Abstract
Purpose
This study aimed to evaluate the cost-effectiveness of cetuximab in different genetic populations of metastatic colorectal carcinoma patients, including KRAS and RAS wild types and mutants, when added to FOLFIRI treatment regimens for evidence-based disease management in Iran.
Method
A Markov decision model was designed in TreeAge software with the three states of stable, progress, and death. Clinical outcomes were extracted from published clinical studies, and costs were extracted from the Iranian local data. The primary outcome was an incremental cost-effectiveness ratio (ICER) in the simulated population.
Results
The cost-utility model from the perspective of the health system indicated that the average direct medical costs of a patient that has not been genetically screened are $56,985.27 and $20,767.74 in FOLFIRI + cetuximab and FOLFIRI regimens, respectively. However, costs per patient in the KRAS wild-type population were $21,845.52 in FOLFIRI and $78,321.22 in FOLFIRI + cetuximab. In RAS wild-type patients, FOLFIRI and FOLFIRI + cetuximab costs per patient were $23,111.62 and $84,976.39, respectively. Incremental QALYs for the above scenarios were 0.069, 0.193, and 0.285, respectively. Therefore, the ICER of add-on cetuximab in Iran compared to the treatment alternatives in the scenarios with and without KRAS screening was $520,771.55/QALY, $292,768.16/QALY, and $217,460.51/QALY.
Conclusion
Although genetic screening in precision medicine reduces costs per outcome, according to the willingness-to-pay threshold of $4349.50 in the Iranian health system, add-on cetuximab to the FOLFIRI regimen is not a cost-effective strategy even with genetic screening and a 20% price reduction.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Colorectal carcinoma (CRC) is the second leading cause of cancer-related deaths after lung cancer, with a mortality rate of 9% of all cancer deaths [1, 2]. The incidence of CRC varies greatly in different parts of the world. The highest incidence of CRC is in North America, Australia, New Zealand, Western Europe, and China [3,4,5]. The early diagnosis of malignancy and pre-metastatic intervention are critical factors in reducing CRC mortality [6, 7]. The 5-year survival rate of CRC is approximately 27–85% in Iran [8, 9]. The incidence of CRC in both sexes has increased in recent years. In Iran, CRC is particularly important for a significant proportion of patients under 50 years, and its increasing incidence, especially in the colon Sect. [10]. Along with other countries, new and expensive medications, particularly monoclonal antibodies for CRC management, have been increasingly introduced to the Iranian pharmaceutical market. Well-known examples are epidermal growth factor receptors (EGFR) inhibitors such as cetuximab panitumumab, nimotuzumab, and necitumumab. Although EGFR is found in approximately 80% of CRCs, this medication class is effective when there is no mutation in the tumor K-RAS gene (wild type K-RAS) as well as the RAS gene (RAS wild type) [11,12,13,14,15].
These new treatments of CRC have shown promising results in saving patients and improving their quality of life. However, they usually incur high costs for patients and health systems. Therefore, evaluating the costs and benefits of these new treatments over current traditional interventions can help health care providers in evidence-based decision-making [4, 16].
Cost-effectiveness studies have been mandatory for marketing authorization in Iran since 2016. However, some medicines, such as cetuximab which entered the market before 2016, have not been evaluated economically. This study aimed to provide scientific evidence on the cost-effectiveness of cetuximab in the Iranian health care system [17, 18].
The present economic evaluation investigates the cost-effectiveness of adding cetuximab to the routine treatment regimen of FOLFIRI (folinic acid, fluorouracil, and irinotecan) in three scenarios: no genetic screening, K-RAS mutation testing, and RAS mutation testing in the first line of treatment of metastatic colorectal carcinoma (mCRC) in Iran.
Method
Overview
This economic evaluation was conducted in 2020 from the perspective of all healthcare payers, with three different scenarios based on Consolidated Health Economic Evaluation Reporting Standards (CHEERS) [19]. Due to the lack of local studies on medicines effectiveness, the efficacy and safety of the drugs were extracted from the published international randomized clinical trials (RCTs) considering two main treatments outcomes, i.e., increasing overall survival (OS) and progression-free survival (PFS), as well as reducing the mortality rate. In Scenario I, the FOLFIRI regimen with or without cetuximab was assessed in a population that has not been genetically screened for K-RAS or RAS mutations. The population in Scenario II was genetically screened for KRAS, and in Scenario III, the RAS gene was screened. The model time horizon was 160 months (approximately 13 years) stratified into 1-month cycles. This is the maximum time where 99% of the 1000 patients in the present hypothetical cohort died [20]. The discount rate for costs was 7.2% based on a local study by Abdoli and 5% for outcomes based on Drummond’s recommendation [21, 22].
