to take other medications on a full stomach or with food.
An early study of adherence to treatment regimens in an elderly patient population found that 60% of patients discontinued a medication without consulting their doctor if they felt the medication was not useful [29]. As such, it is also important to tell patients to take sorafenib continuously unless otherwise directed, even when they do not perceive the drug to be working. Dose interruptions of sorafenib can diminish its potential clinical benefit to patients.
Financial constraints may negatively affect adherence to drug treatment for many patients [30], particularly those who are not working or on a fixed income. To address issues of access to sorafenib, the Resources for Expert Assistance and Care Helpline (REACH) program provides help with explaining insurance coverage and finding other programs to financially assist patients receiving sorafenib [31]. Patients who have trouble affording sorafenib can seek assistance through the REACH program to avoid interruptions in treatment [31].
Quality of life in elderly patients on sorafenib
In a subanalysis of the TARGET trial to measure quality of life, patients on sorafenib, regardless of age, reported a longer delay to health status deterioration, compared to placebo [16]. Compared with patients receiving placebo, a greater proportion of patients receiving sorafenib, regardless of age, reported stable or improved physical well-being at cycles 4 and 5 of treatment [16]. Thus, elderly patients are as likely as younger patients to maintain or improve their quality of life while on sorafenib therapy.
In the clinical setting, quality of life can be assessed at each patient visit by inquiring about the occurrence and severity of adverse events, overall health, and the effect, if any, of sorafenib treatment on a patient’s ability to perform the activities of daily living (Table 3). Scales are available to help assess the ability of patients to carry out the basic and instrumental activities of daily living, and these measure factors that contribute to the inability of patients to care for themselves (Table 4) [32, 33]. Using such scales when inquiring about a patient’s activities of daily living can guide the interview. As is often the case in elder care, maintaining quality of life can be the primary goal of therapy [34]. Striking the proper balance between minimizing adverse effects and maximizing clinical response to therapy is an important aspect of maintaining or improving patient’s quality of life. Elderly patients may report an improvement in their quality of life if they are able to perform more tasks than they could previously.
For elderly patients, maintaining disease control is an important goal of treatment. We recommend that disease control be monitored with regularly scheduled imaging scans (Table 3). In our clinical experience, we have had many elderly patients consider an objective tumor response of stable disease to be an indication of clinical efficacy, because their cancer remains asymptomatic. The potential for disease control to translate into preserved or improved quality of life may be considered a significant measure of clinical efficacy by many elderly patients.
Drug–drug interactions and the elderly patient
Patients with cancer with comorbidities are often treated with multiple agents. In one cancer center in the southeastern US, elderly patients with cancer were taking, on average, six concomitant drugs [6]. The potential exists that drugs used to treat comorbidities may interact with anticancer therapy and increase toxicity and/or reduce efficacy of one or more drugs [4]. It is imperative to inventory all medications and dietary supplements used by a patient before beginning sorafenib therapy.
Angiogenesis inhibitors, such as sorafenib, sunitinib, and bevacizumab, have raised concerns about their potential to increase the risk of bleeding. In the TARGET trial, bleeding (all grades, all causalities) was reported in 15.3 and 8.2% of patients receiving sorafenib or placebo, respectively [11, 28]. There was no significant difference in grade 3 and 4 bleeding reported in 2.0 and 1.5% of patients receiving sorafenib or placebo, respectively [11, 28]. In patients who are taking anticoagulants (such as warfarin) while on sorafenib, infrequent bleeding events or elevations in the international normalized ratio (INR) have been reported [28]. Thus, it is recommended that INR be monitored closely. With appropriate management of anticoagulant therapy, patients may continue to receive sorafenib therapy.
