Abstract
Betulinic acid (BA), a pentacyclic triterpene of natural origin, has been demonstrated to have varied biologic activities including anti-viral, anti-inflammatory, and anti-malarial effects; it has also been found to induce apoptosis in many types of cancer. However, little is known about the effect of BA on normal cells. In this study, the effects of BA on normal neuronal cell apoptosis and the mechanisms involved were studied using differentiated PC12 cells as a model. Treatment with 50 μM BA for 24 h apparently induced PC12 cell apoptosis. In the early stage of apoptosis, the level of intracellular reactive oxygen species (ROS) increased. Afterwards, the loss of the mitochondrial membrane potential, the release of cytochrome c and the activation of caspase-3 occurred. Treatment with antioxidants could significantly reduce BA-induced PC12 cell apoptosis. In conclusion, we report for the first time that BA induced the mitochondrial apoptotic pathway in differentiated PC12 cells through ROS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Betulinic acid (BA), a natural pentacyclic triterpene in abundant resources, can be extracted from the white birch tree, triphyophyllum peltatum and the jujube tree [1–3]. In vitro and in vivo studies have indicated that BA has extensive biological capabilities as anti-viral, anti-inflammatory, anti-lipogenic, anti-malarial and anticancer [2, 4–8]. Among these properties, its anticancer activity has long been a focus of interest.
Recent researches have shown that BA is capable of inducing apoptosis in multiple tumor types, such as melanoma, ovarian cancer, lung cancer and neuroectodermal tumors including neuroblastoma, medulloblastoma and glioblastoma [9–13]. The cytotoxic effect of BA on neuroectodermal tumors has been reported to occur via a direct effect on mitochondria [13]. Some other studies have found that the generation of reactive oxygen species (ROS), the activation of caspases and the upregulation of Bax are involved in the nerve tumor apoptosis induced by BA [10, 14, 15]. Studies on the mechanisms underlying BA-induced cancer cell apoptosis suggest that several signaling pathways and pro-apoptotic factors are involved, such as the MAPK pathway, endoplasmic reticulum stress, and TNF-alpha [16–18]. Furthermore, BA-induced apoptosis is independent of the p53 status in human breast tumor cell lines and human melanoma cells [19, 20].
The cytotoxicity of BA is considered to be selective for tumor cells and not normal cells [17, 21], which makes it possible to be a promising antitumor agent. However, recent studies have suggested that BA also induces eryptosis in human red blood cells [22] and tissue-damaging ROS generation within the CNS [23]. Since BA has a great cytotoxic effect on nervous system tumors [10, 13, 14, 24] and has the potential for application in the treatment of brain tumors, it is necessary to consider the influence of this agent on normal neuronal cells.
In the present study, we aimed to investigate the effect of BA on normal neuronal cells, using the PC12 cell line, which is derived from pheochromocytoma and broadly used as an in vitro model for neurotoxicity research [25]. This study explored whether BA could induce PC12 cell apoptosis. Moreover, we further explored the mechanism of BA-induced PC12 cell apoptosis and searched for the critical factor of the cytotoxicity.
Materials and Methods
Cell Culture
Animal experiments were approved by the Administration Committee of Experimental Animals, Nanjing Medical University. Primary cortical neurons were obtained from the cortices of embryonic day 18 Sprague-Dawley rats. First, the cells (3 × 105 cells/mL) were maintained in poly-l-lysine (Sigma, USA) coated plates in DMEM medium supplemented with 10% heat-inactivated fetal bovine serum. After 4 h of culture, the cells adhered to the wall. Then, the culture medium was replaced by Neurobasal medium supplemented with 2% B27, 0.5 mM glutamine and 1% streptomycin/penicillin at 37 °C with 5% CO2. Half of the medium was changed every 3 days.
PC12 cells were cultured in RPMI 1640 medium supplemented with 10% heat-inactivated horse serum, 5% heat-inactivated fetal bovine serum and 1% streptomycin/penicillin in a humidified atmosphere with 5% CO2 and 95% air at 37 °C. Two days after seeding in the growth medium, the medium was changed to differentiation medium (DMEM supplemented with 1% horse serum, 1% streptomycin/penicillin, 50 ng/mL NGF) for 3 days, and the culture medium was replaced with fresh medium every other day.
