Abstract
The aim of this study was to describe the characteristics and outcomes of a large cohort of patients treated with sorafenib in clinical practice and to identify predictive factors associated with prognosis. Patient data were obtained from the national Czech registry (RenIS). Data of virtually all Czech patients receiving targeted therapies are entered into this non-interventional post-registration database. Demographics and clinical data, as well as all treatment sequences and clinical outcomes, are reported in this registry. A total of 836 patients treated with sorafenib before March 2013 were included in the analysis. Median age was 63 years and 70 % were men. Most patients had received prior treatment with cytokines, sunitinib or both. Sorafenib was the first-line treatment in 15 % of patients. Median overall survival and progression-free survival were 21.7 months and 7.5 months, respectively. Median overall survival and progression-free survival was 26.3 and 8.3 months, respectively, in patients receiving sorafenib as first-line therapy. Cox proportional models identified several parameters associated with poor outcome including time ≤1 year from diagnosis to first-line systemic treatment, performance status ≥2, low hemoglobin, and LDH >1.5 times the upper limit of normal. Our data demonstrate that the outcomes of real-life patients are comparable to those enrolled in clinical trials. Prognostic factors identified in the present study were consistent with previously reported models.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Over 200,000 new patients with renal cell carcinoma (RCC) are diagnosed each year worldwide [1]. RCC predominantly affects men (2:1 ratio) and the median age at diagnosis is approximately 60 years [1]. The outcome of RCC is dependent of the disease stage at diagnosis, with the estimated 5-year survival rates of 96 % for patients diagnosed with stage I, 82 % for stage II, 64 % for stage III, and 23 % for stage IV RCC patients [2].
The principal therapeutic modality for localized and advanced RCC is surgical resection [1, 3, 4]. Systemic therapy is usually considered for patients presenting with unresectable or metastatic RCC (mRCC) [1, 3, 4]. Until recently, cytokines had been the only systemic therapy available for mRCC [5]. However, the activity of cytokines is limited. New targeted agents have been introduced to the management of mRCC over the last decade [6]. The oral multitargeted tyrosine kinase inhibitor sorafenib (Nexavar®) was one of the first targeted agents to demonstrate a statistically significant progression-free survival (PFS) benefit in mRCC [7–9]. Based on findings from phase II and phase III trials, sorafenib was approved for the use in mRCC in 2005 in the USA and in 2006 in the European Union [10–12]. Sorafenib is recommended as second-line treatment after cytokine failure in patients with relapsed or medically unresectable stage IV predominantly clear cell RCC [3, 13]. It has also been used with some success in patients progressing on other targeted agents [14, 15].
The Czech Republic has reported the highest renal cancer and mortality in the world: incidence of 25 cases per 100,000 and mortality of 10 cases per 100,000. All Czech patients treated with targeted therapy are entered into a national registry “RenIS registry” and clinical outcomes are reported [16, 17]. This registry allows for the assessment and monitoring of patients treated with targeted agents in clinical practice. The aim of the present study was (i) to describe the characteristics and outcomes of patients treated with sorafenib in clinical practice, (ii) to compare the outcomes between the different lines of treatment, and (iii) to assess the prognostic factors based on the data from the national RenIS registry.
Patients and methods
The RenIS registry (Renal Information System, http://renis.registry.cz) is a non-interventional post-registration database of RCC patients treated with targeted agents in the Czech Republic. Seven targeted agents are currently approved and reimbursed in the Czech Republic, including sorafenib, sunitinib, temsirolimus, everolimus, bevacizumab, pazopanib, and axitinib. The administration of targeted therapy is restricted to 20 specialized comprehensive cancer centers that accepted to participate in the registry. The project was initiated in June 2007 by the Czech Cancer Society. A detailed description of the RenIS registry has been published earlier [17].
All patients who started treatment with sorafenib before 25 March 2013 were included in the present analysis. Collected data included demographics, tumor characteristics, prior anticancer therapies, clinical parameters, and treatment response and outcomes. Fully anonymized patient data were entered into the database by the participating centers. The database has been approved by Ethical Committees of the participating centers.
