Abstract
Elevated levels of free fatty acids (FFAs) in plasma and increased incidence of chronic systemic inflammation are associated with obesity. In the brain, activated microglia are believed to play different roles during inflammation that may either be neuroprotective or promote neurodegeneration. Here, we have investigated the effects of FFAs on microglial response to inflammatory stimuli. Our results indicate that the saturated FFA palmitate on its own induces alternative activation of BV-2 microglia cells. Further, pre-exposure to palmitate changed the response of microglia to lipopolysaccharide (LPS). We show that palmitate affects the mRNA levels of the pro-inflammatory cytokines interleukin-1β and interleukin-6. The transcription factor CCAAT/enhancer-binding protein δ is also affected by pre-exposure to palmitate. Furthermore, the phagocytic activity of microglia was investigated using fluorescent beads. By analyzing the bead uptake by fluorescence-activated cell sorting, we found that palmitate alone, as well as together with LPS, stimulated the phagocytic activity of microglia. Taken together, our results suggest that exposure of microglia to increased levels of free fatty acids may alter the consequences of classical inflammatory stimuli.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
High-calorie diets with increased proportion of saturated fats, together with lack of exercise, are contributing to a growing number of obese and diabetic people in the world. A correlation between high-fat diets and impaired cognitive function in humans has been proposed (Morris et al. 2004; Solfrizzi et al. 2005; Morris et al. 2006). Further, obesity and type 2 diabetes (T2D) increase the risk for developing dementia and Alzheimer’s disease (AD) (Ott et al. 1999; Biessels et al. 2005). Obesity is also linked to a chronic low-grade systemic inflammation (Mohamed-Ali et al. 1997; Yudkin et al. 1999). High-fat diets have been shown to cause neuroinflammation, i.e., inflammatory responses in the brain, as recently reviewed by Cai (2013), and neuroinflammation is in turn linked to neurodegeneration (Block and Hong 2005).
Elevated levels of circulating free fatty acids (FFAs) are observed in obese individuals, and uptake of FFAs into the brain has been shown to increase in obese subjects with metabolic syndrome (Karmi et al. 2010). One of the most abundant types of saturated fatty acids in mammals is palmitate. A number of studies on the inflammatory response of monocytes or macrophages exposed to FFAs/palmitate have been performed. However, there are contradictory reports showing either that palmitate on its own can induce mRNA expression of inflammatory cytokines (Haversen et al. 2009; Prieur et al. 2011) or that it can only affect maturation and secretion of the cytokines (Wen et al. 2011). In the brain, the inflammatory response is mediated by activated microglia cells and reactive astrocytes. The classically activated microglia cells show increased production and secretion of pro-inflammatory cytokines such as interleukin (IL)-1β, tumor necrosis factor (TNF)-α, and IL-6, while alternatively activated cells express more phagocytic activity and neuroprotective factors.
The neuroinflammatory response is regulated mainly at the level of transcription. The transcription factor nuclear factor-κB (NF-κB) is a major player involved in neuroinflammation and is an early responder to inflammatory stimuli. In addition, CCAAT/enhancer-binding proteins (C/EBPs), which have the ability to modulate neuroinflammation as well as to form complexes with NF-κB, are of importance. C/EBPδ, which is expressed at low levels during normal conditions but is rapidly up-regulated by a variety of extracellular stimuli such as lipopolysaccharide (LPS), IL-1β, and TNF-α (Stein et al. 1993; Yin et al. 1996; Litvak et al. 2009), is of special interest. Previous studies have indicated that C/EBPδ is dysregulated in AD patients (Li et al. 2004) and in an AD mice model (Ramberg et al. 2011). Together NF-κB and C/EBPδ regulate expression of some of the most important pro-inflammatory factors (Tak and Firestein 2001; Valente et al. 2013). As reviewed by Cai (2013), obesity and overnutrition may lead to NF-κB-dependent hypothalamic inflammation and may induce neuronal apoptosis.