Because sorafenib is dosed continuously, patients taking other drugs concurrently may not need to adjust the dosages of those drugs during cancer treatment. This can be particularly important in elderly patients who may be sensitive to the changes in drug levels. For example, patients may experience labile hypertension with sunitinib, a drug that is dosed continuously for 4 weeks followed by a 2-week rest period [35]. In some patients started on antihypertensive treatment associated with their cancer therapy, symptomatic hypotension has been observed during the rest weeks, necessitating intermittent dosing of the antihypertensive [35, 36]. Similarly, the need for readjustment of warfarin dosing during sunitinib therapy has been observed in clinical practice [36]. The need for some elderly patients to regularly adjust the dosages of their medications during intermittent cancer therapy in order to maintain equilibrium can prove burdensome.
Experience with sorafenib tolerability in elderly patients
The incidence of treatment-related adverse events in the TARGET trial was comparable between sorafenib-treated elderly patients and younger patients (Table 1) [16]. Similar observations were made in the NA-ARCCS and EU-ARCCS studies in this regard (Table 2) [13, 17]. Nonetheless, some adverse events associated with sorafenib may be tolerated differently in elderly patients than in younger patients because of differences in physiology and psychology. Because maintaining quality of life is the primary goal of treatment for many elderly patients, any side effect that impinges on quality of life may become intolerable, regardless of severity. Faced with the prospect of diminished quality of life with sorafenib therapy, some patients may opt to discontinue therapy. Close follow-up and aggressive prophylaxis and management of mild degrees of toxicity can minimize their effect on the quality of life for elderly patients.
Another aspect of difference between elderly and younger patients is tolerance to the additive impact of multiple adverse effects. Elderly patients may not be able to withstand an accumulation of multiple adverse effects, even mild ones, as well as younger patients might. For example, elderly patients may suffer more fatigue than usual if they have anemia [37]. Anemia occurred more frequently in elderly patients (11.4%) than in younger patients (6.8%) receiving sorafenib [16].
Similarly, age-related physiological differences can affect tolerance to adverse effects. For example, elderly patients experiencing diarrhea may become dehydrated more easily than younger patients and, therefore, suffer greater morbidity from this side effect [38]. Understanding the individual patient’s level of tolerance and goals of therapy can help tailor the most appropriate treatment regimen for that patient, as well as help guide the level of surveillance and the threshold for intervention for toxicities.
Generally speaking, elderly patients experience the same types of adverse events that younger patients experience. The management of these events, however, may differ slightly to take into account the special needs of this patient population (Fig. 1).
Skin toxicities
Hand–foot skin reaction
The incidence of hand–foot skin reaction (HFSR) was not significantly different between elderly patients and younger patients in the TARGET trial (Table 1) [16]. Similarly, no substantial differences in HFSR incidence were observed in the NA-ARCCS and EU-ARCCS studies (Table 2) [13, 17]. Many of the preventive and management recommendations for handling HFSR associated with sorafenib are universal [39]. Before starting sorafenib therapy, perform a physical examination of the patient to identify areas of hyperkeratotic skin, and we suggest that patients be advised to use pure aloe vera or urea-based creams to treat these areas. If foot problems such as calluses or ingrown toenails are present, advise patients to have these problems resolved [39]. A referral to a podiatrist may be necessary and is strongly recommended particularly for patients who are diabetic and may be predisposed to foot problems. While taking sorafenib, if HFSR occurs, lanolin- or urea-based creams may help alleviate mild symptoms [39]. If symptoms of HFSR persist or are severe, then consider discontinuing therapy until symptoms subside [39]. Then, sorafenib therapy can be restarted.
Skin rash/desquamation
If skin rash or desquamation should develop in the first weeks of treatment with sorafenib, the use of emollient creams may provide relief for mild symptoms. For severe or persistent skin rash, dose reduction or interruption may be necessary to allow symptoms to clear [28]. Then, sorafenib therapy can be re-escalated or restarted. General recommendations for dose modifications are outlined later.
Other potential cutaneous reactions
Cases of keratoacanthoma, actinic keratoses, and squamous cell carcinoma (SCC) occurring in patients taking sorafenib, including patients aged ≥65, have been reported [40–44]. Following these reports, a review of clinical safety data from sorafenib clinical studies (all tumor types) and marketed use has found keratoacanthoma and SCC of the skin to be an uncommon undesirable event occurring in 0.1 to <1% of patients taking sorafenib [28]. It is recommended that prompt recognition and referral to a dermatologist of suspected keratoacanthoma/SCC is important for early intervention to decrease the potential for additional morbidity in patients taking sorafenib [28].