Cell Viability Assay
Cell viability was measured by the Cell Counting Kit-8 (CCK-8) (Dojindo, Japan) according to the manufacturer’s instructions. Briefly, primary cortical neurons and PC12 cells were seeded in 96-well plates with 1 × 104 cells/well, and allowed to attach overnight at 37 °C. The cells were treated with various concentrations of BA for 24 h. Then 100 μl CCK-8 was added to each well. The cells were then incubated at 37 °C for 2 h, and the absorbance was detected at 450 nm by a microplate reader (Thermo Scientific, USA). Cell proliferation was expressed as the mean optical density at 450 nm [±SEM (n = 3)].
Assessment of Apoptosis
Flow cytometry using Annexin V-FITC/PI detection kit was used to assess the apoptosis of the PC12 cells. Briefly, the cells were plated in 6-well culture plates at a density of 3 × 106/well, then cultured with 50 μM BA for 24 h. After that, the cells were trypsinized, rinsed with PBS and re-suspended in 400 μl of 1× binding buffer. Then, the cells were stained with 5 μl annexin V-FITC and 5 μl PI in darkness for 20 min at room temperature. Immediately after Annexin-V/PI staining, the samples were analyzed by flow cytometry (Beckman Coulter, USA). The viable cells were annexin V−/PI−, earlyapoptotic cells were annexin V+/PI−, late apoptotic cells were annexin V+/PI+ and the cell debris was annexin V−/PI+.
Nuclear Morphological Observation
The nuclear morphological changes in BA-treated PC12 cells were evaluated using the Hoechst 33342 stain. In brief, the treated cells were incubated with 50 μM BA in a 6-well plate for 24 h. After washing with phosphate buffered saline (PBS), the cells were stained for 10 min with Hoechst 33342 at a concentration of 10 μg/ml in the dark. The cultures were washed twice more with PBS, and the fluorescence was visualized using a fluorescence microscope (Leica DMI3000B, Germany).
Measurement of Mitochondrial Membrane Potential
The mitochondrial membrane potential (MMP) was analyzed by flow cytometry using the JC-1 assay kit (Beyotime, China). JC-1 exhibits potential-dependent accumulation in mitochondria, indicated by a fluorescence emission shift from green (530 nm, FL-1 channel) to red (590 nm, FL-2 channel). In summary, the loss of MMP was indicated by a decrease in the red/green mean fluorescence intensity ratio. After different treatments, the PC12 cells were incubated in 0.5 mL JC-1 working solution for 25 min at 37 °C, then washed and re-suspended in staining buffer and analyzed by a flow cytometer.
Detection of Intracellular ROS Concentration
The formation of intracellular ROS was evaluated using a fluorescent probe, 2′, 7′-dichlorofluorescin diacetate (DCFH-DA). PC12 cells were seeded in 6-well cell culture plates, then incubated with 50 μM BA for 0, 15, 30, and 60 min. The cells were treated with 10 μM of DCFH-DA for 30 min. After washing with PBS, the DCF fluorescence of the cells was measured by flow cytometry.
Preparation of Mitochondrial and Cytosolic Proteins of PC12 Cells
Mitochondria and cytosol were isolated from PC12 cells using the Mitochondrial/cytosol Fractionation Kit (Beyotime, China). The cells were re-suspended and homogenized in isolation buffer. The homogenates were centrifuged at 1000×g for 5 min at 4 °C to remove the nuclei. After that, the mitochondria were pelleted by centrifuging the supernatant at 11,000×g for 10 min at 4 °C. Then, the pellet was re-suspended in lysis buffer for 30 min to dissolve the mitochondrial proteins in the lysis buffer. The supernatant was transferred to another Eppendorf tube and further centrifuged at 12,000×g for 15 min to obtain the cytosolic proteins. The proteins of the mitochondria and cytosol were analyzed by Western blot to detect the release of cytochrome c.
Western Blot Analysis
After different treatments, the PC12 cells were rinsed thrice with ice-cold PBS and lysed in pre-cooled lysis buffer (KeyGEN, China) supplemented with 1% PMSF. After incubation on ice for 30 min, the cells were centrifuged at 4 °C at 12,000×g for 15 min and the supernatant was stored at −20 °C. The protein concentration was determined by the BCA method (Pierce, USA). Equal amounts of protein per sample was loaded in each well, separated on a 12% SDS-PAGE gel, and transferred to PVDF transfer membranes (Merck Millipore, USA). The membranes were blocked with 5% nonfat milk for 2 h, washed with Tris-buffered saline-Tween20 solution (TBST) and then incubated with primary antibodies overnight at 4 °C. After washing three times with TBST, the membranes were incubated with anti-rabbit or anti-mouse horseradish peroxidase-conjugated secondary antibodies for 2 h at room temperature. Then, the protein bands were detected using enhanced chemiluminescent substrate (Pierce, USA). The density of the respective bands was detected and analyzed by the ChemiDocXRS system with Image Lab software (Bio-Rad, USA).