We have previously shown that palmitate, at a moderately elevated concentration, induced expression of markers for alternative activation in glial cells and that the mRNA expression of the pro-inflammatory cytokine TNF-α in the brain of obese ob/ob mice was lowered (Kappe et al. 2012). Microglial cytokine expression changes depending on extracellular stimuli, and microglia can switch between different activation profiles depending on its microenvironment (cf. Colton 2009). Therefore, it is of great importance to further elucidate the effects and underlying mechanisms in microglia after exposure to increased levels of FFAs. This may also lead to a better understanding of obesity and T2D as risk factors for AD and neurodegeneration.
Here, we have investigated how the microglia response to classical inflammatory stimuli is affected by exposure to palmitate. Our results show that, on the one hand, palmitate on its own seems to promote an alternatively activated phenotype with increased phagocytic activity and, on the other hand, it also alters the response to LPS which otherwise triggers a classical inflammatory reaction.
Material and Methods
Cell Culture and Reagents
Cells from the immortalized murine microglia cell line BV-2 (Blasi et al. 1990) were cultured in Dulbecco’s modified eagle medium Glutamax supplemented with 5 % fetal bovine serum, 10,000 U/ml penicillin, and 10 mg/ml streptomycin sulfate.
Palmitate (sodium palmitate, Sigma-Aldrich) was dissolved in 12.5 % ethanol by heating and thereafter diluted in culture media supplemented with 0.5 % bovine serum albumin (BSA; fatty acid free, Sigma-Aldrich). Cells were treated with 0.125 mM palmitate or vehicle with equal amounts of ethanol for 24 h before analysis and/or 10 ng/ml LPS (Sigma-Aldrich) for 3 h before analysis. In the uptake studies, cells were exposed to palmitate/vehicle for 24 h and, for the last 5 h, co-incubated with 1-μm fluorescent (red 580/605) latex beads (F8821, Invitrogen) with or without LPS.
Immunofluorescence and Confocal Microscopy
Cells were grown on glass coverslips, washed twice in phosphate-buffered saline (PBS; pH 7.4), subsequently fixed on ice in 4 % paraformaldehyde (PFA) for 30 min, and permeabilized with 0.5 % Triton X-100 in PBS for 5 min. Cells were then blocked in PBS containing 5 % milk and 0.1 % Tween-20 (blocking solution) at room temperature for 45 min. Cells were incubated with primary antibody C/EBPδ (C-22)X (1:500 dilution; Santa Cruz Biotechnology) or NF-κB p65 (SC-109) (1:100 dilution; Santa Cruz Biotechnology) in blocking solution at room temperature for 45 min, washed in blocking solution three times, incubated with secondary antibody Alexa 488 goat anti-rabbit IgG (A11008, Invitrogen) and Alexa 568 goat anti-rabbit IgG (A11011, Invitrogen) (1:2,000), respectively, for 45 min at room temperature, and thereafter washed three times in blocking solution. For deoxyribonucleic acid (DNA) staining, cells were incubated with Hoechst (33258) for 5 min before final washes and mounting on glass slides with vector shield (Vector Laboratories, Inc.). The samples were analyzed on a Leica DMIRBE fluorescence microscope or a Zeiss LSM 780 confocal microscope.
In the uptake studies, cells were washed twice in PBS and fixed in 4 % PFA and then washed again twice before addition of fluorescent wheat germ agglutinin (Alexa fluor 633 conjugated, Invitrogen) for 10 min at room temperature. The samples were washed again before mounting on glass slides with vector shield and sealed with nail polish.
Fluorescence-Activated Cell Sorting
Cells were washed with ice-cold PBS, trypsinated, resuspended in culture medium, and centrifuged for 5 min at 16,000 × g. The medium was removed and the cells were resuspended in PBS. Flow cytometric analysis of 100,000 events was made immediately after, using a FACSCalibur instrument (Becton Dickinson Immunocytometry Systems).