Standard descriptive statistics were used to characterize the sample data set. Overall survival (OS) and progression-free survival (PFS) were the primary endpoints of this study. OS was defined as the time from sorafenib treatment initiation to death from any cause. PFS was defined as the time from sorafenib treatment initiation to progression or death from any cause. Both outcome measures were estimated using the Kaplan-Meier method. Statistical significance of the differences in Kaplan-Meier estimates was assessed with the log-rank test. Univariate and multivariate Cox proportional hazards models were used to evaluate the effect of all potential predictive and prognostic factors on the survival measures. Hazard ratio was estimated with appropriate 95 % confidence intervals (CI) and significance levels.
Results
Demographics and patient characteristics
A total of 836 patients treated with sorafenib were included in the present analysis. Baseline characteristics are shown in Table 1. Median age at the initiation of sorafenib was 63 years. Most patients were men (70 %).
Clear cell carcinoma was the most frequent histology (96 %). Almost half of patients had synchronous metastases at diagnosis and 97 % at the time of targeted therapy initiation.
Nephrectomy was performed in 88 % (n = 738) of patients. Most patients received prior immunotherapy (n = 648; 78 %) as neoadjuvant and palliative therapy. Of these patients, 62 % (n = 522) received interferon-α monotherapy (between 5 and 9 MIU three times weekly) and 13 % (n = 110) received an interleukin-2, interferon-α, and 5-fluorouracil combination. Almost one third (n = 290; 35 %) received prior targeted therapy (Fig. 1).
At the time of sorafenib initiation, 165 patients (22 %) were asymptomatic (performance status [PS] 0), 488 (65 %) had minimal symptoms (PS 1), and 96 (13 %) had poor performance status (PS ≥2).
Sorafenib treatment
Details on sorafenib administration and therapeutic response are summarized in Table 2. Sorafenib was used as first-line treatment in 15 % (n = 125) of patients and as second-line treatment in 65 % (n = 541). The prescribed dose was 800 mg orally in two daily doses in most patients (n = 630; 76 %). Median duration of sorafenib treatment was 5.2 months in all patients.
As of the cutoff date for this analysis, treatment with sorafenib was discontinued in 91 % (n = 758) of patients. The most common reasons for sorafenib discontinuation was disease progression (n = 511; 67 %) and occurrence of adverse events (n = 132; 17 %).
Complete and partial remissions were observed in 2 % (n = 17) and 17 % (n = 140) of all patients, respectively. Stable disease was reported in 34 % (n = 280) of patients. The response was not evaluable in 154 patients (19 %).
During sorafenib treatment, adverse events were reported in 405 patients (48 %). Of these, 122 experienced serious adverse events. Main reported adverse events were skin toxicity (28 %) and gastrointestinal complications (19 %) (Table 3).
Overall survival (OS) and progression-free survival (PFS)
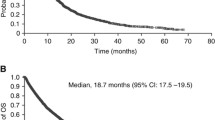
As of 25 March 2013, 446 patients (53 %) died and 138 (17 %) were lost to follow up. Median OS and median PFS was 21.7 and 7.5 months, respectively. One- and 2-year OS rates were 69.0 % (95 % confidence interval (CI): 65.7–72.4) and 47.5 % (95 % CI: 43.7–51.4), respectively. One- and 2-year PFS were 34.1 % (95 % CI: 30.8–37.5) and 16.3 % (95 % CI: 13.6–19.1), respectively. OS and PFS results with Kaplan-Meier survival curves are shown in Fig. 2.
OS and PFS were also analyzed according to the prior treatment. Median OS was significantly better in patients having received no prior therapy or prior cytokines only compared with those with prior sunitinib only or prior cytokines and sunitinib treatment. One-year OS rates were 71.6 % (95 % CI: 63.2; 80.0) and 72.3 % (95 % CI: 67.8–76.8) in patients with no prior therapy and in patients having received prior cytokines only, respectively. Similarly median PFS was significantly longer in patients having received no prior therapy or prior cytokines only. OS and PFS results according to the line of treatment are summarized in Table 4. Kaplan-Meier survival curves are shown in Fig. 3 according to the line of treatment.