Statistical Analysis
Data were presented as the mean ± SEM for three separate experiments. Statistical analyses were performed by one-way ANOVA followed by Tukey’s multiple comparison test to compare values among various groups or by the Student’s t test to compare values between two groups with the use of SPSS statistical software 20.0. In all cases, the difference between groups was considered statistically significant at P < 0.05.
Results
BA Reduced Cell Viability of Primary Cortical Neurons and PC12 Cells as well as Induced PC12 Cell Apoptosis
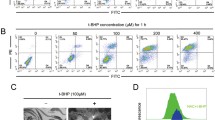
Cell viability was measured by the CCK-8 assay to evaluate the cytotoxic effects of BA on primary cortical neurons and PC12 cells. The cells were treated with different final concentrations (0–100 μM) of BA for 24 h. The primary cortical neuron viability was significantly reduced to 89.79 ± 0.93, 86.59 ± 1.42, 74.33 ± 1.41, 55.68 ± 1.99, and 37.39 ± 0.33% at 5, 10, 25, 50, and 100 μM respectively (Fig. 1a).The PC12 cell viability was significantly reduced to 89.96 ± 1.52, 87.20 ± 2.27, 71.76 ± 1.64, 46.82 ± 1.82, and 29.09 ± 1.83% at 5, 10, 25, 50, and 100 μM respectively (Fig. 1b). These results showed that BA treatment led to a significant decrease in cell viability in a dose-dependent manner. The estimated IC50 values of both primary cortical neurons and PC12 cells were approximately 50 μM. To further investigate the mechanism by which BA reduced normal neuronal cell viability, we used differentiated PC12 cells as the model. First, we studied the effect of BA on apoptosis in PC12 cells using annexin V-FITC/PI double staining followed by flow cytometry analysis. The cells were treated with 50 μM BA or 50 μM DMSO for 24 h. Early apoptotic cells were defined as annexinV+/PI− and late apoptosis cells were defined as annexinV+/PI+. As seen in Fig. 1c, the proportions of apoptotic cells (the summation of early apoptotic cells and late apoptotic cells) were 3.06 ± 0.66 and 33.77 ± 0.58% in the control group and BA-treated group, respectively. BA treatment significantly induced PC12 cell apoptosis (P < 0.05).
BA reduced the viability of primary cortical neurons and induced apoptosis of PC12 cells. Cells were cultured in medium with BA (0–100 μM) for 24 h, and cell viability was measured by CCK-8 assay (a, b). PC12 cells were treated with DMSO or 50 μM BA for 24 h. Cells stained with annexin V and PI were measured with flow cytometry (c). The effects of BA on morphological changes of PC12 cells were detected by treating cells with DMSO or 50 μM BA for 24 h. The morphological changes of the PC12 cell nucleus were detected by Hoechst 33342 stain and observed by fluorescence microscopy (×400) (d). The data are expressed as the mean ± SEM of three separate experiments. **P < 0.01 versus control
BA Induced PC12 Cell Morphological Changes
The morphological change of the PC12 cell nucleus was detected by Hoechst 33342 stain and observed by fluorescence microscopy. Following BA treatment for 24 h, the PC12 cells exhibited an obviously apoptotic morphology, characterized by cell nuclei pyknosis and asymmetric chromatin condensation, compared with control cells (Fig. 1d). These results showed that BA treatment elicited characteristically morphological changes of PC12 cell apoptosis. There was a statistically significant difference between the experimental and control groups regarding the percentage of morphologically changed cells (P < 0.05).
BA Treatment Reduced the Mitochondrial Membrane Potential
To investigate whether BA altered the mitochondrial membrane potential, we treated cells with 50 μM BA for 1 and 3 h. The mitochondrial membrane potential was then investigated with the molecular probe JC-1 using a flow cytometer. As seen in Fig. 2, after incubation with BA for 1 h, the PC12 cells did not show a significant loss of MMP. However, after incubation with BA for 3 h, the PC12 cells then displayed a loss of MMP. The mean fluorescence intensity ratio of red to green was decreased to 50.53 ± 2.09% of that in the control group. The results from flow cytometry suggested that the mitochondrial potential of PC12 cells was significantly decreased with the treatment of BA for 3 h (P < 0.05).