Western Blot
Cells were scraped in lysis buffer (radio-immunoprecipitation assay buffer containing 50 mM Tris–HCl (pH 8), 150 mM NaCl, 1 % NP-40, 0.5 % sodium deoxycholate, 0.1 % sodium dodecyl sulfate, 1× protease inhibitor cocktail (Roche)) and subsequently shaken for 30 min at 4 °C. Thereafter, the samples were centrifuged at 16,000 × g for 10 min. Supernatants were collected and stored in 6× sample buffer. Determination of protein concentrations by bicinchoninic acid (Pierce) and Western blot were performed as described earlier (Ramberg et al. 2011), using primary antibody directed against C/EBPδ ((C-22)X, 1:4,000 dilution; Santa Cruz Biotechnologies) or actin ((I-19)-R, 1:500 dilution; Santa Cruz Biotechnologies) and secondary antibody horseradish peroxidase-coupled anti-rabbit IgG 1:5,000 dilution for the C/EBPδ blots and 1:10,000 for the actin blots (GE Healthcare). Blots were incubated in Thermo Fisher’s SuperSignal West Dura Extended Duration for 5 min and visualized in ChemiDoc imaging system (Bio-Rad).
RNA Extraction, cDNA Synthesis, and qPCR
BV-2 cells were lysed and RNA-extracted using Fermentas GeneJet RNA Purification Kit (Thermo Scientific) according to the manufacturer’s instructions including DNAse treatment (Thermo Scientific). Complementary DNA (cDNA) was synthesized for quantitative polymerase chain reaction (qPCR) using Fermentas RevertAid H Minus First strand cDNA Synthesis Kit (Thermo Scientific) according to the manufacturer’s instructions. All primers were designed using Invitrogen custom primer design software (Invitrogen, Inc). The sequences of primers used are given in Table 1.
A qPCR kit with SYBR Green (Fermenta’s Maxima SYBR Green/Fluorescein qPCR Master Mix, Thermo Scientific) was used for real-time qPCR. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as housekeeping gene for normalization.
Statistical Analysis
Data were analyzed by analysis of variance followed by Tukey’s post hoc test. P < 0.05 was considered statistically significant.
Results
Palmitate-Induced Expression of Arginase 1, a Marker for Alternative Microglial Expression, is Counteracted by LPS
Previously we have observed that moderate levels of palmitate down-regulate the mRNA expression and secretion of TNF-α, a known marker for classically activated microglia, and up-regulate the levels of the alternative marker arginase 1 (Arg1) in BV-2 microglia cells (Kappe et al. 2012). In this study, we aimed to investigate if increased background levels of palmitate could alter the acute response of microglia to a typical inflammatory stimulus. Cells were exposed to 0.125 mM palmitate for 24 h to model an in vivo situation with increased levels of FFAs. During the last 3 h, LPS (10 ng/ml) was added. This concentration is in the lower end of the range commonly used and was chosen to mimic a relatively low-grade inflammation. In agreement with our earlier study (Kappe et al. 2012), we observed significant up-regulation of Arg1 mRNA levels after 24 h of exposure to palmitate (Fig. 1). Addition of LPS during the last 3 h of treatment counteracted this effect of palmitate, which could be due to the decreased rate of mRNA synthesis. However, effects on mRNA stability cannot be excluded. The levels of Arg1 mRNA were already affected by palmitate by the time of LPS addition and did not significantly differ from those of 24-h palmitate-exposed cells (Arg1 mRNA increased by 109 ± 32 % after 21 h and 169 ± 21 % after 24 h compared with control; this was significantly different from control at P < 0.01 and P < 0.001, respectively, n = 3–5). In addition, compared to control, stimulation with LPS in the absence of palmitate even showed a tendency to decrease the levels of Arg1 mRNA. The expression levels of chitinase 3-like 3 mRNA, which is another marker for alternative activation, were also significantly increased by palmitate (by 165 ± 18 %, significantly different from control at P < 0.05, n = 3), and the levels after LPS exposure showed a trend toward down-regulation (not shown), as observed for Arg1.