Assessment of predictive factors for OS and PFS
In the univariate Cox analysis, factors significantly associated with inferior OS included absence of nephrectomy, stage IV disease at diagnosis, time from diagnosis to first-line systemic treatment initiation ≤1 year, PS ≥1, hemoglobin < lower limit of normal (LLN), LDH >1.5× the upper limit of normal (ULN), thrombocytes >400 × 109/l, and neutrophils >7 × 109/l (Table 5). There was a borderline significant association of poor OS with calcium >2.5 mmol/l. All parameters, with the exception of the stage at diagnosis, were then entered into a multivariate model (Table 6). The stage at diagnosis was highly correlated with the time from diagnosis to first-line treatment, and this parameter was not included in the multivariate model. Subsequent multivariate analysis identified five factors that were independently predictive for poorer OS in patients treated with sorafenib: time from diagnosis to first-line systemic therapy ≤1 year, Eastern Cooperative Oncology Group Performance Status (ECOG) PS 1, hemoglobin < LLN, LDH >1.5× ULN, and neutrophil count >7 × 109/l. Given the limited number of patients with available laboratory values, this model was based on data from only 259 patients (31 %). Therefore, another model excluding the laboratory values was planned (n = 749; 90 % patients). In this latter model, three factors were identified as independent predictors of worse OS, including time from diagnosis to first-line therapy ≤1 year, sorafenib as third- or fourth-line treatment, and ECOG PS ≥1.
Factors significantly associated with inferior PFS included time from diagnosis to first-line systemic treatment initiation ≤1 year, poor PS, hemoglobin < LLN, LDH >1.5× ULN, thrombocytes >400 × 109/l, and neutrophils >7 × 109/l (Table 5). Again, there was a borderline significant trend towards the worse PFS in patients with calcium >2.5 mmol/l. As for the OS, two multivariate Cox models were calculated that either included or excluded laboratory parameters. In the first model (n = 259), four parameters found to independently predict inferior PFS included time from diagnosis to first-line therapy ≤1 year, sorafenib as third- or fourth-line treatment, poor ECOG PS (1–3), and LDH >1.5× ULN. Among these four parameters that were significantly predictive of PFS in the first model, the first three variables were also significant predictors of PFS in the second model (Table 7).
Discussion
The present analysis issued from a national population-based registry reflects the clinical practice and the management of patients with metastatic RCC. The results show that the efficacy and safety of sorafenib in real-life practice in the Czech Republic are comparable, or slightly better, than the outcomes observed in selected patients enrolled in clinical trials. This observation may reflect the patient selection process in a health-care system with limited resources. Improved management of side effects and availability of active agents for patients failing sorafenib treatment might have contributed to better outcomes. Since targeted therapy is provided by restricted number of centers in the Czech Republic, the patients are therefore thoroughly followed by the oncologists, without substantial involvement of the general practitioners, allowing for close monitoring and reporting of adverse events and complications.
The present data showed also that the use of sorafenib in the Czech Republic was generally in accordance with the European and US guidelines [3, 13]. No particular safety concerns were raised regarding the use of sorafenib. Almost all patients treated with sorafenib had clear cell RCC. The majority of patients received sorafenib as second-line treatment after cytokines or after sunitinib (65 %), while 20 % were treated in the third-line or higher after cytokines and sunitinib. A minor, but non-negligible proportion of patients were treated with sorafenib as first-line treatment. Clinical outcomes, including OS and PFS, were significantly better in patients on first-line treatment or second-line treatment after cytokines compared with other treatment sequences.