Diarrhea
Treatment-related diarrhea may not occur more frequently in elderly patients than in younger patients receiving sorafenib [13, 16, 17], but the adverse effects may be more burdensome. Although preventive measures are lacking, patients for whom diarrhea is a problem can take a dose of loperamide 30 min before each dose of sorafenib [45]. If patients experience diarrhea, advise them how to remain well hydrated. Additional loperamide can be taken after each loose bowel movement, if needed [45]. Dietary changes, such as eating more bulk-producing foods and dietary supplements, such as soluble fiber, can help to alleviate symptoms [45]. Encourage patients to reach out to a healthcare provider (e.g., nurse or physician) before diarrhea becomes severe or debilitating. If diarrhea is severe, then patients may need intravenous fluids administered to combat dehydration. If diarrhea is chronic and/or severe, then consider a temporary discontinuation of sorafenib therapy.
Anorexia
In the TARGET trial, anorexia (all grades) occurred more frequently in elderly patients than in younger patients receiving sorafenib (Table 1) [16]. In the NA-ARCCS study, however, grade 3 or greater anorexia occurred at comparable rates in elderly and younger patients (Table 2) [13]. A number of physiological, psychological, and social factors can contribute to anorexia in the elderly [46]. In addition, because patients with advanced cancers also tend to be anorexic, have reduced food intake, and have increased weight loss [47], it is important to regularly monitor the weight and appetite of elderly patients with cancer. Elderly patients who live alone or are unable to cook for themselves may be able to take advantage of local social service programs that can provide meals at little or no cost to patients. A number of orexigenic agents have been studied in younger patients with cancer, but data are lacking for their efficacy and safety in elderly patients with cancer [48].
Hypertension
While the incidence of hypertension in elderly patients (aged ≥70) was comparable to that seen in younger patients receiving sorafenib in the TARGET trial (Table 1) [16] and in the NA-ARCCS and EU-ARCCS studies (Table 2) [13, 17], the potential for hypertension to develop in the elderly receiving sorafenib remains a clinically important consideration to be monitored closely. Up to 50% of patients with cancer over the age of 60 have hypertension [5, 49]. Before starting sorafenib therapy, it is important to determine baseline blood pressure and advise patients with preexisting hypertension to have their blood pressure controlled before starting on sorafenib. If hypertension worsens with sorafenib therapy, then increasing the dose of the patient’s current antihypertensive medication or adding a new medication may be necessary. If hypertension develops with sorafenib therapy and blood pressure rises above 150/90 mm Hg, then aggressive management may be warranted. According to guidelines put forth by the World Health Organization and the International Society of Hypertension, blood pressure should be maintained below 160/90 mm Hg, if possible [50]. Consider starting patients on an antihypertensive medication, such as a beta-blocker or an ACE inhibitor. As in all older patients with hypertension, choice of therapy should take into account coexisting conditions. For severe or persistent hypertension that does not improve with antihypertensive medication, a temporary or permanent discontinuation of sorafenib should be considered [28].
Therapeutic modification in the elderly patient
Dose modifications of sorafenib may be necessary in elderly patients who experience intolerable or unmanageable adverse effects. In the TARGET trial, dose reductions occurred in 21.4% of elderly patients and 11.3% of younger patients. Most dose reductions in the elderly patient group were attributable to skin toxicity, gastrointestinal events, and cardiac adverse events [16]. Dose discontinuations occurred in 21.4% of elderly patients and 8.1% of younger patients [16]. Younger patients discontinued mainly because of pulmonary or upper respiratory disorders and constitutional disorders (e.g., fatigue, fever) and elderly patients because of gastrointestinal and dermatologic concerns [16].