Effect of BA on mitochondrial membrane potential loss in PC12 cells. Cells were incubated with BA (50 μM) for 0–3 h. Mitochondrial membrane potential was detected by JC-1 assay using flow cytometry. The ratio of red/green (FL2/FL1) fluorescence represented the MMP of PC12 cell. The data (% of control) are expressed as the mean ± SEM of three independent experiments. **P < 0.01 versus control
BA Increased the Expression of Bax and Reduced the Expression of Bcl-2
We analyzed the expression of Bax and Bcl-2 in PC12 cells after incubation with 50 μM BA for 0.5–3 h by Western blot analysis. As shown in Fig. 3, BA treatment increased the expression of Bax and decreased the expression of Bcl-2 in a time-dependent manner. Furthermore, the expression of Bax and Bcl-2 were obviously changed after even 1 h of BA treatment (P < 0.05).
The effects of BA on Bax and Bcl-2 expression in PC12 cells. Cells were treated with 50 μM BA for 0, 0.5, 1 and 3 h. Whole cell protein was extracted and the levels of Bax and Bcl-2 were detected by Western blotting analysis. The data are expressed as the mean ± SEM of three independent experiments. *P < 0.05 versus control, **P < 0.01 versus control
BA Induced Cytochrome c Release and Caspase-3 Activation
We measured the expression of cytochrome c and cleaved caspase-3 in BA induced programmed PC12 cell death. The result (Fig. 4a) showed that after exposure to BA, the level of cytochrome c in the mitochondria decreased in a time-dependent fashion, while the level of cytochrome c in the cytosol significantly increased at 6–24 h. These results indicated that BA induced the release of cytochrome c from the mitochondrial inner membrane into the cytosol.
Cytochrome c release and caspase-3 activation after treatment with BA. PC12 cells were treated with 50 μM BA for 0, 6, 12 and 24 h. The mitochondrial and cytosolic fractions were extracted and the level of cytochrome c was detected by Western blotting analysis (a). For evaluating active caspase-3, whole cell protein was harvested followed by Western blotting analysis (b). The data are expressed as the mean ± SEM of three separate experiments. *P < 0.05 versus control, **P < 0.01 versus control
The expression of cleaved caspase-3 increased in a time-dependent manner. Cleaved caspase-3 slightly increased following 6 h of BA treatment, and then significantly increased at 12–24 h, as shown in Fig. 4b. These results suggested that the mitochondrial apoptosis pathway was involved in the BA-induced programmed PC12 cells death.
Effects of BA on Intracellular ROS Production
To examine the effects of BA on intracellular ROS production in PC12 cells, we measured the level of ROS in the treatment and control groups by staining cells with DCFH-DA and using flow cytometry to analyze them. As demonstrated in Fig. 5, the levels of ROS were significantly increased when treated with BA (50 μM) for 15 min and continued to rise from 15 to 60 min after treatment compared to the control (P < 0.05). The results suggested that BA may induce a significant accumulation of ROS in PC12 cells.
The effects of BA on ROS generation in PC12 cells. Cells were treated with 50 μM BA for different time points (0–60 min). The positive control group was incubated with Rosup for 30 min. After treatment, the cells were stained with DCFH-DA and measured by flow cytometry. The data are expressed as the mean fluorescence intensity of three independent experiments. **P < 0.01 versus control
The Role of ROS in the Apoptosis of PC12 Cells Induced by BA
In our study, we found that the intracellular ROS generation was the initial event that occurred in the course of PC12 cell apoptosis induced by BA. Therefore, we suspected that ROS may play a crucial role in the apoptosis. The increase of ROS accumulation could be completely arrested by two different antioxidants, N-acetyl-l-cysteine (NAC) (5 mM) and Trolox (500 μM), as shown in Fig. 6a. Then, we estimated the impact of ROS on BA-induced PC12 cell apoptosis. BA was added to PC12 cells after pretreatment with antioxidants or solvent for half an hour. Afterwards, we found that the apoptosis rate was obviously decreased by both NAC and Trolox, and the antioxidants themselves did not have a remarkable impact on PC12 cell apoptosis (Fig. 6b). The results suggested that the accumulation of intracellular ROS had a major influence on BA-induced apoptosis of PC12 cells.