In a recent phase III randomized trial evaluating the efficacy and safety of sorafenib versus axitinib, a second-generation VEGFR inhibitor, as first-line treatment in patients with mRCC, PFS was comparable between groups [18]. Other studies showed also that PFS and OS were not significantly different between the sorafenib and sunitinib groups, as first-line treatment for patients with mRCC [19]. The recently completed phase III SWITCH study showed that the sequence sorafenib-sunitinib is equivalent to sunitinib-sorafenib, in terms of OS and combined PFS [20]. Sorafenib has also shown activity in other randomized studies, including those patients progressing on sunitinib [21] and on VEGF-targeted agent or a mammalian target of rapamycin (mTOR) inhibitors [15]. These reported results together with our results suggest that sorafenib might be a fair alternative as a VEGF-targeted therapy for the second and later lines and possibly even in the first line.
Given the fact that sunitinib and sorafenib were the first two targeted agents for mRCC introduced in the Czech Republic, it is not surprising that most patients receiving sorafenib were also treated, in sequence with sunitinib. Of note, the optimal sequence for sorafenib and sunitinib was recently evaluated in mRCC [22]; the sorafenib-sunitinib sequence reported a longer combined PFS compared with the sunitinib-sorafenib sequence. In general, physicians would feel more confident prescribing sorafenib and sunitinib, than newly discovered agents; which may explain the use of sorafenib as a first-line treatment in a number of patients (15 %), although it is not commonly recommended. Data from other analyses based on the RenIS registry showed that over 10 % of patients were treated with everolimus in second- or third-line setting. Bevacizumab and temsirolimus were rarely administered. It should be noted that reimbursement of pazopanib and axitinib was approved in the Czech Republic only in 2011 and 2013, respectively.
Compared with the general population of Czech patients diagnosed with RCC, this analysis revealed that sorafenib tended to be administered to younger patients, indicating possible patient selection. According to the epidemiological data from the Czech National Cancer Registry [23], almost 53 % of patients are aged over 65 years at diagnosis, while in the present report, the proportion of patients aged over 65 years at sorafenib treatment initiation was lower (41 %). The majority of patients had a good performance status at sorafenib initiation (PS 0–1).
The present strategy of mRCC management is, to a large extent, determined by patient stratification. The Memorial Sloan-Kettering Cancer Center (MSKCC) model is the most widely used prognostic system [3, 13]. This model, established by Motzer et al., classifies patients according to the presence or absence of five adverse prognostic factors, including Karnofsky performance status of 70 or less, serum LDH level greater than 1.5× ULN, low hemoglobin level, corrected serum calcium level above the ULN, and time from diagnosis and nephrectomy to therapy of less than 1 year [24]. Another study confirmed the validity of this model in patients treated with targeted agents [25]. Independent prognostic parameters identified in the present study are almost identical to MSKCC criteria, despite the fact that the population in the present analysis was very heterogeneous, with most patients pre-treated with different agents and only a minority received sorafenib as first-line treatment.
However, our study has some limitations, including the retrospective design and the number of missing data, particularly in laboratory values. Like other retrospective studies, a potential selection bias could not be excluded. The treatment was administered at the discretion of the attending medical oncologist in the centers, and there was no source data verification. Consequently, the data on progression-free survival may be biased, while the data on overall survival that are checked against the national database of deaths must be considered as reliable. On the other hand, the strength of this study was its ability to reproduce the outcomes observed in prospective trials. Despite all these potential biases, these real-world results acquired from a nationwide Czech registry could be useful either for comparing the routine practice with trials results or for generating valuable hypotheses.
In conclusion, the present data describe the use of sorafenib in the management of mRCC in clinical practice. The outcomes of real-life patients, including PFS and OS data, were comparable to those achieved in prospective trials [4, 8, 26, 27].