If a grade 3 or 4 sorafenib-related adverse event occurs, then a temporary discontinuation of treatment may be necessary to allow time for symptoms to subside. If adverse effects are severe and/or chronic, then permanent discontinuation may be warranted (Table 5) [28]. If, however, sorafenib-related adverse events are grade 1 or 2, then a dose reduction may be sufficient to alleviate symptoms. Reduce the sorafenib dose by 50% [28], to 400 mg once daily, and maintain this dose level until symptoms subside. If symptoms persist, then reduce dose by 50% again, to 400 mg once every other day (Table 5) [28].
Once symptoms clear up, sorafenib therapy can be restarted and/or re-escalated to reach the full standard dose (400 mg BID). Dose modifications should be undertaken on a case-by-case basis, with regular monitoring of patients for response and adverse reactions. Based on our clinical experience, we suggest restarting sorafenib at a reduced dose determined by clinical judgment. If the reduced dose is well tolerated and the patient’s cancer is stable, then consider maintaining this dose level for as long as the patient remains stable. If dose escalation is necessary, then increase the sorafenib steadily by 200 mg/day for several days until the full standard dose (400 mg BID) is reached (Table 5). Generally speaking, it is common for patients who have had sorafenib dose modifications to restart and/or re-escalate to the full standard dose without the return of adverse effects (Table 6).
The details of disclosures and conflicts of interest are given in “Appendix”.
Conclusion
Half of all new diagnoses of RCC are made in patients aged 65 or older. Until recently, therapies available to treat RCC were associated with toxicity severe enough to exclude many elderly patients from treatment. Sorafenib is the first multikinase inhibitor approved for use in RCC in the United States and in Europe. In a phase III clinical study, sorafenib was shown to significantly prolong PFS, extend OS, and improve quality of life, compared with placebo, regardless of patient age. Importantly, the overall incidence of adverse events was not significantly different between elderly and younger patients receiving sorafenib. Thus, elderly patients with RCC may receive clinical benefit from treatment with sorafenib, without an increase in the frequency or severity of adverse events.
Nonetheless, many elderly patients require special considerations when devising a treatment plan. Age-related physiological, cognitive, and social characteristics seen in this patient population may influence patient selection, goals of treatment, response to therapy, and the management of adverse effects. None of these factors, however, should necessarily preclude an elderly patient from treatment with sorafenib. With a proper understanding of these particular considerations, appropriate preparation and patient education, and regular monitoring for and management of adverse effects, elderly patients with RCC can benefit from sorafenib therapy.
References
Wilkins M. Cancer in the elderly patient. In: Pathy MSJ, Sinclair AJ, Morley JE, editors. Principles and Practices of Geriatric Medicine. Chichester: Wiley; 1991. p. 1385–96.
National Cancer Institute SEER Database. Cancer: renal and kidney pelvis. 2009. Available at: http://www.seer.cancer.gov./statfacts/html/kidrp.html. Accessed 16 Feb 2009.
Martin JE, Sheaff MT. The pathology of ageing: concepts and mechanisms. J Pathol. 2007;211:111–3.
Bellmunt J, et al. The medical treatment of metastatic renal cell cancer in the elderly: position paper of a SIOG Taskforce. Crit Rev Oncol Hematol. 2009;69:64–72.
Coebergh JW, Janssen-Heijnen ML, Post PN, Razenberg PP. Serious co-morbidity among unselected cancer patients newly diagnosed in the southeastern part of The Netherlands in 1993–1996. J Clin Epidemiol. 1999;52:1131–6.
Extermann M. Interaction between comorbidity and cancer. Cancer Control. 2007;14:13–22.
Taccoen X, et al. Renal cell carcinoma in adults 40 years old or less: young age is an independent prognostic factor for cancer-specific survival. Eur Urol. 2007;51:980–7.
National Cancer Institute. FDA approval for Sorafenib Tosylate. National Cancer Institute. 2007. Available at: http://www.cancer.gov/cancertopics/druginfo/fda-sorafenib-tosylate#Anchor-Kidne-39939. Accessed 7 July 2009.
European Medicines Agency. Public summary of positive opinion for orphan designation of sorafenib tosylate for the treatment of renal cell carcinoma. 2009. Available at: http://www.emea.europa.eu/pdfs/human/comp/opinion/029404en.pdf. Accessed 10 Feb 2009.
