Abstract
Mesenchymal stem cells (MSCs)-derived exosomes play significant roles in alleviating spinal cord injury (SCI). Previous study showed that long non-coding RNA tectonic family member 2 (TCTN2) was able to relieve SCI. Herein, whether TCTN2 exerted its roles in functional recovery after SCI via exosomes derived from MSCs was explored. The SCI model was established in rats, and the neurological function was evaluated using the Basso, Beattie, and Bresnahan (BBB) scoring. Lipopolysaccharide (LPS)-induced differentiated PC12 cells were used as an in vitro model for neurotoxicity research. The expression of genes and proteins was detected by qRT-PCR and Western blot. Exosomes were isolated by ultracentrifugation and qualified by TEM and Western blot. In vitro assays were performed using CCK-8 assay, EdU assay, and flow cytometry, respectively. Dual-luciferase reporter assay and RIP assay were used to confirm the target relationship between miR-329-3p and TCTN2 or insulin-like growth factor1 receptor (IGF1R). TCTN2 expression was down-regulated in SCI model rat and lipopolysaccharide (LPS)-stimulated PC12 cells. MSCs produced exosomes and could package TCTN2 into secreted exosomes. Tail vein injection of TCTN2 exosomes into rats significantly improved functional recovery of SCI. Meanwhile, TCTN2 exosomes treatment alleviated LPS-induced neuronal apoptosis, inflammation, and oxidative stress in vitro. Additionally, TCTN2 targeted miR-329-3p and subsequently regulated the expression of its target IGF1R. Rescue assays suggested that miR-329-3p/IGF1R axis mediated the beneficial effects of TCTN2 exosomes on LPS-treated PC12 cells. In all, exosomes derived from TCTN2-modified MSCs could improve functional recovery of SCI in vivo and attenuate LPS-induced neuronal apoptosis, inflammation, and oxidative stress in vitro via miR-329-3p/IGF1R axis, suggesting a novel insight into the development of MSC-exosomes-based therapy for SCI.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Traumatic spinal cord injury (SCI) is a kind of fatal injury resulting in irreversible neurological damage, dysfunction, and necrosis (Cui et al. 2017). It affects more than 2.5 million people through the world and can cause permanent changes in strength, sensation, and other body functions (Fakhoury 2015). SCI is the consequence of primary mechanical injury followed by secondary injury, a cascade of biochemical and cellular processes, including the activation of neuroinflammation, apoptosis, free radical-mediated peroxidation, vascular ischemia, and lipid peroxidation (Chang et al. 2014; Popovich 2014; Witiw and Fehlings 2015). Current treatment options are largely limited to supportive measures, and development of effective treatments for SCI is urgent.
Recently, cell transplantation, such as mesenchymal stem cells (MSCs), has been investigated as promising strategy for improving functional recovery after SCI (Mothe and Tator 2012; Assinck et al. 2017). MSCs are self-renewing multi-potent stem cells, which possess potent immunomodulatory properties and multi-directional differentiation ability, holding great potential for cellular therapy and regenerative medicine (Horwitz et al. 2005; Samsonraj et al. 2017). However, the direct transplantation of MSCs to target sites remains challenging due to its low survival rate in ischemic area (Phinney and Prockop 2007; Martin-Rendon et al. 2008). Interestingly, recent findings have suggested that transplanted MSCs exert therapeutic effects mainly through a paracrine mechanism (Katsuda et al. 2013; Camussi et al. 2010). Exosomes are important components of cell paracrine secretion and play a crucial role in paracrine effects (Hessvik and Llorente 2018). Exosomes are nano-sized liposomes with a diameter of 40–150 nm, which are actively secreted from different mammalian cell types (Zhou et al. 2018). They are natural information carriers and mediate the transport of exosomal surface proteins and complex biofunctional cargoes, including lipids, proteins, and RNAs, to cells in local environments or distant metastatic sites, affecting the biological function of recipient cells (Lugea and Waldron 2017; Roma-Rodrigues et al. 2014; Milane et al. 2015; Kosaka et al. 2016). MSCs are the most common cell types known to produce exosomes; MSC-derived exosomes have been demonstrated to improve SCI via regulating inflammation, suppressing apoptosis, and facilitating angiogenesis; more importantly, MSC-exosomes can be also employed to deliver drugs or genetic material to target cells (Ren et al. 2020).
Long non-coding RNAs (lncRNAs) are non-coding transcripts with a length of more than 200 nucleotides, which are demonstrated to be involved in various physiological and pathological processes, and abnormal expression of lncRNAs is associated with the process of SCI (Zhang et al. 2018; Bai et al. 2020). LncRNA tectonic family member 2 (TCTN2) is a functional RNA; Ren et al. revealed that TCTN2 stimulated autophagy in neurons, thereby moderating neuronal apoptosis and improving SCI (Ren et al. 2019). However, whether TCTN2 exerted its roles in functional recovery after SCI via MSC-exosomes remains vague.
Here, the goal of this research was to clarify the potential effects of exosomes from TCTN2-modified MSCs on SCI, using the lipopolysaccharide (LPS)-induced differentiated PC12 cell line, which is derived from pheochromocytoma and broadly used as an in vitro model for neurotoxicity research (Greene et al. 1987), and further investigate the possible underlying mechanisms.
Materials and Methods
Animals
Adult male Sprague-Dawley (SD) rats (N = 30, age: 6–8 weeks; weight: 250–300 g) were purchased from Charles River Labs (Beijing, China). The animal experimental protocols were approved by the Institutional Animal Care and Use Committee of Hainan General Hospital and complied in line with the Guide for the Care and Use of Laboratory Animals proposed by the National Institutes of Health (NIH).
Cell Culture
PC12 cells were purchased from American Type Culture Collection (ATCC, CRL-1721, Rockville, MD, USA) and grown in 5% CO2 atmosphere at 37 ℃ with RPMI-1640 medium (ATCC) containing 5% fetal bovine serum (FBS) (ATCC) and 10% heat-inactivated horse serum (ATCC). Two days after culture in the growth medium, the medium was changed to differentiation medium (DMEM supplemented with 1% horse serum, 1% streptomycin/penicillin, 50 ng/mL NGF) for 3 days, and the culture medium was replaced with fresh medium every other day (Wang et al. 2017).