References
Rini BI, Campbell SC, Escudier B (2009) Renal cell carcinoma. Lancet 373(9669):1119–1132. doi:10.1016/S0140-6736(09)60229-4
Hudes GR, Carducci MA, Choueiri TK, Esper P, Jonasch E, Kumar R, Margolin KA, Michaelson MD, Motzer RJ, Pili R, Roethke S, Srinivas S (2011) NCCN Task Force report: optimizing treatment of advanced renal cell carcinoma with molecular targeted therapy. J Natl Compr Cancer Netw 9(Suppl 1):S1–S29
Escudier B, Eisen T, Porta C, Patard JJ, Khoo V, Algaba F, Mulders P, Kataja V (2012) Renal cell carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 23(Suppl 7):vii65–vii71. doi:10.1093/annonc/mds227
Escudier B, Szczylik C, Hutson TE, Demkow T, Staehler M, Rolland F, Negrier S, Laferriere N, Scheuring UJ, Cella D, Shah S, Bukowski RM (2009) Randomized phase II trial of first-line treatment with sorafenib versus interferon Alfa-2a in patients with metastatic renal cell carcinoma. J Clin Oncol 27(8):1280–1289. doi:10.1200/JCO.2008.19.3342
Krown SE (1987) Interferon treatment of renal cell carcinoma. Current status and future prospects. Cancer 59(3 Suppl):647–651
Coppin C, Kollmannsberger C, Le L, Porzsolt F, Wilt TJ (2011) Targeted therapy for advanced renal cell cancer (RCC): a Cochrane systematic review of published randomised trials. BJU Int 108(10):1556–1563. doi:10.1111/j.1464-410X.2011.10629.x
Escudier B, Eisen T, Stadler WM, Szczylik C, Oudard S, Siebels M, Negrier S, Chevreau C, Solska E, Desai AA, Rolland F, Demkow T, Hutson TE, Gore M, Freeman S, Schwartz B, Shan M, Simantov R, Bukowski RM (2007) Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med 356(2):125–134. doi:10.1056/NEJMoa060655
Escudier B, Eisen T, Stadler WM, Szczylik C, Oudard S, Staehler M, Negrier S, Chevreau C, Desai AA, Rolland F, Demkow T, Hutson TE, Gore M, Anderson S, Hofilena G, Shan M, Pena C, Lathia C, Bukowski RM (2009) Sorafenib for treatment of renal cell carcinoma: final efficacy and safety results of the phase III treatment approaches in renal cancer global evaluation trial. J Clin Oncol 27(20):3312–3318. doi:10.1200/JCO.2008.19.5511
Ratain MJ, Eisen T, Stadler WM, Flaherty KT, Kaye SB, Rosner GL, Gore M, Desai AA, Patnaik A, Xiong HQ, Rowinsky E, Abbruzzese JL, Xia C, Simantov R, Schwartz B, O’Dwyer PJ (2006) Phase II placebo-controlled randomized discontinuation trial of sorafenib in patients with metastatic renal cell carcinoma. J Clin Oncol 24(16):2505–2512. doi:10.1200/JCO.2005.03.6723
FDA (2005) FDA approves new treatment for advanced kidney cancer. Silver Spring, MD 20993
Kane RC, Farrell AT, Saber H, Tang S, Williams G, Jee JM, Liang C, Booth B, Chidambaram N, Morse D, Sridhara R, Garvey P, Justice R, Pazdur R (2006) Sorafenib for the treatment of advanced renal cell carcinoma. Clin Cancer Res 12(24):7271–7278. doi:10.1158/1078-0432.CCR-06-1249
EMA (2006) Sorafenib Marketing Authorisation
Motzer RJ, Agarwal N, Beard C, Bhayani S, Bolger GB, Carducci MA, Chang SS, Choueiri TK, Hancock SL, Hudes GR, Jonasch E, Josephson D, Kuzel TM, Levine EG, Lin DW, Margolin KA, Michaelson MD, Olencki T, Pili R, Ratliff TW, Redman BG, Robertson CN, Ryan CJ, Sheinfeld J, Spiess PE, Wang J, Wilder RB (2011) Kidney cancer. J Natl Compr Cancer Netw 9(9):960–977