LPS counteracts palmitate-induced expression of arginase 1 (Arg1) mRNA. Cells were exposed to palmitate (Palm)/vehicle for 24 h in the absence or presence of LPS during the last 3 h. Arg1 mRNA levels were measured by qPCR and normalized against the levels of GAPDH mRNA. ***P < 0.001, significantly different compared to control or as otherwise indicated; n = 3
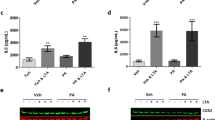
C/EBPδ Expression Levels Increase in Cells Co-treated with Palmitate and LPS
We also investigated the effect of palmitate in combination with LPS on markers and regulators of neuroinflammation. NF-κB and C/EBPs are major players in the inflammatory response and regulate expression of several pro-inflammatory cytokines. C/EBPδ has been shown to regulate the expression of pro-inflammatory factors including IL-6 in glial cells (Valente et al. 2013). Our results show that palmitate alone did not have any effect on C/EBPδ mRNA or protein levels (Fig. 2a–c). Treatment with LPS resulted in significant up-regulation of C/EBPδ mRNA (Fig. 2a), in accordance with a previous study performed on the same type of cells (Ejarque-Ortiz et al. 2010). Further, we found that pre-treatment with palmitate led to significantly higher LPS-induced mRNA expression and a trend toward an up-regulation of protein levels of C/EBPδ (Fig. 2a, b). These effects were further confirmed by immunofluorescence microscopy (Fig. 2c). C/EBPδ immunoreactivity was increased by LPS and appeared even stronger after palmitate and LPS co-treatment.
Palmitate potentiates LPS-induced expression of C/EBPδ mRNA. a–c Cells were exposed as described in Fig. 1. a C/EBPδ mRNA levels were measured by qPCR and normalized against the levels of GAPDH mRNA. b C/EBPδ protein levels were analyzed by Western blot and normalized against actin. c C/EBPδ expression was analyzed by fluorescence microscopy. d Cells were exposed to palmitate/vehicle for 24 h in the presence or absence of LPS during the last 30 min. Cellular localization of NF-κB was analyzed by confocal microscopy using antibodies directed to p65. *P < 0.05, ***P < 0.001, significantly different compared to control or as otherwise indicated; n = 3
It has been shown that C/EBPδ and NF-κB can form heteromers and work in concert. Translocation of NF-κB into the nucleus is considered a measure of its activation and was analyzed by confocal microscopy after stimulation with palmitate. As expected, a larger fraction of NF-κB was observed in the nucleus after exposure to LPS. However, our results indicate neither any clear effects of palmitate by itself compared to control nor does the nuclear localization of NF-κB seem to differ between the LPS-treated cells compared to LPS- and palmitate-exposed cells (Fig. 2d).
Palmitate Increases LPS-Induced IL-1β mRNA Expression but Negatively Affects IL-6 mRNA Levels
The inflammatory cytokines IL-1β, TNF-α, and IL-6 are commonly up-regulated in classically activated microglia. We did not observe any effect on IL-1β mRNA expression by palmitate alone (Fig. 3a). However, pre-exposure to palmitate did increase the mRNA expression of IL-1β in response to LPS (Fig. 3a). Interestingly, palmitate did not have any effect on LPS-induced TNF-α expression. LPS alone and LPS together with palmitate both increased TNF-α production by 35–40-fold compared to control (data not shown). Contrary to other studies showing palmitate-induced up-regulation of IL-6 in monocytes, in our study no changes in IL-6 mRNA levels were observed in the BV-2 microglia cells in response to palmitate (Fig. 3b). Further, palmitate reduced the LPS-induced increase of IL-6 mRNA levels (Fig. 3b). This indicates that exposure to elevated levels of FFAs could result in an altered inflammatory response that involves enhanced up-regulation of IL-1β expression and reduced up-regulation of IL-6.
Palmitate differentially affects LPS-induced IL-1β and IL-6 mRNA levels. Cells were treated as described in Fig. 1. mRNA levels of a IL-1β and b IL-6 were measured by qPCR and normalized against the levels of GAPDH mRNA. *P < 0.05, ***P < 0.001, significantly different compared to control or as otherwise indicated; n = 3
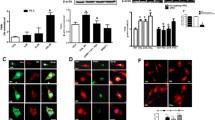
Phagocytic Activity of Microglia is Triggered by Palmitate and Further Enhanced by LPS
It is known that LPS stimulates the phagocytic activity of microglia. Here, we wanted to investigate if palmitate can alter this function. BV-2 microglia cells were exposed to palmitate or vehicle for 24 h with or without LPS stimulation during the last 5 h. Fluorescent beads were added to the cultures during the last 5 h of treatment, and the uptake was analyzed by confocal microscopy and fluorescence-activated cell sorting (FACS) analysis. Our results show that both palmitate and LPS induced the uptake of beads and that co-exposure to palmitate and LPS results in an increased effect (Fig. 4a–d).