Primary rat bone marrow MSCs were isolated from SD rat by flushing the femurs and tibias with cold phosphate-buffered saline (PBS). After centrifugation, the pellet was suspended in culture medium containing Dulbecco’s Modified Eagle Medium (DMEM; Life Technologies, Scotland, UK) plus 100 U/mL of penicillin and streptomycin (Life Technologies) and 10% FBS (ATCC) at 37 ℃ with 5% CO2. Fresh culture medium was replaced every 3 days. MSCs from passages 3–5 were used for exosomes isolation.
Cell Treatment
The PC12 cells were stimulated with LPS (1 μg/mL, Invitrogen, Hemel Hempstead, UK) for 24 h to mimic the situation of SCI in vivo. After that, cells were collected for subsequent experiments.
Cell Transfection
The pcDNA3.1 TCTN2 overexpressing plasmid (TCTN2) and negative control (pcDNA), siRNA for TCTN2 (si-TCTN2) or IGF1R (si-IGF1R) and the negative control (si-NC), miR-329-3p mimic (miR-329-3p), and inhibitor (anti-miR-329-3p) or negative control (miR-NC or anti-miR-NC) were synthesized by GeneCopoepia Biosciences (Shanghai, China). Then, transient transfected in PC12 cells with 50 nM of miRNA mimic, inhibitor, or negative control or 100 nM of si-TCTN2, si-IGF1R, or si-NC was implemented using Lipofectamine 3000 (Invitrogen). MSCs were transfected with 200 ug of TCTN2 or pcDNA in serum-free medium with Lipofectamine 3000 (Invitrogen). Then, transfected cell were collected for functional experiments or exosomes isolation.
Preparation of MSC-Derived TCTN2 Exosomes (EXO-TCTN2)
When transfected MSCs reached ~ 80% confluency, an exosome-depleted FBS-DMEM was applied, and cells were incubated for another 48 h. To harvest exosomes, the conditioned culture medium was centrifuged sequentially at 4000×g for 10 min and 17,000×g for 1 h at 4 ℃. After filtering with a 0.22-μm pore filter, the filtrate was further ultracentrifuged at 4 ℃at 200,000×g for 1 h. The resulting pellet was resuspended in PBS and again re-ultrafiltrated at 200,000×g for 1 h at 4 ℃. Then exosomes (EXO-TCTN2 or EXO-pcDNA) were collected and resuspended in PBS at a dilution of 10:1, and the morphology and the specific surface markers (CD81, CD63, and CD9) of exosomes were identified using transmission electron microscopy (TEM) (FEI, Hillsboro, OR, USA) (× 100) and Western blot, respectively.
Preparation of Contusive SCI Rat Model
Male SD rats were anaesthetized by 10% chloral hydrate (400 mg/kg body weight). Then, the spinal cord was exposed by a laminectomy at T9-11 spinous process. Thereafter, SCI was inflicted by dropping a 10 g rod from a height of 6.5 cm onto the spinal cord. The muscles were sutured in layers after administration. The bladders were manually voided thrice every day until the recovery of the reflexive control of bladder function. After SCI, rats received treatments by tail vein injection of EXO-TCTN2, EXO-pcDNA (100 μg exosomes in 500 mL of PBS) or 500 μL PBS immediately.
The rats were randomly divided into four groups: Sham group (rats only received laminectomy), SCI (rats subjected to SCI and received PBS treatment), SCI + EXO-pcDNA group (rats subjected to SCI and received EXO-pcDNA treatment), or SCI + EXO-TCTN2 (rats subjected to SCI and received EXO-TCTN2 treatment) (N = 6/group). Neurological function was evaluated using the Basso, Beattie, and Bresnahan (BBB) scoring based on the locomotion following SCI (Basso et al. 1995) at 1, 3, 5, 7, and 9 days post-injury. Two independent examiners blinded to the treatment regimen assessed the movement of the hindlimbs of rats. At day 14, rats were sacrificed, and the spinal cord tissues were harvested for molecular analysis.
Subcellular Localization Assay
The cytoplasm RNA and nucleus RNA from PC12 cells were extracted using PARIS Kit (Invitrogen). Then, the levels of TCTN2, U6 (nucleus control) and GAPDH (cytoplasm control) were examined by quantitative real-time PCR (qRT-PCR).
qRT-PCR
Total RNA was isolated from cultured cells, exosomes, and spinal cord tissues of rats using Trizol reagent (Life Technologies). The single-stranded cDNA was acquired utilizing the M-MLV reverse transcriptase (TaKaRa, Tokyo, Japan) with 1 μg of RNA. Then quantitative PCR was accomplished using a SYBR® Select Master Mix (Takara). The relative gene expression was assessed with U6 or GAPDH, respectively, as a housekeeping gene. The primers were synthesized by Sangon Biotech (Shanghai, China):
-
IGF1R: F 5′-TGACATCCGCAACGACTATCA-3′, R 5′-CCAGTGCGTAGTTGTAGAAGAGT-3′;
-
GAPDH: F 5′-CCCACATGGCCTCCAAGGAGTA-3′, R 5′-GTGTACATGGCAACTGTGAGGAGG-3′;
-
miR-329-3p: F 5′-GCGCGAACACACCCAGCTAACCTTTTT-3′, R 5′- CAGTGCAGGGTCCGAGGTA-3′;
-
U6: F 5′-GCTTCGGCAGCACATATACTAAAAT-3′, R 5′- CGCTTCACGAATTTGCGTGTCAT-3′.
Cell Counting Kit-8 (CCK-8) Assay
PC12 cells were cultured in 96-well plates (3000 cells/well). Forty-eight hours later, the addition of 10 µL CCK-8 reagent (Solarbio, Beijing, China) in per well was accomplished. After incubation for 2 h, the optical density was read by a microplate reader at 450 nm.
5-Ethynyl-2’-deoxyuridine (EdU) Assay
The proliferation of PC12 cells was evaluated using the EdU incorporation assay kit (RiboBio, Guangdong, China) referring to the manufacturer protocol. DAPI or Merge (Invitrogen) dyed cells, severally. A fluorescence microscope (Nikon, Tokyo, Japan) was utilized to obtain images.