Wilhelm SM, et al. Preclinical overview of sorafenib, a multikinase inhibitor that targets both Raf and VEGF and PDGF receptor tyrosine kinase signaling. Mol Cancer Ther. 2008;7:3129–40.
Escudier B, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007;356:125–34.
Escudier B, et al. Sorafenib for treatment of renal cell carcinoma: final efficacy and safety results of the phase III Treatment Approaches in Renal Cancer Global Evaluation Trial. J Clin Oncol. 2009;27(20):3312–8.
Stadler W, et al. Safety and efficacy results of the advanced renal cell carcinoma sorafenib (ARCCS) expanded access program in North America. Cancer. 2009. (in press).
Beck J, et al. A large open-label, non-comparative, phase III study of the multi-targeted kinase inhibitor sorafenib in European patients with advanced renal cell carcinoma. Presented at ECCO 14, The European Cancer Conference, Barcelona, Spain; 23–27 September 2007. Abstract 4506.
Rosenberg SA, et al. Experience with the use of high-dose interleukin-2 in the treatment of 652 cancer patients. Ann Surg. 1989;210:474–84.
Eisen T, et al. Sorafenib for older patients with renal cell carcinoma: subset analysis from a randomized trial. J Natl Cancer Inst. 2008;100:1454–63.
Porta C, et al. Efficacy and safety of sorafenib in elderly patients: results from a large open-label, non-comparative phase III study in European patients with advanced RCC (EU-ARCCS). Ann Oncol. 2008;19(suppl 8):viii193.
National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: kidney cancer. 2009. Available at: http://www.nccn.org/professionals/physician_gls/PDF/kidney.pdf. Accessed 26 Mar 2009.
Droz JP, Chaladaj A. Management of metastatic prostate cancer: the crucial role of geriatric assessment. BJU Int. 2008;101(suppl 2):23–9.
Bulpitt CJ, et al. The assessment of biological age: a report from the Department of Environment Study. Aging (Milano). 1994;6:181–91.
Aapro MS. The frail are not always elderly. J Clin Oncol. 2005;23:2121–2.
Walter LC, et al. Development and validation of a prognostic index for 1-year mortality in older adults after hospitalization. JAMA. 2001;285:2987–94.
Maas HA, Janssen-Heijnen ML, Olde Rikkert MG, Machteld Wymenga AN. Comprehensive geriatric assessment and its clinical impact in oncology. Eur J Cancer. 2007;43:2161–9.
Passage KJ, McCarthy NJ. Critical review of the management of early-stage breast cancer in elderly women. Intern Med J. 2007;37:181–9.
Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–83.
Schmidinger M, et al. Cardiac toxicity of sunitinib and sorafenib in patients with metastatic renal cell carcinoma. J Clin Oncol. 2008;26:5204–12.
Lonardi S, Bortolami A, Stefani M, Monfardini S. Oral anticancer drugs in the elderly: an overview. Drugs Aging. 2007;24:395–410.
Bayer HealthCare Pharmaceuticals, Inc. Nexavar: highlights of prescribing information. 2009. Available at: http://www.univgraph.com/bayer/inserts/nexavar.pdf. Accessed 16 Mar 2009.
Gebhardt MW, Governali JF, Hart EJ. Drug-related behavior, knowledge, and misconceptions among a selected group of senior citizens. J Drug Educ. 1978;8:85–92.
Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353:487–97.
Bayer HealthCare Pharmaceuticals, Inc. REACH Program. 2009. Available at: http://www.bayeroncology.com/patient_resources/reach.jsp. Accessed 23 Mar 2009.
Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9:179–86.
Katz S, Akpom CA. A measure of primary sociobiological functions. Int J Health Serv. 1976;6:493–508.
Wedding U, Pientka L, Höffken K. Quality-of-life in elderly patients with cancer: a short review. Eur J Cancer. 2007;43:2203–10.