Both palmitate and LPS stimulate microglia phagocytosis and co-exposure further enhances the effect. Cells were exposed to palmitate/vehicle for 24 h in the absence or presence of LPS during the last 5 h. Fluorescent latex beads were added for the last 5 h. a–c Uptake of beads monitored by FACS. a Cells were gated based on their forward and side scatter to exclude cell debris as well as cell aggregates. b Representative graph showing the uptake of beads by microglia. c Quantification and statistical analysis of cells taking up two or more beads. d Confocal image of phagocytosed beads after LPS treatment. *P < 0.05, ***P < 0.001, significantly different compared to control or otherwise indicated; n = 3–4
Discussion
Obesity is a growing problem with increased risk for T2D and other chronic metabolic disorders. A consequence of obesity is elevated levels of FFAs, including the saturated FFA palmitate, which have been shown to affect the peripheral immune system (Mohamed-Ali et al. 1997; Yudkin et al. 1999). LPS-induced secretion of the pro-inflammatory cytokines IL-1β and TNF-α has been shown to increase in macrophages exposed to palmitate (Haversen et al. 2009; Wen et al. 2011; Schilling et al. 2013). Microglia, which are considered the main immune cells of the brain, have been attributed similar properties and functions as macrophages. However, it is not clear how palmitate affects microglia. In this study, we show that palmitate increases markers for alternative activation in BV-2 microglia cells and facilitates phagocytosis. This suggests that although palmitate does have an effect on microglia, it does not act as a classical pro-inflammatory stimulus. However, here we demonstrate that it can change the response to classical pro-inflammatory stimuli. When co-incubated with LPS, the palmitate-induced expression of the marker for alternative activation Arg1 is abolished. At this point, we cannot say whether the effects of palmitate and LPS are on the rate of synthesis or on the stability of the mRNA. Moreover, although palmitate alone does not induce any increase in pro-inflammatory cytokines, it changes the profile of pro-inflammatory cytokine mRNA expression in microglia in response to inflammatory stimuli. It should be noted that palmitate and LPS could act on the same receptors—toll-like receptor 4 (Wang et al. 2012), and therefore, the involvement of desensitization of receptors or signaling pathways upon co-stimulation cannot be excluded. However, the levels of IL-1β mRNA were elevated, the mRNA levels of IL-6 were decreased, while the mRNA levels of TNF-α remained unchanged. It is possible that an increase in IL-1β and a decrease in IL-6 expression might affect the outcome of a potential neuroinflammation. IL-6, although most often viewed as a pro-inflammatory cytokine, can also have anti-inflammatory and neuroprotective functions, as reviewed by, e.g., Jüttler et al. (2002). One could speculate that the response to other inflammatory stimuli such as Aβ peptides (which are accumulated in AD and known to induce a neuroinflammatory response) could also be affected by elevated levels of FFAs. This would be of great interest to further investigate.
As mentioned above, we did not observe any effects of palmitate on its own on mRNA levels of the cytokines investigated. Previous studies have reported contradictory results. On the one hand, enhanced mRNA expression of inflammatory cytokines induced by palmitate has been demonstrated in microglia (Wang et al. 2012). However, lower ratios of BSA to palmitate, compared to most other studies, were used in that study. On the other hand, several studies on macrophages/monocytes support our finding that palmitate does not have any effect on cytokine mRNA levels (Wen et al. 2011; Little et al. 2012; Schilling et al. 2013); instead, palmitate has been proposed to increase their secretion. In mice macrophages pre-treated with LPS, the effect of palmitate on IL-1β secretion was shown to be mediated through inflammasome activation (Wen et al. 2011). In agreement with this, in the present study, palmitate on its own did not seem to increase the expression levels of C/EBPδ nor the nuclear translocation of NF-κB. However, we observed a trend toward an increase in C/EBPδ protein levels and a significant increase in its mRNA levels after concomitant exposure of the cells to palmitate and LPS, indicating that palmitate has the ability to strengthen some effects of inflammatory stimuli on microglia. In addition, we observed that both palmitate and LPS on their own stimulated the phagocytic activity of BV-2 microglia. This effect was further enhanced upon co-treatment with palmitate and LPS. Since they can act on the same receptor, it will be of interest to investigate if palmitate and LPS stimulate phagocytosis by common or by distinct signaling pathways.