Enzyme-Linked Immunosorbent Assay (ELISA)
The commercial ELISA kits (Solarbio) were employed to detect the concentrations of interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α) from the culture supernatants of PC12 cells referring to the manufacturer protocol.
Flow Cytometry
PC12 cells were placed in 6-well plates, rinsed with PBS, and suspended in binding buffer. Then, the cell suspension was mixed with 10 µL Annexin V-fluorescein isothiocyanate (FITC) (BD Biosciences. Heidelberg, Germany) and 10 µL propidium iodide (PI) (Life Technologies), followed by incubation for 15 min under darkness at 37 ℃. Finally, the apoptotic rates of PC12 cells were analyzed by FACSCalibur II sorter (BD Biosciences).
Western Blot
Protein was prepared using RIPA lysis buffer (Solarbio), separated with 10% SDS-PAGE gel, and then transferred onto a PVDF membrane (Solarbio). Thereafter, the membrane was incubated with primary antibodies included CD81 (1: 2000, ab109201), CD9 (1:2000, ab92726), CD63 (1:2000, ab68418,), B-cell lymphoma-2 (Bcl-2) (1:1000, ab194583), and Bcl-2-associated X protein (Bax) (1:1000, ab32503) (all from Abcam, Cambridge, MA, USA) at 4 ℃ overnight. After incubation with HRP-conjugated goat anti-rabbit (1:2000, A0208, Beyotime, Beijing, China) or goat anti-mouse (1:1000, A0216, Beyotime) for 2 h, immunolabelling was visualized using the ECL system (Amersham, Bucks, UK).
Measurement of MDA and SOD
The level of malondialdehyde (MDA) and superoxide dismutase (SOD) were detected following the recommended manufacturers of commercial assay kits (Solarbio).
Dual-Luciferase Reporter Assay
The fragments of TCTN2 or IGF1R 3’UTR encompassing miR-329-3p complementary base paring were inserted into the pGL3-Basic Vector (Ambion, Thermo Fisher Scientific. Runcorn, UK). When cell density reached 70% confluency, PC12 cells, infected with miR-329-3p mimic or mimic control, were transfected with 50 ng pGL3 Vector and 10 ng pRL-TK Renilla. Dual-luciferase reporter assay system (Ambion) was applied for the analysis of luciferase activity.
RNA Immunoprecipitation (RIP) Assay
RIP assay was performed according to the instructions of Magna RIP kit (Millipore, Billerica, MA, USA). The co-precipitated RNAs were separated for the measurement of TCTN2, miR-329-3p or IGF1R expression with qRT-PCR.
Statistical Analyses
Data were expressed as mean ± standard deviation. Statistical analyses were performed with GraphPad Prism 6.0 software (GraphPad, San Diego, CA, USA). Statistical comparisons were made by Student’s t test (two-sided) or analysis of variance. P < 0.05 were considered significant differences.
Results
TCTN2 Is Down-Regulated in SCI Model Rat and LPS-Stimulated PC12 Cells
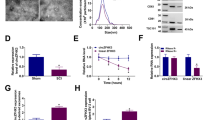
The expression pattern of TCTN2 was firstly investigated. We constructed an SCI rat model, the neurological function was assessed with BBB scoring, and the results showed that BBB score was significantly decreased in SCI rat relative to the control (Fig. 1A). Then, the spinal tissues were obtained; qRT-PCR analysis exhibited that TCTN2 expression was decreased in rats of SCI group in comparison to the sham group (Fig. 1B). Thereafter, LPS was used to expose to neuron PC12 cells to mimic the SCI condition in vitro. We found that TCTN2 level was decreased in LPS-induced PC12 cells compared with the normal control (Fig. 1C). Besides that, the distribution of TCTN2 in PC12 cells was investigated. It was proved that TCTN2 was mainly located in the cytoplasm in PC12 cells (Fig. 1D). These data suggested that TCTN2 decrease might be associated with SCI.
TCTN2 is down-regulated in SCI model rat and LPS-stimulated neurons. (A) Hindlimb functional recovery was monitored after SCI using BBB scoring. (B) Relative expression of TCTN2 at the lesion site was detected after SCI using qRT-PCR. (C) Relative expression of TCTN2 in PC12 cells with or without LPS stimulation was measured by qRT-PCR. (D) qRT-PCR for TCTN2 expression in the nuclear and cytoplasm fractions of PC12 cells. **P < 0.01, ***P < 0.001
MSCs Packages TCTN2 into Secreted Exosomes and TCTN2 Exosomes Improve Functional Recovery After SCI In Vivo
MSCs were transfected with TCTN2 overexpression plasmid and negative pcDNA; then, an increased TCTN2 expression in TCTN2-transfetced MSCs was observed (Fig. 2A). Then, the exosomes were isolated from the supernatants of transfected MSCs, named as EXO-pcDNA or EXO-TCTN2. Morphology of exosomes was confirmed by TEM (Fig. 2B), and surface hallmarks (CD81, CD63, and CD9) were identified by Western blot (Fig. 2C). Thereafter, we found that TCTN2 expression was higher in exosomes isolated from TCTN2-transfetced MSCs (EXO-TCTN2) compared with exosomes isolated from pcDNA-transfected MSCs (EXO-TCTN2) (Fig. 2D). Subsequently, the role of exosomal TCTN2 in SCI progression in vivo was validated. The EXO-TCTN2 or EXO-pcDNA was injected into SCI rats via tail vein injection. In comparison to the rats injected with EXO-pcDNA, the BBB score of rats with EXO-TCTN2 was remarkably increased (Fig. 2E). Moreover, with the spinal cord taken, the levels of TCTN2 were determined using qRT-PCR; it was discovered that TCTN2 expression was significantly elevated in the injured spinal cord of SCI rats with EXO-TCTN2 (Fig. 2F). Besides that, we detected the viability of normal human astrocytes (NHAs) and the levels of GFAP and CCL2 in NHAs. Astrocytes are regarded as the most abundant glial cell type within the central nervous system (CNS), reactive astrocyte proliferation post-SCI results in the formation of glial scars, thus hindering axon regeneration (Silver and Miller 2004; Li et al. 2020). After injury, astrocytes show changes in morphology and phenotype, and reinforce the expression of intermediate filament glial fibrillary acidic proteins (GFAP), besides that, CCL2, a typically highly modulated inflammatory chemokine, is mainly released by reactive astrocytes in CNS injury and diseases (Li et al. 2020). The qRT-PCR analysis suggested that EXO-TCTN2 treatment rescued LPS-induced decrease of TCTN2 expression (Fig. S1A). Thereafter, we proved that TCTN2 overexpression via exosomes suppressed the viability and reduced the levels of GFAP and CCL2 in LPS-stimulated NHAs (Fig. S1B, C), implying the abilities of TCTN2 exosomes to induce axon crossings through the glial scars indirectly. Taken together, exosomal TCTN2 derived from MSCs promoted recovery after SCI in vivo.