The roles of BA in BA-induced apoptosis of PC12 cells. Cells were pretreated with NAC (5 mM), Trolox (500 μM) or solvent for 30 min. Then, the cells were exposed to 50 μM BA for different time points. After staining with DCFH-DA, the level of intracellular ROS was measured by flow cytometry (a). After exposure to BA for 24 h, the cell apoptosis was detected with annexin V-FITC/PI double staining followed by flow cytometry (b). The bar chart shows the levels of mitochondrial membrane potential of PC12 cells after different treatments (c). The data are the mean ± SEM of three separate experiments. **P < 0.01 versus corresponding solvent-treated groups. The levels of cytochrome c in mitochondria and cytosol were detected by Western blotting analysis after exposure to BA for 6 h with NAC, Trolox or solvent pretreatment (d). The levels of cleaved caspase-3 were measured with Western blotting after different treatments for 12 h (e). The blots are representative of three independent experiments. Statistical results are presented below the figures. Mean values were derived from three independent experiments. *P < 0.05 versus control, **P < 0.01 versus control, #P < 0.01 compared with indicated groups
To further understand the mechanism of ROS on the apoptosis of PC12 cells induced by BA, we investigated the relationship between ROS and the mitochondrial apoptotic pathways. According to the time sequence of the mitochondrial apoptosis pathway, we first investigated the relevance of the ROS level and the loss of mitochondrial membrane potential. The bar chart revealed that after treatment with antioxidants and after the ROS levels dropped back to normal, the loss of the mitochondrial membrane potential was apparently alleviated (Fig. 6c). Subsequently, this investigation found that pre-incubation of PC12 cells with NAC or Trolox before exposure to BA for 6 h caused a significant decrease in cytochrome c release compared with cells without antioxidant treatment, as shown in Fig. 6d. In addition, the Western blotting analysis showed that BA-induced caspase-3 activation was markedly inhibited by treatment with NAC or Trolox (Fig. 6e). These finding suggested that ROS may trigger PC12 cell apoptosis through the mitochondrial apoptotic pathway, which can be significantly blocked by antioxidants.
Discussion
This study was carried out to investigate the influence of BA on normal neuronal cells. BA exerted cytotoxicity on both primary cortical cells and differentiated PC12 cells, with IC50 values of approximately 50 μM. A significant apoptotic phenomenon was observed when PC12 cells were treated with 50 μM BA for 24 h. We showed that BA increased the intracellular ROS levels, activated the mitochondrial apoptosis pathway and triggered caspase-3 activation.
Previous studies have shown that BA can induce programmed cell death in many types of tumors, with IC50 values of approximately 10–30 μM [14, 15, 26]. Similarly, this study showed that PC12 cell apoptosis occurred after treatment with BA, and the IC50 value was 50 μM after 24 h. This result suggests that BA is more toxic to tumor cells than neuronal cells. However, our observations indicated the cytotoxicity of BA to normal neuronal cells, which conflicts with some previous views [21]. At the same time, some recent studies have also reported that BA had cytotoxic effects on normal cells [22, 23].
BA has been reported to directly target mitochondria during induced cell apoptosis [13, 26, 27]. After the mitochondrial membrane potential decreased, the release of soluble mitochondrial proteins occurred, which then mediated the cytosolic caspase activation [27–29]. Thus, we investigated whether the mitochondrial apoptosis pathway was involved in BA-induced PC12 cell apoptosis. A significant loss of the mitochondrial membrane potential was observed 3 h after exposure of PC12 cells to 50 μM BA. Additionally, the Western blot analysis indicated that the release of cytochrome c from the mitochondrial inner membrane into the cytosol occurred after treatment with BA for 6 h. Meanwhile, we observed that the level of cleaved caspase-3 increased slightly, and the level of these pro-apoptotic proteins significantly increased 12 h after treating PC12 cells with BA. These results suggested that the mitochondrial apoptosis pathway was involved in BA-induced programmed PC12 cell death. In addition, we found that at the early stage of BA treatment, the level of Bax was upregulated and the level of Bcl-2 was downregulated. This finding is in accordance with the conclusion of previous studies that BA treatment resulting in the upregulation of death-promoting proteins from Bcl-2 family [15, 20]. However, these studies did not report the downregulation of Bcl-2. Further, an upregulation of Bcl-2 was observed in glioblastoma cells incubated with BA [15], and a suppression of Bax expression was reported in malignant head and neck cancer cells treated with BA [30]. These contradictory views suggest that Bcl-2 family proteins may have an influence on the mitochondrial apoptosis pathway, but they are not the determining factor in initiating the mitochondrial apoptotic pathway.