Model Structure
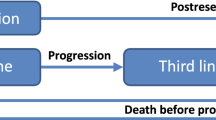
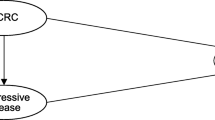
In this study, a Markov model (Fig. 1) was developed based on the leading disease states, OS, PFS, and death, which are frequently used in cancer economic models [23]. The cost-utility model was developed using the TreeAge software. Costs were introduced in US dollars (42,000 Iranian Rials) and outcomes in QALY. The patient could move between these states in each 1-month cycle based on the effectiveness probabilities.
Cost Input
Based on the perspectives of the study, direct medical costs, including drug prices, oncologist visits, diagnostic imaging, laboratory tests, diagnostic examinations, and iatrogenic side effects management, were included in the study, considering current treatment guidelines and routine practices in Iran. Table 1 shows the cost input in detail.
Transition Probabilities
OS and PFS probabilities were extracted from the CRYSTAL study using Plot Digitalizer. Their Weibull distribution was defined based on the Hoyle study and Henley Scale method using R software [24].
Utility
Due to the lack of a domestic study regarding colorectal patients’ quality of life, utility scores were also extracted from the CRYSTAL clinical trial. Patients’ utility scores were assumed as 0.81 and 0.57 in the stable and progressive states, respectively [25].
Sensitivity Analysis
The robustness of the model output against the uncertainty of the critical parameters of the cost-effectiveness model was assessed through deterministic and probabilistic sensitivity analyses (PSA). Tornado diagrams were drawn based on ± 20% variation in the main model variables, including costs, probabilities, utilities, time horizon, and discount rates. For PSA, costs were entered into the model as a gamma distribution in the scheduled intervals, and all probabilities were entered as a beta distribution according to the SD reported in the studies.
Results
In Scenario I, the FOLFIRI regimen with or without cetuximab was assessed in a population of patients who had not been genetically screened for K-RAS or RAS mutation. In terms of effectiveness, the mean discounted QALY in the FOLFIRI treatment regimen in this scenario was 1086, and the FOLFIRI with cetuximab was 1155. Therefore, the FOLFIRI regimen plus cetuximab was more effective than the FOLFIRI alone, with an increment QALY of 0.069 and an incremental cost per patient of 36,217.53 USD (1,521,136,252 IRR). The calculated ICER is 520,771.55 USD/QALY (21,872,405,120 IRR/QALY). Therefore, considering the willingness to pay (WTP) threshold in Iran ($4349.50), adding cetuximab to the FOLFIRI regimen is not a cost-effective strategy in a patient population that has not been genetically screened. The major costs in each category were the cost of the medications, which constituted 89% of the total costs.
Next, precision medicine was considered by adding some genetic screening.
In Scenario II, cost-effectiveness was evaluated in a patient population that underwent KRAS genetic screening. The KRAS-WT population associated with the FOLFIRI regimen was treated with or without cetuximab. In terms of effectiveness, discounted QALY in the FOLFIRI regimen was 1165, and the FOLFIRI + cetuximab was 1358. According to the results, FOLFIRI + cetuximab was more effective than the FOLFIRI alone by at least 0.169 QALYs in patients and increased the costs per patient by 56,475.70 USD (2,371,979,228 IRR). The ICER was 292,768.16 USD/QALY (12,296,262,931 IRR/QALY), which is again significantly higher than the Iran WTP threshold. Therefore, adding cetuximab to the FOLFIRI regimen at the current price in Iran is not a cost-effective strategy, even in the KRAS-WT population. The major cost in each category was related to the cost of the medications, which included 96% of the total costs.
Scenario III showed the results in the RAS-WT population for the FOLFIRI treatment regimen with or without cetuximab. In terms of effectiveness, the average discounted QALY in the FOLFIRI treatment regimen in this scenario was 1523, and the FOLFIRI + cetuximab was 1519. FOLFIRI + cetuximab, in addition to being more effective than the FOLFIRI regimen by at least 0.285 QALYs per patient, FOLFIRI + cetuximab increased the costs per patient by at least 61,864.77 USD (2,598,320,337 IRR). The ICER was 217,460.51 USD/QALY (9,133,341,253 IRR/QALY). Therefore, adding cetuximab to FOLFIRI is not a cost-effective strategy for the RAS-WT mCRC population. The high costs in each category were related to the cost of the medications, which accounted for 96% of the total costs.
Although gene screening decreases the incremental cost per incremental effect, it is not cost-effective in the Iranian health system due to the high cost of cetuximab.
