Summary
Background
In metastatic colorectal cancer (mCRC), multimodal therapeutic strategies and diagnostics have continuously improved patient survival. The aim of our investigation was to relate this enhanced clinical outcome to treatment costs based on predictive biomarker scenarios guiding epidermal growth factor receptor (EGFR) targeting in a developed country.
Methods
We performed a cost-effectiveness analysis for the combination of EGFR inhibitors with chemotherapy in the first-line treatment of mCRC. Resource use estimates were based on actual data from two oncological departments and on clinical outcomes adapted from published trials. Comparative analyses for the use of EGFR inhibitors were based on three biomarker scenarios (sensitivity: 35%, 55% and 75%) to estimate their incremental cost-effectiveness and were completed by sensitivity analyses.
Results
Using FOLFIRI+cetuximab, preselection for EGFR therapy with KRAS testing prolonged progression-free survival with average savings of 913 €/month/patient (scenario 1) and average savings of 1811 €/month/patient when testing the whole RAS-family (scenario 2). In a future but realistic scenario, up 39% of treatment costs could be saved with almost three life–years gained (LYG).
The incremental cost/LYG was 212,083 € (116,646–1,866,332 €) for unselected EGFR therapy, 32,251 € (30,294–43,488 €) for EGFR following KRAS testing, 19,172 € (15,369–28,611 €) for the all RAS scenario, and 12,369 € (3865–18,533 €) for a future biomarker scenario.
Conclusions
In the therapy of mCRC, predictive biomarker testing has shown to be effective and cost saving. For further improvement, a strong research focus on predictive biomarkers is considered highly efficient to promote precision oncology by alleviating the pressure on the healthcare system.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cancer is an overwhelming disease, affecting about 14.1 million people worldwide/year [1] and has become a main cost issue owing to the high costs of novel drugs and the extent of care. Rising therapy costs (USA: US$157 billion total costs per year; EU: 124 billion € per year) require better policies to make cancer “affordable” for the coming decades [2, 3]. As the third most common type of cancer in the world, colorectal cancer (CRC) affected almost 1.4 million people worldwide in 2012 [4]. In Austria, about 4700 patients are diagnosed annually with CRC [5]. The median survival rate of patients with metastatic CRC (mCRC) has improved in the last 2 decades from 6 months to 2 years and higher. This increase can be attributed mainly to treatment advances in the introduction of new cytotoxics (e.g. oxaliplatin, approved in 2002) and biologicals (e.g. cetuximab, approved in 2004) and the establishment of a multidisciplinary approach [6]. As a consequence, survival rates increased steadily but at the same time treatment costs exploded. In the USA, the annual expenditure for CRC was conservatively estimated at US$14 billion in 2010 by the National Institutes of Health (NIH) and is expected to increase to US$17 billion for 2020 being the second most expensive tumour in the ranking of national cost for cancer care [7]. Unfortunately, no similar information is available for Austria. One promising way of cost reduction in the era of personalised medicine is to apply predictive biomarkers to direct targeted therapy. The selection of patients according to their specific cancer profile is supposed to enhance treatment efficiency, spare toxicities for patients who are unlikely to benefit from a given therapy and also minimise unnecessary therapy costs. A common argument against, however, is the additional cost of enhanced diagnosis, which needs to be weighed against additional benefit and cost saving potential of targeted treatment.
Currently two main groups of biologicals assume a role in the treatment of mCRC. First the angiogenesis inhibitors bevacizumab and aflibercept, second the epidermal growth factor receptor (EGFR) inhibitors cetuximab and panitumumab. Before systemic treatment, biomolecular testing is needed to select the right patient for the right therapy. No validated predictive biomarkers are yet available for angiogenesis inhibitors. In contrast, patients without a mutation in the RAS oncogene (wild-type RAS) show benefit to EGFR-inhibiting therapies. These patients, with so-called “all RAS wild-type (wt)” tumour receive often an EGFR-inhibiting antibody as first-line treatment together with chemotherapy. RAS testing is meanwhile a well-established companion diagnostic and has changed the indication for both EGFR inhibitors. Mostly for operational problems, like duration of the testing, logistic but also due to economic reasons, testing is not routinely done in all countries.