MSCs packages TCTN2 into secreted exosomes and TCTN2 exosomes improves functional recovery after SCI in vivo. (A) qRT-PCR for TCTN2 expression in MSCs transfected with TCTN2 or negative pcDNA. (B) TEM was used to analyze isolated exosomes (red arrows). Scale bar, 100 nm. (C) Western blot analysis of the levels of extracellular vesicle-related protein markers CD81, CD63, and CD9. (D) qRT-PCR for TCTN2 expression in isolated exosomes. (E, F) The EXO-TCTN2 or EXO-pcDNA was injected into SCI rats via tail vein injection. (E) Hindlimb functional recovery was monitored after SCI using BBB scoring. (F) qRT-PCR for TCTN2 expression in the injured spinal cord of SCI rats. ***P < 0.001, ****P < 0.0001
TCTN2 Exosomes Suppresses Neuronal Apoptosis, Inflammation, and Oxidative Stress In Vitro
To determine whether exosomal TCTN2 had therapeutic effects in vitro, PC12 cells were stimulated with LPS (1 μg/mL) for 24 h to mimic the situation of SCI in vivo, followed by the treatment of EXO-pcDNA or EXO-TCTN2. The qRT-PCR analysis suggested that LPS stimulation induced a decrease of TCTN2 expression in PC12 cells, which was rescued by EXO-TCTN2 treatment (Fig. 3A). Then, functional experiments were performed. The results of CCK-8 and EdU assays showed that the viability and DNA synthesis activity of PC12 cells were significantly decreased by LPS treatment, while EXO-TCTN2 reversed LPS-induced suppression of PC12 cell proliferation (Fig. 3B, C). ELISA analysis suggested that the concentration of pro-inflammatory cytokines IL-6 and TNF-α was increased by LPS treatment in PC12 cells, which was reduced by EXO-TCTN2 (Fig. 3D). Besides that, the apoptosis rate was increased in LPS-stimulated PC12 cells accompanied by the co-dependent increase of Bax protein level and decrease of Bcl-2 protein level, while this condition was reversed by EXO-TCTN2 (Fig. 3E, F). Additionally, we also found that the level of SOD was decreased, while MDA was increased in LPS-induced PC12 cells; however, the levels of them showed opposite trend in LPS-induced PC12 cells co-treated with EXO-TCTN2 (Fig. 3G, H). Altogether, exosomal TCTN2 derived from MSCs protected PC12 cells from LPS-induced apoptosis, inflammation, and oxidative stress in vitro.
TCTN2 exosomes suppresses neuronal apoptosis, inflammation and oxidative stress in vitro. (A–H) PC12 cells were stimulated with LPS (1 μg/mL) for 24 h, followed by the treatment of EXO-pcDNA or EXO-TCTN2. (A) qRT-PCR for TCTN2 expression in cells. (B) CCK-8 assay for cell viability. (C) EdU assay for cell DNA synthesis activity. (D) Detection of IL-6 and TNF-α levels in cells using ELISA. (E) Flow cytometry for cell apoptosis rate. (F) Western blot analysis for the protein levels of Bax and Bcl-2. (G, H) Measurement of SOD and MDA levels in cells using commercial assay kits. **P < 0.01, ***P < 0.001, ****P < 0.0001
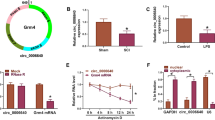
Mir-329-3p Is A Target Of TCTN2
Given that TCTN2 was predominantly distributed in the cell cytoplasm, we hypothesized that TCTN2 might act as a miRNA sponge to exert its effects. According to the prediction of LncBase Predicted v.2, miR-329-3p was found to have the complementary sequences on TCTN2 (Fig. 4A). To verify this prediction, WT-TCTN2 and MUT-TCTN2 with luciferase reporter were constructed and co-transfected into PC12 cells with miR-329-3p. The transfection efficiency of miR-329-3p mimics was firstly validated (Fig. 4B). Then, we found that overexpression of miR-329-3p significantly declined the luciferase activity of MEG3-WT in PC12 cells, while there was no significant difference in cells co-transfected with MUT-TCTN2 and miR-329-3p (Fig. 4C). Moreover, RIP assay suggested that TCTN2 and miR-329-3p were pulled down by antibody against Ago relative to the control IgG antibody (Fig. 4D). All these results confirmed that TCTN2 targeted miR-329-3p. After that, it was observed that miR-329-3p expression was increased in SCI rat and LPS-induced PC12 cells (Fig. 4E, F). To further investigate the relationship between TCTN2 and miR-329-3p, overexpression and knockdown TCTN2 plasmids were transfected into LPS-induced PC12 cells, and qRT-PCR suggested the up- and down-regulation of TCTN2 expression in cells (Fig. 4G). Subsequently, the level of miR-329-3p was detected; the results showed that miR-329-3p expression was higher in TCTN2-decreased PC12 cells, but lower in TCTN2-increased PC12 cells in the presence of LPS compared with the respective negative controls (Fig. 4H). In all, these data confirmed that TCTN2 targeted miR-329-3p to down-regulate its expression.