Porta C, Paglino C, Imarisio I, Bonomi L. Uncovering Pandora’s vase: the growing problem of new toxicities from novel anticancer agents. The case of sorafenib and sunitinib. Clin Exp Med. 2007;7:127–34.
Kollmannsberger C, et al. Sunitinib therapy for metastatic renal cell carcinoma: recommendations for management of side effects. Can Urol Assoc J. 2007;1(2 suppl):S41–54.
Balducci L. Epidemiology of anemia in the elderly: information on diagnostic evaluation. J Am Geriatr Soc. 2003;51(3 suppl):S2–9.
Gastrointestinal disorders: constipation, diarrhea, and fecal incontinence. In: Beers MH, Berkow R, editors. The Merck manual of geriatrics. Whitehouse Station: Merck Research Laboratories; 2000. pp. 1080–1094.
Lacouture ME, et al. Evolving strategies for the management of hand-foot skin reaction associated with the multitargeted kinase inhibitors sorafenib and sunitinib. Oncologist. 2008;13:1001–11.
Lacouture ME, et al. Inflammation of actinic keratoses subsequent to therapy with sorafenib, a multitargeted tyrosine-kinase inhibitor. Clin Exp Dermatol. 2006;31:783–5.
Kong HH, et al. Keratoacanthomas associated with sorafenib therapy. J Am Acad Dermatol. 2007;56:171–2.
Autier J, Escudier B, Wechsler J, Spatz A, Robert C. Prospective study of the cutaneous adverse effects of sorafenib, a novel multikinase inhibitor. Arch Dermatol. 2008;144:886–92.
Dubauskas Z, et al. Cutaneous squamous cell carcinoma and inflammation of actinic keratoses associated with sorafenib. Clin Genitourin Cancer. 2009;7:20–3.
Arnault JP, et al. Keratoacanthomas and squamous cell carcinomas in patients receiving sorafenib. J Clin Oncol. 2009;27(23):e59–61.
Wood LS, Manchen B. Sorafenib: a promising new targeted therapy for renal cell carcinoma. Clin J Oncol Nurs. 2007;11:649–56.
Morley JE. Pathophysiology of anorexia. Clin Geriatr Med. 2002;18:661–73.
Tchekmedyian NS, Zahyna D, Halpert C, Heber D. Clinical aspects of nutrition in advanced cancer. Oncology. 1992;49(suppl 2):3–7.
Golden AG, Daiello LA, Silverman MA, Llorente M, Preston RA. University of Miami Division of Clinical Pharmacology Therapeutic Rounds: medications used to treat anorexia in the frail elderly. Am J Ther. 2003;10:292–8.
McNeil JJ, Silagy CA. Hypertension in the elderly: epidemiology and pathophysiology. Cardiovasc Drugs Ther. 1991;4(suppl 6):1197–201.
World Health Organization, International Society of Hypertension Writing Group. World Health Organization (WHO)/International Society of Hypertension (ISH) statement on management of hypertension. J Hypertens. 2003;21:1983–92.
Acknowledgments
The development of this article was supported by Bayer HealthCare Pharmaceuticals. This article is based upon an expert panel discussion with the authors held on February 26, 2009, supported by Bayer HealthCare. Envision Communications, LLC, provided writing and editorial support in the development of this article, funded by Bayer HealthCare.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
Disclosures/Conflicts of interest | JPD | NT | JB | BE |
|---|---|---|---|---|
Employment/leadership position | – | – | – | – |
Intellectual property rights/inventor/patent holder | – | – | – | – |
Consultant/advisory role | b,c,e,g | – | – | a,e,f |
Honoraria | b,d,e,g | b | a,d,e–g | a,c–f |
Research funding/contracted research | b–e,g | – | – | – |
Ownership interest | – | – | – | – |
Expert testimony | – | – | – | – |
Other | – | – | – | – |
Rights and permissions
About this article
Cite this article
Dutcher, J.P., Tannir, N., Bellmunt, J. et al. Experience with sorafenib and the elderly patient. Med Oncol 27, 1359–1370 (2010). https://doi.org/10.1007/s12032-009-9388-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12032-009-9388-4