Table 2 shows the overall results of cost-effectiveness analysis in three scenarios. In all scenarios, the addition of cetuximab to the treatment regimen of mCRC patients resulted in higher incremental QALY and lower incremental cost. However, ICERs were higher than WTP in all cases, and cetuximab add-on therapy is not a cost-effective strategy in Iran with the current cetuximab price.
Figure 2 shows the cost-effectiveness distribution of cetuximab in a hypothetical cohort of 1000 mCRC patients by the Monte Carlo simulation. Monte Carlo curve provides more detailed information in individual comparisons. The figures show the movement of iterations to lower costs and higher effects after gene screening. However, most points are much higher than the WTP threshold, so the chart scale could not accommodate the WTP line.
As ICER is affected by many variables with some degrees of uncertainty, a tornado diagram is illustrated to determine which variable is more challenging to make the result unstable. The tornado diagram is a method to find the most effective changes of variables in the model outcomes. As seen in Fig. 3, changing the variables up to + _20% will not change the final result of the cost-effectiveness of cetuximab; in other words, the model is not sensitive to variables.
Discussion
This study evaluated the cost-effectiveness of add-on cetuximab to the conventional oncotherapy regimen in Iran by designing a Markov model. Screening tests and the detection of genetic mutations are critical in reducing costs and improving outcomes. Precision medicine can facilitate market access by improving the cost-effectiveness of expensive and high-tech medications. However, the present study found that cetuximab might not be a cost-effective strategy for Iranian mCRC patients even with a gene screening in different scenarios and a 20% discount in drug prices in the sensitivity analysis. Therefore, other market access strategies, such as manage entry agreements (MEA)/risk-sharing agreements, might be used.
In a study in 2021, Petrou and Koilakou conducted a systematic review of the economic evaluation studies of monoclonal antibodies in mCRC. Two studies examined the cost-effectiveness of cetuximab add-on treatment to the FOLFIRI diet in China and Iran. Despite better efficacy on PFS, OS, and life-years gained (LYG), treatment costs were very high, and ICER exceeded WTP. Also, cetuximab has been cost-effective in patients with KRAS and RAS-WT mCRC compared to cetuximab and bevacizumab in addition to the FOLFIRI. Cetuximab is more expensive than panitumumab and much less effective as a last resort treatment. Cetuximab was not cost-effective compared to standard therapy and panitumumab but cost-effective compared to bevacizumab in mCRC patients [26].
Given the considerable cost difference of adding cetuximab to FOLFIRI and the importance of personalized drug therapy based on individual genetic characteristics, it is expected that genetic screening with a significant reduction in the price of cetuximab or the production of in-house biosimilars can reduce costs. Also, regulatory organizations can define the WTP threshold in cancer and end-stage diseases as higher than other ailments (e.g., 3 × GDP/Capita) to increase the probability of cost-effectiveness. The MEAs are extensively considered in developing countries to ease access to expensive medicines. The results of the present study indicate that although K-RAS and RAS screening tests reduce the treatment costs of mCRC patients in Iran, the ICER is still higher than the WTP threshold. Therefore, if the manufacturing company cannot have a high direct discount on the price of cetuximab, it is possible to use the managed entry agreement (MEA) programs [27,28,29]. MEA allows market access to high-priced medications, especially oncotherapeutics and orphan drugs, by sharing the cost of uncertainty between the prescribing stakeholders and payers. Discounts, money-back guarantees, and outcome guarantees are among the most famous MEA typologies for expensive anticancer drugs [30,31,32,33].
This study faced limitations and challenges in design and implementation. No direct clinical trials were performed in Iran, and only clinical trial data of drugs and patients’ utilities published in European countries were used. Clinical conditions and events were simulated in software using Iranian indigenous costs and standard practices performed in the country. According to the treatment guidelines, cetuximab should only be used in KRAS-WT patients, but in Iran, KRAS and RAS are not routine tests.
In conclusion, the advantage of applying gene screening for precision medicine and considering the manage entry agreement for reimbursement are highlights of the current study.
Data Availability
Available if required.
References
Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Gut. 2017;66(4):683–91.
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108.
Park S, Jee SH. Epidemiology of colorectal cancer in Asia-Pacific Region. Surg Treat Color Cancer Asian Perspect Optim Stand [Internet]. 2018 May 2 [cited 2022 Apr 29];3–10. https://doi.org/10.1007/978-981-10-5143-2_1.
Hull R, Francies FZ, Oyomno M, Dlamini Z. Colorectal cancer genetics, incidence and risk factors: in search for targeted therapies. Cancer Manag Res [Internet]. 2020 [cited 2022 Apr 29];12:9869. /pmc/articles/PMC7553623/.