MiR-329-3p is a target of TCTN2. (A) The putative binding sites of TCTN2 and miR-329-3p. (B) qRT-PCR for miR-329-3p expression in PC12 cells transfected with miR-NC or miR-329-3p. (C, D) The interaction between TCTN2 and miR-329-3p was confirmed using dual-luciferase reporter assay and RIP assay. (E) Relative expression of miR-329-3p at the lesion site was detected after SCI using qRT-PCR. (F) Relative expression of miR-329-3p in PC12 cells with or without LPS stimulation was measured by qRT-PCR. (G, H) qRT-PCR for TCTN2 and miR-329-3p expression in LPS-stimulated PC12 cells transfected with si-NC, si-TCTN2, TCTN2, or pcDNA. ***P < 0.001, ****P < 0.0001
TCTN2 Exosomes Suppresses Neuronal Apoptosis, Inflammation, and Oxidative Stress via miR-329-3p
To explore whether miR-329-3p mediated the effects of TCTN2 exosomes, miR-329-3p was forced in LPS-induced PC12 cells with the incubation of EXO-TCTN2. The qRT-PCR analysis exhibited that miR-329-3p mimic rescued EXO-TCTN2-induced decrease of miR-329-3p expression in LPS-induced PC12 cells (Fig. 5A). Then, functional experiments indicated that overexpression of miR-329-3p blocked the promoting effects of EXO-TCTN2 on cell proliferation (Fig. 5B, C) and the suppressive effects on cell inflammation (Fig. 5D), apoptosis (Fig. 5E–G), and oxidative stress (Fig. 5H, I) in LPS-induced PC12 cells. Therefore, we demonstrated that TCTN2 exosomes regulated neuronal apoptosis, inflammation, and oxidative stress via miR-329-3p.
TCTN2 exosomes suppresses neuronal apoptosis, inflammation and oxidative stress via miR-329-3p. (A–I) PC12 cells were stimulated with LPS for 24 h, followed by the treatment of EXO-TCTN2 and miR-329-3p. (A) qRT-PCR for miR-329-3p expression in cells. (B) CCK-8 assay for cell viability. (C) EdU assay for cell DNA synthesis activity. (D) Detection of IL-6 and TNF-α levels in cells using ELISA. (E, F) Flow cytometry for cell apoptosis rate. (G) Western blot analysis for the protein levels of Bax and Bcl-2. (H, I) Measurement of SOD and MDA levels in cells using commercial assay kits. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001
Mir-329-3p Targeted IGF1R To Down-Regulate Its Expression
To clarify the underlying mechanism of miR-329-3p in neuron regulation, starBase v2.0 database was used to identify the target genes with miR-329-3p biding sites, which showed a strong binding between miR-329-3p and IGF1R (Fig. 6A). Dual-luciferase reporter assay suggested that miR-329-3p reduced the luciferase activity of WT-IGF1R 3’ UTR, but not mutant IGF1R (Fig. 6B). RIP assay further confirmed that miR-329-3p and IGF1R were largely captured by anti-Ago2 compared with the negative control in IgG-induced PC12 cells (Fig. 6C). Then, the expression pattern of IGF1R was investigated; it was found that IGF1R expression was decreased in SCI rat and LPS-induced PC12 cells (Fig. 6D, E). After confirming the interference efficiency of miR-329-3p inhibitor (Fig. 6F), we observed that IGF1R expression level in LPS-induced PC12 cells was decreased by miR-329-3p mimic but elevated by miR-329-3p inhibitor (Fig. 6G). These results demonstrated that miR-329-3p interacted with and sequestered IGF1R.
MiR-329-3p targeted IGF1R to down-regulate its expression. (A) The putative binding sites of IGF1R and miR-329-3p. (B, C) The interaction between IGF1R and miR-329-3p was confirmed using dual-luciferase reporter assay and RIP assay. (D) Relative expression of IGF1R at the lesion site was detected after SCI using Western blot. (E) Relative expression of IGF1R in PC12 cells with or without LPS stimulation was measured by Western blot. (F) qRT-PCR for miR-329-3p expression in PC12 cells transfected with anti-miR-NC or anti-miR-329-3p. (G) Western blot for IGF1R expression in LPS-stimulated PC12 cells transfected with miR-NC, miR-329-3p, anti-miR-NC, or anti-miR-329-3p. **P < 0.01, ***P < 0.001, ****P < 0.0001
Inhibition of miR-329-3p Suppresses Neuronal Apoptosis, Inflammation, and Oxidative Stress via IGF1R
We then elucidated whether miR-329-3p/IGF1R axis was engaged in PC12 cell injury. To verify this hypothesis, the transfection efficiency of IGF1R siRNA (si-IGF1R) was first verified (Fig. 7A). Then, miR-329-3p inhibitor together with IGF1R siRNA was co-transfected into LPS-induced PC12 cells; the results of qRT-PCR suggested that the inhibition of miR-329-3p led to an increase of IGF1R expression, which was reduced by the knockdown of IGF1R in treated PC12 cells (Fig. 7B). Thereafter, functional experiments were performed. CCK-8 and EdU assays showed that miR-329-3p inhibition exhibited the highest cell proliferative capacity, whereas cell activity of anti-miR-329-3p + si-IGF1R was significantly inhibited (Fig. 7C, D). The results from ELISA implied that miR-329-3p inhibition caused a decrease of IL-6 and TNF-α expression in LPS-induced PC12 cells, which was rescued by IGF1R knockdown (Fig. 7E). Flow cytometry analysis revealed that miR-329-3p inhibition reduced cell apoptosis accompanied by the down-regulation of Bax level and up-regulation of Bcl-2 level in LPS-induced PC12 cells, while this condition was attenuated by IGF1R knockdown (Fig. 7F–H). Moreover, we also found that the level of SOD was increased, while MDA level was decreased in miR-329-3p-decreased PC12 cells under LPS stimulation, while there were opposite content trends in cells co-transfection with anti-miR-329-3p and si-IGF1R in the presence of LPS (Fig. 7I, J). Collectively, miR-329-3p inhibition impaired neuronal apoptosis, inflammation, and oxidative stress via IGF1R.
Inhibition of miR-329-3p suppresses neuronal apoptosis, inflammation and oxidative stress via IGF1R. (A) Western blot for IGF1R expression in PC12 cells transfected with si-NC or si-IGF1R. (B–J) PC12 cells were stimulated with LPS for 24 h, followed by the transfection of anti-miR-329-3p and si-IGF1R. (B) Western blot analysis for the expression of IGF1R protein. (C) CCK-8 assay for cell viability. (D) EdU assay for cell DNA synthesis activity. (E) Detection of IL-6 and TNF-α levels in cells using ELISA. (F, G) Flow cytometry for cell apoptosis rate. (H) Western blot analysis for the protein levels of Bax and Bcl-2. (I, J) Measurement of SOD and MDA levels in cells using commercial assay kits. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001
TCTN2/miR-329-3p Axis Can Regulate IGF1R Expression
To assay whether TCTN2 could regulate IGF1R expression via miR-329-3p, miR-329-3p was forced in LPS-induced PC12 cells with the incubation of EXO-TCTN2. Then, qRT-PCR and Western blot analysis revealed that EXO-TCTN2 led to an elevation of IGF1R expression level, which was reversed by miR-329-3p overexpression in LPS-induced PC12 cells (Fig. 8A, B), suggesting that TCTN2/miR-329-3p axis mediated the expression of IGF1R.