As classical damaging agents, ROS have been reported to generate not only in PC12 cell apoptosis induced by some cytotoxic drugs, but also in several kinds of tumor cells induced by BA [14, 16, 31–34]. Studies have reported that the activation of caspase and the loss of the mitochondrial membrane potential is preceded by generation of ROS [15, 35–37]. Therefore, we examined the effects of BA on PC12 cells’ intracellular ROS. When treating PC12 cells with 50 μM BA for 15 min, the levels of ROS were significantly increased by almost 100% than that of the control, and the increase was in a time-dependent manner from 15 to 60 min. It was found that the generation of ROS preceded the activation of the mitochondrial apoptotic pathway in BA-induced PC12 cell apoptosis. Thus, we suspected that the accumulation of ROS may play a part in triggering mitochondrial apoptotic way. We used NAC and Trolox to scavenge ROS and inhibit the ROS generation. The results showed that both NAC and Trolox could inhibit the PC12 cell apoptosis induced by BA. Meanwhile, the antioxidants significantly blocked the BA-induced loss of MMP, the release of cytochrome c and the activation of caspase-3. These results support the speculation that ROS triggers the mitochondrial apoptotic pathway of PC12 cell apoptosis. However, the mitochondrial pathway is not completely blocked by antioxidants, so we suspect that this pathway is also influenced by other factors independent of ROS.
The mechanism of ROS generation induced by BA has not been clarified. The source of intracellular ROS includes peroxisomes, NADPH oxidase and the mitochondrial electron transport chain [38–41]. Further research is needed to understand the mechanism between BA and ROS generation. Previous research has found that nanoderivatives of betulinic acid could readily and effectively cross the blood–brain barrier (BBB) [42]. However, the ability of BA to cross the BBB was not clearly demonstrated and requires further study and discussion for clinical application.
In conclusion, the present study was the first to note that BA induces the apoptosis of PC12 cells. The generation of ROS occurs in the early stage of PC12 cell apoptosis and triggers the mitochondrial apoptotic pathway. However, additional factors are involved in the activation of this pathway, and the mechanism of ROS generation induced by BA needs to be further determined. Our findings have some degree of significance and value for the continuing research, development and clinical application of BA.
References
Dubey KK, Goel N (2013) Evaluation and optimization of downstream process parameters for extraction of betulinic acid from the bark of Ziziphus jujubae L. Sci World J. doi:10.1155/2013/469674
Bringmann G, Saeb W, Assi LA, Francois G, Sankara Narayanan AS, Peters K, Peters EM (1997) Betulinic acid: isolation from Triphyophyllum peltatum and Ancistrocladus heyneanus, antimalarial activity, and crystal structure of the benzyl ester. Planta Med 63:255–257
Son LB, Kaplun AP, Spilevskii AA, Andiia-Pravdivyi Iu E, Alekseeva SG, Gribor’ev VB, Shvets VI (1998) Synthesis of betulinic acid from betulin and study of its solubilization usingliposomes. Bioorganicheskaia khimiia 24:787–793
Hong EH, Song JH, Kang KB, Sung SH, Ko HJ, Yang H (2015) Anti-influenza activity of betulinic acid from Zizyphus jujuba on influenza A/PR/8 virus. Biomol Ther 23:345–349
Qian K, Yu D, Chen CH, Huang L, Morris-Natschke SL, Nitz TJ, Salzwedel K, Reddick M, Allaway GP, Lee KH (2009) Anti-AIDS agents. 78. Design, synthesis, metabolic stability assessment, and antiviral evaluation of novel betulinic acid derivatives as potent anti-human immunodeficiency virus (HIV) agents. J Med Chem 52:3248–3258
Quan HY, Kim do Y, Kim SJ, Jo HK, Kim GW, Chung SH (2013) Betulinic acid alleviates non-alcoholic fatty liver by inhibiting SREBP1 activity via the AMPK-mTOR-SREBP signaling pathway. Biochem Pharmacol 85:1330–1340
Costa JF, Barbosa-Filho JM, Maia GL, Guimaraes ET, Meira CS, Ribeiro-dos-Santos R, de Carvalho LC, Soares MB (2014) Potent anti-inflammatory activity of betulinic acid treatment in a model of lethal endotoxemia. Int Immunopharmacol 23:469–474