Rini BI, Escudier B, Tomczak P, Kaprin A, Szczylik C, Hutson TE, Michaelson MD, Gorbunova VA, Gore ME, Rusakov IG, Negrier S, Ou YC, Castellano D, Lim HY, Uemura H, Tarazi J, Cella D, Chen C, Rosbrook B, Kim S, Motzer RJ (2011) Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (AXIS): a randomised phase 3 trial. Lancet 378(9807):1931–1939. doi:10.1016/S0140-6736(11)61613-9
Motzer RJ, Porta C, Vogelzang NJ, Sternberg CN, Szczylik C, Zolnierek J, Kollmannsberger C, Rha SY, Bjarnason GA, Melichar B, De Giorgi U, Grunwald V, Davis ID, Lee JL, Esteban E, Urbanowitz G, Cai C, Squires M, Marker M, Shi MM, Escudier B (2014) Dovitinib versus sorafenib for third-line targeted treatment of patients with metastatic renal cell carcinoma: an open-label, randomised phase 3 trial. Lancet Oncol 15(3):286–296. doi:10.1016/S1470-2045(14)70030-0
Buchler T, Klapka R, Melichar B, Brabec P, Dusek L, Vyzula R, Abrahamova J (2012) Sunitinib followed by sorafenib or vice versa for metastatic renal cell carcinoma—data from the Czech registry. Ann Oncol 23(2):395–401. doi:10.1093/annonc/mdr065
Poprach A, Bortlicek Z, Buchler T, Melichar B, Lakomy R, Vyzula R, Brabec P, Svoboda M, Dusek L, Gregor J (2012) Patients with advanced and metastatic renal cell carcinoma treated with targeted therapy in the Czech Republic: twenty cancer centres, six agents, one database. Med Oncol 29(5):3314–3320. doi:10.1007/s12032-012-0286-9
Hutson TE, Lesovoy V, Al-Shukri S, Stus VP, Lipatov ON, Bair AH, Rosbrook B, Chen C, Kim S, Vogelzang NJ (2013) Axitinib versus sorafenib as first-line therapy in patients with metastatic renal-cell carcinoma: a randomised open-label phase 3 trial. Lancet Oncol 14(13):1287–1294. doi:10.1016/S1470-2045(13)70465-0
Park SJ, Lee JL, Park I, Park K, Ahn Y, Ahn JH, Lee DH, Ahn S, Song C, Hong JH, Kim CS, Ahn H (2012) Comparative efficacy of sunitinib versus sorafenib as first-line treatment for patients with metastatic renal cell carcinoma. Chemotherapy 58(6):468–474. doi:10.1159/000346484
Michel SM, Vervenne W, de Santis M, von Weikersthal LF, Goebell PJ, Lerchenmueller J, Zimmermann U (2014) SWITCH: a randomized sequential open-label study to evaluate efficacy and safety of sorafenib (SO)/sunitinib (SU) versus SU/SO in the treatment of metastatic renal cell cancer (mRCC). J Clin Oncol 32(suppl 4; abstr 393)
Hutson TE, Escudier B, Esteban E, Bjarnason GA, Lim HY, Pittman KB, Senico P, Niethammer A, Lu DR, Hariharan S, Motzer RJ (2014) Randomized phase III trial of temsirolimus versus sorafenib as second-line therapy after sunitinib in patients with metastatic renal cell carcinoma. J Clin Oncol 32(8):760–767. doi:10.1200/JCO.2013.50.3961
Stenner F, Chastonay R, Liewen H, Haile SR, Cathomas R, Rothermundt C, Siciliano RD, Stoll S, Knuth A, Buchler T, Porta C, Renner C, Samaras P (2012) A pooled analysis of sequential therapies with sorafenib and sunitinib in metastatic renal cell carcinoma. Oncology 82(6):333–340. doi:10.1159/000338001
Epidemiology of malignant tumors in the Czech Republic (1977–2008) http://www.svod.cz/