TCTN2 Exosomes Improve Functional Recovery After SCI In Vivo via miR-329-3p/IGF1R Axis
Subsequently, we probed whether TCTN2 exosomes regulated functional recovery after SCI in vivo via miR-329-3p/IGF1R axis. Mice were subjected to SCI, followed by tail vein injection of EXO-TCTN2 and lentiviral vectors carrying miR-329-3p or si-IGF1R; the BBB score of rats with EXO-TCTN2 was remarkably increased, which was attenuated by miR-329-3p overexpression or IGF1R knockdown (Fig. 9A). Besides that, TCTN2 overexpression by EXO led to an increase of IGF1R expression in the injured spinal cord of SCI rats, while this condition was abolished by miR-329-3p overexpression or IGF1R knockdown (Fig. 9B). Thus, TCTN2 exosomes also improved functional recovery after SCI via miR-329-3p/IGF1R axis in vivo.
TCTN2 exosomes improve functional recovery after SCI in vivo via miR-329-3p/IGF1R axis. Rats were subjected to SCI, followed by tail vein injection of EXO-TCTN2 and lentiviral vectors carrying miR-329-3p or si-IGF1R. (A) Hindlimb functional recovery was monitored after SCI using BBB scoring. (B) Western blot for IGF1R expression in the injured spinal cord of SCI rats. **P < 0.01, ***P < 0.001, ****P < 0.0001
Discussion
SCI is a devastating traumatic event, leading to severe motor, sensory, and autonomic deficits, which remains the major cause of long-term physical damage (Ambrozaitis et al. 2006). MSCs are commonly used as a source of cellular therapy; it has been uncovered that MSC efficacy appears to derive from their paracrine effects, which are mediated by both MSC-derived exosomes and soluble paracrine factors (Hessvik and Llorente 2018; Mendt et al. 2019; Abreu et al. 2016). Exosomes are smaller and less complex relative to their parent cells; they are stable in body fluids, present no risk of tumor formation, and do not elicit adverse immune responses (Liu et al. 2017). MSCs can produce abundant amounts of exosomes; transplantation of MSC-secreted exosomes exhibits similar functional properties and therapeutic effects to direct stem cell transplantation without apparent adverse effects (Tao et al. 2017; Lou et al. 2017) and yielded beneficial effects in SCI. Liu et al. demonstrated that the application of MSC-exosomes favored functional behavioral recovery of spinal cord after SCI (Liu et al. 2019). Li et al. showed that the transplantation of MSC-exosomes improved recovery of spinal cord function and reduced neuronal apoptosis through the Wnt/β-catenin pathway (Hu et al. 2019). Thus, we suggest that exosomes derived from MSCs may be a promising therapeutic for SCI.
Exosomes are small vesicles mediating intercellular communication by delivering RNAs, DNA, and proteins between cells (Meldolesi 2018). In addition, increasing evidence has revealed that exosomes can be manufactured in culture by the shuttle of therapeutic lncRNAs to exosome-producing cells, such as MSCs, and MSCs simultaneously shed lncRNA-containing exosomes. Su et al. showed that HAND2-AS1 exosomes from MSCs suppressed the activation of rheumatoid arthritis fibroblast-like synoviocytes by blocking NF-κB pathway (Su et al. 2021). MSCs-originated exosomal KLF3-AS1 accelerated cartilage repair and chondrocyte proliferation in osteoarthritis (Liu et al. 2018). TCTN2 lncRNA was found to be involved in functional recovery after SCI (Ren et al. 2019). Here, we further investigated whether TCTN2 exerted its roles in spinal cord functional recovery via MSC-exosomes. We demonstrated that TCTN2 expression was decreased in spinal cord of SCI model rats and LPS-stimulated PC12 cells. The tail vein injection of TCTN2 exosomes from MSCs improved functional recovery of spinal cord in animal models of SCI, evidenced by the higher BBB scores. Further in vitro analysis showed that administration of TCTN2 exosomes suppressed NHA proliferation and activation and alleviated LPS-induced neuronal apoptosis, inflammation, and oxidative stress.
TCTN2 was verified to be distributed predominantly in the cell cytoplasm. Accumulating evidence has clarified that lncRNAs in cell cytoplasm can act as miRNA sponges to protect the target mRNAs from repression (Chen and Wang 2019; Chen et al. 2019). Subsequently, we confirmed that TCTN2 functioned as a sponge for miR-329-3p to up-regulate IGF1R expression. Recently, a study showed that a high expression of miR-329-3p was elevated in the rat bladders of SCI (Shang et al. 2020). IGF1R is a pivotal regulator of normal growth and progression, showing potent anti-apoptotic and pro-survival capacities (Riedemann and Macaulay 2006), which is considered essential for axonal regeneration of neurons in the adult central nervous system (Dupraz et al. 2013). Previous study implied that IGF1R improved the recovery of motor function and prevented paraplegia in the rabbit model of spinal cord ischemia (Nakao et al. 2001). Besides that, IGF1R yielded beneficial effects in neural stem cells-mediated recovery of motor function after spinal cord transection (Hwang et al. 2018; Zhao et al. 2019). In the current study, an increase of miR-329-3p level in spinal cord of SCI model rats and LPS-stimulated PC12 cells was observed. Inhibition of miR-329-3p reversed LPS-induced neuronal apoptosis, inflammation, and oxidative stress, which was counteracted by IGF1R knockdown. Moreover, we also proved that miR-329-3p overexpression attenuated the neuroprotection of TCTN2 exosomes. Besides that, TCTN2 exosomes improved the functional recovery after SCI via miR-329-3p/IGF1R axis in vivo.