Yasukawa K, Takido M, Matsumoto T, Takeuchi M, Nakagawa S (1991) Sterol and triterpene derivatives from plants inhibit the effects of a tumor promoter, and sitosterol and betulinic acid inhibit tumor formation in mouse skin two-stage carcinogenesis. Int Soc Cell 48:72–76
Pisha E, Chai H, Lee IS, Chagwedera TE, Farnsworth NR, Cordell GA, Beecher CW, Fong HH, Kinghorn AD, Brown DM et al (1995) Discovery of betulinic acid as a selective inhibitor of human melanoma that functions by induction of apoptosis. Nat Med 1:1046–1051
Fulda S, Jeremias I, Steiner HH, Pietsch T, Debatin KM (1999) Betulinic acid: a new cytotoxic agent against malignant brain-tumor cells. Int J Cancer 82:435–441
Wang YJ, Liu JB, Dou YC (2015) Sequential treatment with betulinic acid followed by 5-fluorouracil shows synergistic cytotoxic activity in ovarian cancer cells. Int J Clin Exp Pathol 8:252–259
Hsu TI, Wang MC, Chen SY, Huang ST, Yeh YM, Su WC, Chang WC, Hung JJ (2012) Betulinic acid decreases specificity protein 1 (Sp1) level via increasing the sumoylation of sp1 to inhibit lung cancer growth. Mol Pharmacol 82:1115–1128
Fulda S, Debatin KM (2000) Betulinic acid induces apoptosis through a direct effect on mitochondria in neuroectodermal tumors. Med Pediatr Oncol 35:616–618
Wick W, Grimmel C, Wagenknecht B, Dichgans J, Weller M (1999) Betulinic acid-induced apoptosis in glioma cells: a sequential requirement for new protein synthesis, formation of reactive oxygen species, and caspase processing. J Pharmacol Exp Ther 289:1306–1312
Fulda S, Friesen C, Los M, Scaffidi C, Mier W, Benedict M, Nunez G, Krammer PH, Peter ME, Debatin KM (1997) Betulinic acid triggers CD95 (APO-1/Fas)- and p53-independent apoptosis via activation of caspases in neuroectodermal tumors. Cancer Res 57:4956–4964
Tan Y, Yu R, Pezzuto JM (2003) Betulinic acid-induced programmed cell death in human melanoma cells involves mitogen-activated protein kinase activation. Clin Cancer Res 9:2866–2875
Hsu TI, Chen YJ, Hung CY, Wang YC, Lin SJ, Su WC, Lai MD, Kim SY, Wang Q, Qian K, Goto M, Zhao Y, Kashiwada Y, Lee KH, Chang WC, Hung JJ (2015) A novel derivative of betulinic acid, SYK023, suppresses lung cancer growth and malignancy. Oncotarget 6:13671–13687
Wang P, Li Q, Li K, Zhang X, Han Z, Wang J, Gao D, Li J (2012) Betulinic acid exerts immunoregulation and anti-tumor effect on cervical carcinoma (U14) tumor-bearing mice. Die. Pharmazie 67:733–739
Tiwari R, Puthli A, Balakrishnan S, Sapra BK, Mishra KP (2014) Betulinic acid-induced cytotoxicity in human breast tumor cell lines MCF-7 and T47D and its modification by tocopherol. Cancer Invest 32:402–408
Selzer E, Pimentel E, Wacheck V, Schlegel W, Pehamberger H, Jansen B, Kodym R (2000) Effects of betulinic acid alone and in combination with irradiation in human melanoma cells. J Invest Dermatol 114:935–940
Zuco V, Supino R, Righetti SC, Cleris L, Marchesi E, Gambacorti-Passerini C, Formelli F (2002) Selective cytotoxicity of betulinic acid on tumor cell lines, but not on normal cells. Cancer Lett 175:17–25
Gao M, Lau PM, Kong SK (2014) Mitochondrial toxin betulinic acid induces in vitro eryptosis in human red blood cells through membrane permeabilization. Arch Toxicol 88:755–768
Blazevski J, Petkovic F, Momcilovic M, Paschke R, Kaluderovic GN, Mostarica Stojkovic M, Miljkovic D (2013) Betulinic acid regulates generation of neuroinflammatory mediators responsible for tissue destruction in multiple sclerosis in vitro. Acta Pharmacol Sin 34:424–431
Bache M, Zschornak MP, Passin S, Kessler J, Wichmann H, Kappler M, Paschke R, Kaluderovic GN, Kommera H, Taubert H, Vordermark D (2011) Increased betulinic acid induced cytotoxicity and radiosensitivity in glioma cells under hypoxic conditions. Radiat Oncol 6:111
Greene LA, Aletta JM, Rukenstein A, Green SH (1987) PC12 pheochromocytoma cells: culture, nerve growth factor treatment, and experimental exploitation. Methods Enzymol 147:207–216
Mullauer FB, Kessler JH, Medema JP (2010) Betulinic acid, a natural compound with potent anticancer effects. Anticancer Drugs 21:215–227