Motzer RJ, Bacik J, Murphy BA, Russo P, Mazumdar M (2002) Interferon-alfa as a comparative treatment for clinical trials of new therapies against advanced renal cell carcinoma. J Clin Oncol 20(1):289–296
Patil S, Figlin RA, Hutson TE, Michaelson MD, Negrier S, Kim ST, Huang X, Motzer RJ (2011) Prognostic factors for progression-free and overall survival with sunitinib targeted therapy and with cytokine as first-line therapy in patients with metastatic renal cell carcinoma. Ann Oncol 22(2):295–300. doi:10.1093/annonc/mdq342
Bracarda S, Porta C, Boni C, Santoro A, Mucciarini C, Pazzola A, Cortesi E, Gasparro D, Labianca R, Di Costanzo F, Falcone A, Cinquini M, Caserta C, Paglino C, De Angelis V (2013) Could interferon still play a role in metastatic renal cell carcinoma? A randomized study of two schedules of sorafenib plus interferon-alpha 2a (RAPSODY). Eur Urol 63(2):254–261. doi:10.1016/j.eururo.2012.08.027
Naito S, Tsukamoto T, Murai M, Fukino K, Akaza H (2011) Overall survival and good tolerability of long-term use of sorafenib after cytokine treatment: final results of a phase II trial of sorafenib in Japanese patients with metastatic renal cell carcinoma. BJU Int 108(11):1813–1819. doi:10.1111/j.1464-410X.2011.10281.x
Acknowledgments
The authors would like to thank the following department representatives of the Comprehensive Cancer Centers which contributed to the RENIS project: Prof. MUDr. Jiří Petera, Ph.D (Fakultní nemocnice Hradec Králové); Prof. MUDr. Jindřich Fínek, Ph.D (Fakultní nemocnice Plzeň); Prof. MUDr. Luboš Petruželka, CSc (Všeobecná fakultní nemocnice v Praze); Prim. MUDr. Jiří Bartoš, MBA (Krajská nemocnice Liberec, a.s); Doc. MUDr. David Feltl, Ph.D., MBA (Fakultní nemocnice Ostrava); Prim. MUDr. Jana Katolická, Ph.D. (Fakultní nemocnice u sv. Anny v Brně); Prim. MUDr. Lubomír Slavíček (Nemocnice Jihlava); Prim. MUDr. Milan Kohoutek (Krajská nemocnice T. Bati, a.s.); Prim. MUDr. Václav Janovský (Nemocnice České Budějovice, a.s.); Prof. MUDr. Bohuslav Melichar, Ph.D. (Fakultní nemocnice Olomouc); Prof. MUDr. Jiří Vorlíček, CSc. (Fakultní Masarykův onkologický ústav Brno); Prof. MUDr. Jiří Mayer, CSc (Fakultní nemocnice Brno); Prim. doc. MUDr. Renata Soumarová, Ph.D., MBA (Nemocnice s poliklinikou v Novém Jičíně); Prim. MUDr. Milan Lysý (Masarykova nemocnice v Ústí nad Labem, o.z); Prim. MUDr. Martina Chodacká (Nemocnice Chomutov); Prim. doc. MUDr. Jaroslav Vaňásek, CSc. (Pardubická krajská nemocnice, a.s.); MUDr. Martin Šafanda (Nemocnice Na Homolce); and Prim. pplk. MUDr. Petr Cínek, Ph.D. (Ústřední vojenská nemocnice Praha).
The authors also thank Abir Tadmouri, PhD (ClinSearch) for providing editorial assistance in the preparation of the manuscript.
Conflict of interest
Katerina Kubackova received travel grants and lecture honoraria from Bayer and Pfizer.
Author information
Authors and Affiliations
Consortia
Corresponding author
Rights and permissions
About this article
Cite this article
Kubackova, K., Bortlicek, Z., Pavlik, T. et al. Prognostic factors in renal cell carcinoma patients treated with sorafenib: results from the Czech registry. Targ Oncol 10, 385–392 (2015). https://doi.org/10.1007/s11523-014-0343-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11523-014-0343-8