In conclusion, this work for the first time demonstrated that TCTN2 exosomes derived from MSCs promoted spinal cord function recovery in vivo and relieved LPS-induced neuronal apoptosis, inflammation, and oxidative stress in vitro. Our findings have some degree of significance and value for the development of osomes-based therapy in SCI; however, continuing investigations of TCTN2 exosomes on clinical application need further in vivo.
Data and Materials Availability
The analyzed datasets generated during the present study are available from the corresponding author on reasonable request.
References
Abreu SC, Weiss DJ, Rocco PR (2016) Extracellular vesicles derived from mesenchymal stromal cells: a therapeutic option in respiratory diseases? Stem Cell ResTher 7(1):53
Ambrozaitis KV, Kontautas E, Spakauskas B, Vaitkaitis D (2006) Pathophysiology of acute spinal cord injury. Medicina (Kaunas, Lithuania) 42(3):255–261
Assinck P, Duncan GJ, Hilton BJ, Plemel JR, Tetzlaff W (2017) Cell transplantation therapy for spinal cord injury. Nat Neurosci 20(5):637–647
Bai G, Jiang L, Meng P, Li J, Han C, Wang Y, Wang Q (2020) LncRNA neat1 promotes regeneration after spinal cord injury by targeting miR-29b. J Mol Neurosci MN
Basso DM, Beattie MS, Bresnahan JC (1995) A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma 12(1):1–21
Camussi G, Deregibus MC, Tetta C (2010) Paracrine/endocrine mechanism of stem cells on kidney repair: role of microvesicle-mediated transfer of genetic information. Curr Opin Nephrol Hypertens 19(1):7–12
Chang CK, Chou W, Lin HJ, Huang YC, Tang LY, Lin MT, Chang CP (2014) Exercise preconditioning protects against spinal cord injury in rats by upregulating neuronal and astroglial heat shock protein 72. Int J Mol Sci 15(10):19018–19036
Chen S, Wang J (2019) HAND2-AS1 inhibits invasion and metastasis of cervical cancer cells via microRNA-330-5p-mediated LDOC1. Cancer Cell Int 19:353
Chen J, Lin Y, Jia Y, Xu T, Wu F, Jin Y (2019) LncRNA HAND2-AS1 exerts anti-oncogenic effects on ovarian cancer via restoration of BCL2L11 as a sponge of microRNA-340-5p. J Cell Physiol 234(12):23421–23436
Cui M, Ma X, Sun J, He J, Shen L, Li F (2017) Effects of STAT3 inhibitors on neural functional recovery after spinal cord injury in rats. Biosci Trends 10(6):460–466
Dupraz S, Grassi D, Karnas D, Nieto Guil AF, Hicks D, Quiroga S (2013) The insulin-like growth factor 1 receptor is essential for axonal regeneration in adult central nervous system neurons. PloS One 8(1):e54462
Fakhoury M (2015) Spinal cord injury: overview of experimental approaches used to restore locomotor activity. Rev Neurosci 26(4):397–405
Greene LA, Aletta JM, Rukenstein A, Green SH (1987) PC12 pheochromocytoma cells: culture, nerve growth factor treatment, and experimental exploitation. Methods Enzymol 147:207–216
Hessvik NP, Llorente A (2018) Current knowledge on exosome biogenesis and release. Cell Mol Life Sci CMLS 75(2):193–208
Horwitz EM, Le Blanc K, Dominici M, Mueller I, Slaper-Cortenbach I, Marini FC, Deans RJ, Krause DS, Keating A (2005) Clarification of the nomenclature for MSC: the International Society for Cellular Therapy position statement. Cytotherapy 7(5):393–395
Hu X, Wu D, He X, Zhao H, He Z, Lin J, Wang K, Wang W, Pan Z, Lin H et al (2019) circGSK3β promotes metastasis in esophageal squamous cell carcinoma by augmenting β-catenin signaling. Mol Cancer 18(1):160
Hwang DH, Park HH, Shin HY, Cui Y, Kim BG (2018) Insulin-like growth factor-1 receptor dictates beneficial effects of treadmill training by regulating survival and migration of neural stem cell grafts in the injured spinal cord. Exp Neurobiol 27(6):489–507
Katsuda T, Kosaka N, Takeshita F, Ochiya T (2013) The therapeutic potential of mesenchymal stem cell-derived extracellular vesicles. Proteomics 13(10–11):1637–1653
Kosaka N, Yoshioka Y, Fujita Y, Ochiya T (2016) Versatile roles of extracellular vesicles in cancer. J Clin Investig 126(4):1163–1172
Li P, Li Y, Dai Y, Wang B, Li L, Jiang B, Wu P, Xu J (2020) The LncRNA H19/miR-1-3p/CCL2 axis modulates lipopolysaccharide (LPS) stimulation-induced normal human astrocyte proliferation and activation. Cytokine 131:155106
Liu L, Jin X, Hu CF, Li R, Zhou Z, Shen CX (2017) Exosomes derived from mesenchymal stem cells rescue myocardial ischaemia/reperfusion injury by inducing cardiomyocyte autophagy via AMPK and Akt pathways. Cell Physiol Biochem Int J Exp Cell Physiol Biochem Pharmacol 43(1):52–68
Liu Y, Zou R, Wang Z, Wen C, Zhang F, Lin F (2018) Exosomal KLF3-AS1 from hMSCs promoted cartilage repair and chondrocyte proliferation in osteoarthritis. Biochem J 475(22):3629–3638
Liu W, Wang Y, Gong F, Rong Y, Luo Y, Tang P, Zhou Z, Zhou Z, Xu T, Jiang T et al (2019) Exosomes derived from bone mesenchymal stem cells repair traumatic spinal cord injury by suppressing the activation of a1 neurotoxic reactive astrocytes. J Neurotrauma 36(3):469–484
Lou G, Chen Z, Zheng M, Liu Y (2017) Mesenchymal stem cell-derived exosomes as a new therapeutic strategy for liver diseases. Exp Mol Med 49(6):e346
Lugea A, Waldron RT (2017) Exosome-mediated intercellular communication between stellate cells and cancer cells in pancreatic ductal adenocarcinoma. Pancreas 46(1):1–4