Fulda S, Scaffidi C, Susin SA, Krammer PH, Kroemer G, Peter ME, Debatin KM (1998) Activation of mitochondria and release of mitochondrial apoptogenic factors by betulinic acid. J Biol Chem 273:33942–33948
Kluck RM, Bossy-Wetzel E, Green DR, Newmeyer DD (1997) The release of cytochrome c from mitochondria: a primary site for Bcl-2 regulation of apoptosis. Science 275:1132–1136
Sato A, Fujiwara H, Oku H, Ishiguro K, Ohizumi Y (2004) Alpha-mangostin induces Ca2+-ATPase-dependent apoptosis via mitochondrial pathway in PC12 cells. J Pharmacol Sci 95:33–40
Thurnher D, Turhani D, Pelzmann M, Wannemacher B, Knerer B, Formanek M, Wacheck V, Selzer E (2003) Betulinic acid: a new cytotoxic compound against malignant head and neck cancer cells. Head Neck 25:732–740
Lanju X, Jing X, Shichang L, Zhuo Y (2014) Induction of apoptosis by antimycin A in differentiated PC12 cell line. Journal of applied toxicology : JAT 34:651–657
Lee YJ, Choi SY, Yang JH (2014) NMDA receptor-mediated ERK 1/2 pathway is involved in PFHxS-induced apoptosis of PC12 cells. Sci Total Environ 491–492:227–234
Jiang H, Li J, Zhou T, Wang C, Zhang H, Wang H (2014) Colistin-induced apoptosis in PC12 cells: involvement of the mitochondrial apoptotic and death receptor pathways. Int J Mol Med 33:1298–1304
Kashyap MP, Singh AK, Siddiqui MA, Kumar V, Tripathi VK, Khanna VK, Yadav S, Jain SK, Pant AB (2010) Caspase cascade regulated mitochondria mediated apoptosis in monocrotophos exposed PC12 cells. Chem Res Toxicol 23:1663–1672
Kim HJ, Park C, Han MH, Hong SH, Kim GY, Hoon Hong S, Deuk Kim N, Choi YH (2016) Baicalein Induces Caspase-dependent Apoptosis Associated with the Generation of ROS and the Activation of AMPK in Human Lung Carcinoma A549 Cells. Drug Dev Res 77:73–86
Lim EJ, Heo J, Kim YH (2015) Tunicamycin promotes apoptosis in leukemia cells through ROS generation and downregulation of survivin expression. Apoptosis 20:1087–1098
Kwon SJ, Lee JH, Moon KD, Jeong IY, Ahn DU, Lee MK, Seo KI (2014) Induction of apoptosis by isoegomaketone from Perilla frutescens L. in B16 melanoma cells is mediated through ROS generation and mitochondrial-dependent, -independent pathway. Food Chem Toxicol 65:97–104
Sandalio LM, Rodriguez-Serrano M, Romero-Puertas MC, del Rio LA (2013) Role of peroxisomes as a source of reactive oxygen species (ROS) signaling molecules. Sub-Cell Biochem 69:231–255
Choi DH, Lee KH, Kim JH, Seo JH, Kim HY, Shin CY, Han JS, Han SH, Kim YS, Lee J (2014) NADPH oxidase 1, a novel molecular source of ROS in hippocampal neuronal death in vascular dementia. Antioxid Redox Signal 21:533–550
Huang W, Li D, Liu Y (2014) Mitochondrial electron transport chain is involved in microcystin-RR induced tobacco BY-2 cells apoptosis. J Environ Sci (China) 26:1930–1935
Drose S, Brandt U (2012) Molecular mechanisms of superoxide production by the mitochondrial respiratory chain. Adv Exp Med Biol 748:145–169
Das J, Samadder A, Das S, Paul A, Khuda-Bukhsh AR (2016) Nanopharmaceutical approach for enhanced anti-cancer activity of betulinic acid in lung-cancer treatment via activation of PARP: interaction with DNA as a target: anti-cancer potential of nano-betulinic acid in lung cancer. J Pharmacopunct 19:37–44
Acknowledgements
This study was supported by grants from the National Natural Science Foundation of China (No. 81171147), “Xingwei Project” Key Personal Medical Research Foundation of Health Department of Jiangsu Province (No. RC201156), “Six Categories of Key Person” Research Foundation of Jiangsu Province (No. 069), and the Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (No. JX10231801).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interests.
Additional information
Xi Wang and Xiaocheng Lu have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Wang, X., Lu, X., Zhu, R. et al. Betulinic Acid Induces Apoptosis in Differentiated PC12 Cells Via ROS-Mediated Mitochondrial Pathway. Neurochem Res 42, 1130–1140 (2017). https://doi.org/10.1007/s11064-016-2147-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-016-2147-y