Exactly how obesity impacts the inflammatory response in the brain is yet unknown. One study shows that high-fat diets affect the protein levels of pro-inflammatory cytokines and glia reactivity in mice (Pistell et al. 2010). Here, we show that exposure to moderately increased levels of the saturated FFA palmitate significantly alters microglial inflammatory response. For future studies, it will be of great importance to further delineate the consequences of obesity and high-fat diets on neuroinflammation.
References
Biessels GJ, Kappelle LJ, Utrecht Diabetic Encephalopathy Study Group (2005) Increased risk of Alzheimer’s disease in type II diabetes: insulin resistance of the brain or insulin-induced amyloid pathology? Biochem Soc Trans 33:1041–1044
Blasi E, Barluzzi R, Bocchini V, Mazzolla R, Bistoni F (1990) Immortalization of murine microglial cells by a v-raf/v-myc carrying retrovirus. J Neuroimmunol 27:229–237
Block ML, Hong JS (2005) Microglia and inflammation-mediated neurodegeneration: multiple triggers with a common mechanism. Prog Neurobiol 76:77–98
Cai D (2013) Neuroinflammation and neurodegeneration in overnutrition-induced diseases. Trends Endocrinol Metab 24:40–47
Colton CA (2009) Heterogeneity of microglial activation in the innate immune response in the brain. J Neuroimmune Pharmacol 4:399–418
Ejarque-Ortiz A, Gresa-Arribas N, Straccia M, Mancera P, Sola C, Tusell JM, Serratosa J, Saura J (2010) CCAAT/enhancer binding protein delta in microglial activation. J Neurosci Res 88:1113–23
Haversen L, Danielsson KN, Fogelstrand L, Wiklund O (2009) Induction of proinflammatory cytokines by long-chain saturated fatty acids in human macrophages. Atherosclerosis 202:382–393
Jüttler E, Tarabin V, Schwaninger M (2002) Interleukin-6 (IL-6): a possible neuromodulator induced by neuronal activity. Neuroscientist 8:268–275
Kappe C, Tracy LM, Patrone C, Iverfeldt K, Sjoholm A (2012) GLP-1 secretion by microglial cells and decreased CNS expression in obesity. J Neuroinflammation 9:276
Karmi A, Iozzo P, Viljanen A, Hirvonen J, Fielding BA, Virtanen K, Oikonen V, Kemppainen J, Viljanen T, Guiducci L, Haaparanta-Solin M, Nagren K, Solin O, Nuutila P (2010) Increased brain fatty acid uptake in metabolic syndrome. Diabetes 59:2171–2177
Li R, Strohmeyer R, Liang Z, Lue LF, Rogers J (2004) CCAAT/enhancer binding protein delta (C/EBPdelta) expression and elevation in Alzheimer’s disease. Neurobiol Aging 25:991–999
Little JP, Madeira JM, Klegeris A (2012) The saturated fatty acid palmitate induces human monocytic cell toxicity toward neuronal cells: exploring a possible link between obesity-related metabolic impairments and neuroinflammation. J Alzheimers Dis 30(Suppl 2):S179–83
Litvak V, Ramsey SA, Rust AG, Zak DE, Kennedy KA, Lampano AE, Nykter M, Shmulevich I, Aderem A (2009) Function of C/EBPdelta in a regulatory circuit that discriminates between transient and persistent TLR4-induced signals. Nat Immunol 10:437–443
Mohamed-Ali V, Goodrick S, Rawesh A, Katz DR, Miles JM, Yudkin JS, Klein S, Coppack SW (1997) Subcutaneous adipose tissue releases interleukin-6, but not tumor necrosis factor-alpha, in vivo. J Clin Endocrinol Metab 82:4196–4200
Morris MC, Evans DA, Tangney CC, Bienias JL, Schneider JA, Wilson RS, Scherr PA (2006) Dietary copper and high saturated and trans fat intakes associated with cognitive decline. Arch Neurol 63:1085–1088