Martin-Rendon E, Sweeney D, Lu F, Girdlestone J, Navarrete C, Watt SM (2008) 5-Azacytidine-treated human mesenchymal stem/progenitor cells derived from umbilical cord, cord blood and bone marrow do not generate cardiomyocytes in vitro at high frequencies. Vox Sang 95(2):137–148
Meldolesi J (2018) Exosomes and ectosomes in intercellular communication. Curr Biol CB 28(8):R435-r444
Mendt M, Rezvani K, Shpall E (2019) Mesenchymal stem cell-derived exosomes for clinical use. Bone Marrow Transplant 54(Suppl 2):789–792
Milane L, Singh A, Mattheolabakis G, Suresh M, Amiji MM (2015) Exosome mediated communication within the tumor microenvironment. J Control Release Official J Control Release Soc 219:278–294
Mothe AJ, Tator CH (2012) Advances in stem cell therapy for spinal cord injury. J Clin Investig 122(11):3824–3834
Nakao Y, Otani H, Yamamura T, Hattori R, Osako M, Imamura H (2001) Insulin-like growth factor 1 prevents neuronal cell death and paraplegia in the rabbit model of spinal cord ischemia. J Thorac Cardiovasc Surg 122(1):136–143
Phinney DG, Prockop DJ (2007) Concise review: mesenchymal stem/multipotent stromal cells: the state of transdifferentiation and modes of tissue repair–current views. Stem Cells (Dayton, Ohio) 25(11):2896–2902
Popovich PG (2014) Neuroimmunology of traumatic spinal cord injury: a brief history and overview. Exp Neurol 258:1–4
Ren XD, Wan CX, Niu YL (2019) Overexpression of lncRNA TCTN2 protects neurons from apoptosis by enhancing cell autophagy in spinal cord injury. FEBS Open Bio 9(7):1223–1231
Ren Z, Qi Y, Sun S, Tao Y, Shi R (2020) Mesenchymal stem cell-derived exosomes: hope for spinal cord injury repair. Stem Cells Dev 29(23):1467–1478
Riedemann J, Macaulay VM (2006) IGF1R signalling and its inhibition. Endocr Relat Cancer 13(Suppl 1):S33-43
Roma-Rodrigues C, Fernandes AR, Baptista PV (2014) Exosome in tumour microenvironment: overview of the crosstalk between normal and cancer cells. BioMed Res Int 2014:179486
Samsonraj RM, Raghunath M, Nurcombe V, Hui JH, van Wijnen AJ, Cool SM (2017) Concise review: multifaceted characterization of human mesenchymal stem cells for use in regenerative medicine. Stem Cells Transl Med 6(12):2173–2185
Shang Z, Ou T, Xu J, Yan H, Cui B, Wang Q, Wu J, Jia C, Cui X, Li J (2020) MicroRNA expression profile in the spinal cord injured rat neurogenic bladder by next-generation sequencing. Transl Androl Urol 9(4):1585–1602
Silver J, Miller JH (2004) Regeneration beyond the glial scar. Nat Rev Neurosci 5(2):146–156
Su Y, Liu Y, Ma C, Guan C, Ma X, Meng S (2021) Mesenchymal stem cell-originated exosomal lncRNA HAND2-AS1 impairs rheumatoid arthritis fibroblast-like synoviocyte activation through miR-143-3p/TNFAIP3/NF-κB pathway. J Orthop Surg Res 16(1):116
Tao SC, Yuan T, Zhang YL, Yin WJ, Guo SC, Zhang CQ (2017) Exosomes derived from miR-140-5p-overexpressing human synovial mesenchymal stem cells enhance cartilage tissue regeneration and prevent osteoarthritis of the knee in a rat model. Theranostics 7(1):180–195
Wang X, Lu X, Zhu R, Zhang K, Li S, Chen Z, Li L (2017) Betulinic acid induces apoptosis in differentiated PC12 cells via ROS-mediated mitochondrial pathway. Neurochem Res 42(4):1130–1140
Witiw CD, Fehlings MG (2015) Acute Spinal Cord Injury. J Spinal Disord Tech 28(6):202–210
Zhang H, Li D, Zhang Y, Li J, Ma S, Zhang J, Xiong Y, Wang W, Li N, Xia L (2018) Knockdown of lncRNA BDNF-AS suppresses neuronal cell apoptosis via downregulating miR-130b-5p target gene PRDM5 in acute spinal cord injury. RNA Biol 15(8):1071–1080
Zhao XM, He XY, Liu J, Xu Y, Xu FF, Tan YX, Zhang ZB, Wang TH (2019) Neural stem cell transplantation improves locomotor function in spinal cord transection rats associated with nerve regeneration and IGF-1 R expression. Cell Transpl 28(9–10):1197–1211
Zhou R, Chen KK, Zhang J, Xiao B, Huang Z, Ju C, Sun J, Zhang F, Lv XB, Huang G (2018) The decade of exosomal long RNA species: an emerging cancer antagonist. Mol Cancer 17(1):75
Author information
Authors and Affiliations
Contributions
Conceptualization and methodology: Mingxia Lin and Feng Qiao; formal analysis and data curation: Feng Qiao and Chenghua Zhang; validation and investigation: Jian Liu and Feng Qiao; writing-original draft preparation and writing-review and editing: Jian Liu, Mingxia Lin, and Feng Qiao; approval of final manuscript: all authors.
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
The present study was approved by the ethical review committee of Hainan General Hospital with grant No. 20190628. Written informed consent was obtained from all enrolled patients.
Patient Consent for Publication
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlight
• TCTN2 is down-regulated in SCI model rat and LPS-stimulated PC12 cells
• MSCs packages TCTN2 into secreted exosomes
• TCTN2 exosomes improve functional recovery after SCI in vivo
• TCTN2 exosomes suppress neuronal apoptosis, inflammation, and oxidative stress in vitro
• TCTN2 exosomes exert its neuroprotective effects via miR-329-3p/IGF1R axis
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Liu, J., Lin, M., Qiao, F. et al. Exosomes Derived from lncRNA TCTN2-Modified Mesenchymal Stem Cells Improve Spinal Cord Injury by miR-329-3p/IGF1R Axis. J Mol Neurosci 72, 482–495 (2022). https://doi.org/10.1007/s12031-021-01914-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-021-01914-7