Zhou J, Zheng R, Zhang S, Zeng H, Wang S, Chen R, et al. Colorectal cancer burden and trends: comparison between China and major burden countries in the world. Chinese J Cancer Res [Internet]. 2021 [cited 2022 Apr 29];33(1):1. /pmc/articles/PMC7941684/.
Hiller JG, Perry NJ, Poulogiannis G, Riedel B, Sloan EK. Perioperative events influence cancer recurrence risk after surgery. Nat Rev Clin Oncol 2017 154 [Internet]. 2017 Dec 28 [cited 2022 Apr 29];15(4):205–18. Available from: https://www.nature.com/articles/nrclinonc.2017.194.
Seely KD, Morgan AD, Hagenstein LD, Florey GM, Ottaiano A, Caraglia M, et al. Bacterial involvement in progression and metastasis of colorectal neoplasia. Cancers 2022, Vol 14, Page 1019 [Internet]. 2022 Feb 17 [cited 2022 Apr 29];14(4):1019. https://www.mdpi.com/2072-6694/14/4/1019/htm.
Taheri M, Tavakol M, Akbari ME, Almasi-Hashiani A, Abbasi M. Associations of demographic, socioeconomic, self-rated health, and metastasis in colorectal cancer in Iran. Med J Islam Repub Iran [Internet]. 2019 [cited 2022 Apr 29];33(1):17. /pmc/articles/PMC6662537/.
Maajani K, Khodadost M, Fattahi A, Shahrestanaki E, Pirouzi A, Khalili F, et al. Survival rate of colorectal cancer in Iran: a systematic review and meta-analysis. Asian Pac J Cancer Prev [Internet]. 2019 Jan 1 [cited 2022 Apr 29];20(1):13. /pmc/articles/PMC6485573/.
Moghimi-Dehkordi B, Safaee A, Zali MR. Prognostic factors in 1,138 Iranian colorectal cancer patients. Int J Colorectal Dis [Internet]. 2008 Jul [cited 2022 Apr 29];23(7):683–8. https://pubmed.ncbi.nlm.nih.gov/18330578/.
Rosenkranz AA, Slastnikova TA. Epidermal growth factor receptor: key to selective intracellular delivery. Biochem 2020 859 [Internet]. 2020 Sep 22 [cited 2022 Apr 29];85(9):967–93. https://doi.org/10.1134/S0006297920090011.
Kaufman NEM, Dhingra S, Jois SD, Da Vicente MGH. Molecular targeting of epidermal growth factor receptor (EGFR) and vascular endothelial growth factor receptor (VEGFR). Mol 2021, Vol 26, Page 1076 [Internet]. 2021 Feb 18 [cited 2022 Apr 29];26(4):1076. https://www.mdpi.com/1420-3049/26/4/1076/htm.
Guardiola S, Varese M, Sánchez-Navarro M, Giralt E. A third shot at EGFR: new opportunities in cancer therapy. Trends Pharmacol Sci. 2019;40(12):941–55.
Chanprapaph K, Vachiramon V, Rattanakaemakorn P. Epidermal growth factor receptor inhibitors: a review of cutaneous adverse events and management. Dermatol Res Pract [Internet]. 2014 [cited 2022 Apr 29];2014. /pmc/articles/PMC3958662/.
Wasif Saif M, Shah M, Saif MW. K-Ras mutations in colorectal cancer: a practice changing discovery. Clin Adv Hematol Oncol. 2009;7(1).
Blake J, Costescu D, Dunn S, Leyland N, Rheault K. Health technology assessment at health quality Ontario. Ont Health Technol Assess Ser [Internet]. 2016;16(18). https://www.scopus.com/inward/record.uri?eid=2-s2.0-85042000507&partnerID=40&md5=383c0ff10a0bcaea776587552b6c7852.
Olyaaeemanesh A, Jaafaripooyan E, Abdollahiasl A, Davari M, Mousavi SM, Delpasand M. Pharmaceutical subsidy policy in Iran: a qualitative stakeholder analysis. Heal Res Policy Syst [Internet]. 2021;19(1):1–17 [cited 2022 Apr 29]. https://doi.org/10.1186/s12961-021-00762-6.
Yousefi N, Moradi N, Dinarvand R, Ghiasi G, Inanloo H, Peiravian F. Policies to improve access to pharmaceutical products in shortage: the experience of Iran food and drug administration. DARU J Pharm Sci [Internet]. 2019 [cited 2022 Apr 29];27(1):169. Available from: /pmc/articles/PMC6593011/.