Morris MC, Evans DA, Bienias JL, Tangney CC, Wilson RS (2004) Dietary fat intake and 6-year cognitive change in an older biracial community population. Neurology 62:1573–1579
Ott A, Stolk RP, van Harskamp F, Pols HA, Hofman A, Breteler MM (1999) Diabetes mellitus and the risk of dementia: The Rotterdam Study. Neurology 53:1937–1942
Pistell PJ, Morrison CD, Gupta S, Knight AG, Keller JN, Ingram DK, Bruce-Keller AJ (2010) Cognitive impairment following high fat diet consumption is associated with brain inflammation. J Neuroimmunol 219:25–32
Prieur X, Mok CY, Velagapudi VR, Nunez V, Fuentes L, Montaner D, Ishikawa K, Camacho A, Barbarroja N, O’Rahilly S, Sethi JK, Dopazo J, Oresic M, Ricote M, Vidal-Puig A (2011) Differential lipid partitioning between adipocytes and tissue macrophages modulates macrophage lipotoxicity and M2/M1 polarization in obese mice. Diabetes 60:797–809
Ramberg V, Tracy LM, Samuelsson M, Nilsson LN, Iverfeldt K (2011) The CCAAT/enhancer binding protein (C/EBP) delta is differently regulated by fibrillar and oligomeric forms of the Alzheimer amyloid-beta peptide. J Neuroinflammation 8:34
Schilling JD, Machkovech HM, He L, Sidhu R, Fujiwara H, Weber K, Ory DS, Schaffer JE (2013) Palmitate and lipopolysaccharide trigger synergistic ceramide production in primary macrophages. J Biol Chem 288:2923–2932
Solfrizzi V, D’Introno A, Colacicco AM, Capurso C, Del Parigi A, Capurso S, Gadaleta A, Capurso A, Panza F (2005) Dietary fatty acids intake: possible role in cognitive decline and dementia. Exp Gerontol 40:257–270
Stein B, Cogswell PC, Baldwin AS Jr (1993) Functional and physical associations between NF-kappa B and C/EBP family members: a Rel domain-bZIP interaction. Mol Cell Biol 13:3964–3974
Tak PP, Firestein GS (2001) NF-kappaB: a key role in inflammatory diseases. J Clin Invest 107:7–11
Valente T, Straccia M, Gresa-Arribas N, Dentesano G, Tusell JM, Serratosa J, Mancera P, Sola C, Saura J (2013) CCAAT/enhancer binding protein delta regulates glial proinflammatory gene expression. Neurobiol Aging, in press
Wang Z, Liu D, Wang F, Liu S, Zhao S, Ling EA, Hao A (2012) Saturated fatty acids activate microglia via toll-like receptor 4/NF-kappaB signalling. Br J Nutr 107:229–241
Wen H, Gris D, Lei Y, Jha S, Zhang L, Huang MT, Brickey WJ, Ting JP (2011) Fatty acid-induced NLRP3-ASC inflammasome activation interferes with insulin signaling. Nat Immunol 12:408–415
Yin M, Yang SQ, Lin HZ, Lane MD, Chatterjee S, Diehl AM (1996) Tumor necrosis factor alpha promotes nuclear localization of cytokine-inducible CCAAT/enhancer binding protein isoforms in hepatocytes. J Biol Chem 271:17974–17978
Yudkin JS, Stehouwer CD, Emeis JJ, Coppack SW (1999) C-reactive protein in healthy subjects: associations with obesity, insulin resistance, and endothelial dysfunction: a potential role for cytokines originating from adipose tissue? Arterioscler Thromb Vasc Biol 19:972–978
Acknowledgments
We thank Dr. Ricardo Figueroa for the help with confocal microscopy and FACS analysis and Dr. Christina Svensson and Jessica Lundqvist for the help with qPCR analysis. This work was supported by the Swedish Research Council (2012–2367), Gun and Bertil Stohnes Foundation, and the Swedish Dementia Foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tracy, L.M., Bergqvist, F., Ivanova, E.V. et al. Exposure to the Saturated Free Fatty Acid Palmitate Alters BV-2 Microglia Inflammatory Response. J Mol Neurosci 51, 805–812 (2013). https://doi.org/10.1007/s12031-013-0068-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-013-0068-7