CHEERS Checklist Items to include when reporting economic evaluations of health interventions. [cited 2022 Jan 16]; Available from: http://www.ispor.org/TaskForces/EconomicPubGuidelines.asp.
Van Hees F, Habbema DF, Meester RG, Lansdorp-Vogelaar I, Van Ballegooijen M, Zauber AG. Should colorectal cancer screening be considered in elderly persons without previous screening?: A cost-effectiveness analysis. Ann Intern Med. 2014;160(11):750–9.
Ghahraman A. Estimation of social discount rate for Iran [Internet]. Economic Research Review; 2009 [cited 2021 Aug 6]. 10:135–56. https://www.sid.ir/en/Journal/ViewPaper.aspx?ID=178933.
Drummond report: Don Drummond recommends a radical overhaul to get Ontario back to balanced budgets | The Star [Internet]. [cited 2022 Jul 12]. https://www.thestar.com/opinion/editorials/2012/02/15/drummond_report_don_drummond_recommends_a_radical_overhaul_to_get_ontario_back_to_balanced_budgets.html.
Tappenden P, Jones R, Paisley S, Carroll C. The cost-effectiveness of bevacizumab in the first-line treatment of metastatic colorectal cancer in England and Wales. Eur J Cancer. 2007;43(17):2487–94.
Hoyle MW, Henley W. Improved curve fits to summary survival data: application to economic evaluation of health technologies. BMC Med Res Methodol [Internet]. 2011 Oct 10 [cited 2022 Apr 29];11(1):1–14. https://doi.org/10.1186/1471-2288-11-139.
Van Cutsem E, Köhne C-H, Hitre E, Zaluski J, Chang Chien C-R, Makhson A, et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med. 2009;360(14):1408–17.
Koilakou S, Petrou P. Economic evaluation of monoclonal antibodies in metastatic colorectal cancer: a systematic review. Mol Diagnosis Ther 2021 256 [Internet]. 2021 Nov 24 [cited 2022 May 11];25(6):715–34. https://doi.org/10.1007/s40291-021-00560-4.
Vitry A, Roughead E. Managed entry agreements for pharmaceuticals in Australia. Health Policy (New York) [Internet]. 2014;117(3):345–52. Available from: https://www.sciencedirect.com/science/article/pii/S0168851014001341.
Aguiar PN. Cost-effectiveness and affordability of anticancer treatment in Brazil. Ecancermedicalscience [Internet]. 2020;14. Available from: https://www.scopus.com/inward/record.uri?eid=2-s2.0-85084148028&doi=10.3332%2FECANCER.2020.ED96&partnerID=40&md5=695e4709f8dc92dfb1b1c3461f7b8a40.
Giuliani J, Bonetti A. The pharmacological costs of first-line therapies in unselected patients with advanced colorectal cancer: a review of published phase III trials. Clin Colorectal Cancer. 2016;15(4):277–84.
Pauwels K, Huys I, Vogler S, Casteels M, Simoens S. Managed entry agreements for oncology drugs: lessons from the European experience to inform the future. Front Pharmacol. 2017 Apr 4;8(APR):171.
Chapman S, Reeve E, Rajaratnam G, Neary R. Setting up an outcomes guarantee for pharmaceuticals: new approach to risk sharing in primary care. BMJ Br Med J [Internet]. 2003 Mar 3 [cited 2022 Jul 27];326(7391):707. Available from: /pmc/articles/PMC1125604/.
Vreman RA, Broekhoff TF, Leufkens HGM, Mantel-Teeuwisse AK, Goettsch WG. Application of managed entry agreements for innovative therapies in different settings and combinations: a feasibility analysis. Int J Environ Res Public Heal 2020, 17, Page 8309 [Internet]. 2020 Nov 10 [cited 2022 Jul 27];17(22):8309. https://www.mdpi.com/1660-4601/17/22/8309/htm.
Bonetti A, Giuliani J. Implications of drugs with rebate in Europe. Lancet Reg Health Eur. 2021;26(3):100060. https://doi.org/10.1016/j.lanepe.2021.100060.
Author information
Authors and Affiliations
Contributions
N.Y. provided the economic model, S.T. conducted the statistical and model development analyses, G.M. drafted the manuscript, A.S. provided the data about the model, and F.P. supervised all the authors.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yousefi, N., Salimi, A., Mohammadnezhad, G. et al. A Cost-effectiveness Analysis of Adding Cetuximab to the First-line Treatment of Metastatic Colorectal Carcinoma in Iran; Considering Genetic Screening for Precision Medicine. J Gastrointest Canc 54, 1212–1219 (2023). https://doi.org/10.1007/s12029-022-00904-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12029-022-00904-1