Predictive biomarkers emphasize the role of EGFR-downstream signalling molecules such as KRAS (Kirsten rat sarcoma, a member of the RAS [rat sarcoma family]), which is already routinely used as a diagnostic tool in developed countries [8]. The proof-of-concept for this biomarker driven approach has already been demonstrated by several studies including CRYSTAL (Cetuximab combined with irinotecan in first-line therapy for metastatic colorectal cancer), OPUS (oxaliplatin and cetuximab in first-line treatment of metastatic colorectal cancer) and PRIME (panitumumab randomized trial in combination with chemotherapy for metastatic colorectal cancer) [9,10,11]. All those trials have substantiated that patients with wild-type KRAS benefit significantly from therapy with cetuximab or panitumumab, in contrast to patients with mutant KRAS. Even more, patients with tumours that harbour mutant-type KRAS are more likely to have a worse response, progression-free survival (PFS) and overall survival (OS) when treated with cetuximab [12]. Other members of the RAS family such as NRAS (neuroblastoma rat sarcoma with focus on exons 2, 3 and 4) and KRAS (with focus on exons 2, 3 and 4) are also already routinely used in developed countries because trials data showed that comprehensive RAS family testing allows for refined selection of patient groups with additional benefit in response rate and PFS [13,14,15].
Despite progress in research, the use of current biomarker tests remains limited in routine clinical practice due to their poor predictive power observed in clinical validation studies. Implementation of more sensitive predictive markers to direct the application is not only supposed to reduce costs, but is an absolute prerequisite to further develop strategies aiming at the long-term objective of personalised medicine.
A systematic review of cost-effectiveness (CE) of all approved monoclonal antibodies in mCRC was published. There was a clear benefit in CE for testing KRAS prior to treatment with EGFR inhibitors. In this retrospective analysis, no markers other than KRAS were included [16]. Beside this, another study demonstrated that FOLFOX + panitumumab is cost-effective in wt-RAS mCRC [17].
The aim of this article is to assess the potential cost and outcome impact of using predictive biomarkers to guide systemic EGFR-inhibitor treatment in mCRC within the Austrian context based on real-life treatment data. To add to the actual knowledge, current and future aspects of potential resource savings are calculated based on the implementation of three different biomarker scenarios in therapy decisions. Here we show that patient selection provides benefit for patients and relieves the financial burden on the healthcare system.
Methods
Model structure
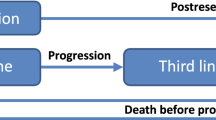
This economic evaluation compared three different testing scenarios: (1) KRAS only, (2) all-RAS (KRAS and NRAS) and (3) a future marker scenario (FM), all-RAS plus other biomarkers, to direct EGFR inhibitors in combination with chemotherapy as first line therapy of mCRC to generalised EGFR inhibitors without biomarker testing, and a baseline control of generalised chemotherapy only. Strategy A: KRAS only testing (test accuracy: 35%) followed by directed treatment. Strategy B: Improved biomarker testing (test accuracy: 55%) by using additional predictive markers from the RAS family [13]. Strategy C: Hypothetical future but realistic marker scenario (FM that is currently under development with promising results [predicted test accuracy: 75%]) (Fig. 1).
Flow chart of the various scenarios of biomarker testing versus no testing; *includes KRAS and NRAS testing, ** includes KRAS, NRAS and further markers testing; numbers in percent (%) give the selection accuracy of each marker. KRAS Kirsten rat sarcoma, RAS Rat sarcoma family, WT wild type, MT mutated type, CHT chemotherapy, AB antibody
Clinical outcomes
Long-term outcome data were based on trials with level 1 clinical evidence. Data for chemotherapy ± cetuximab containing therapies were used for efficacy estimations for the KRAS-wt population (CRYSTAL study: FOLFIRI ± cetuximab [11]; FIRE 3 study: FOLFIRI + cetuximab versus FOLFIRI + bevacizumab [18]; CALGB/SWOG 80405 study: FOLFIRI or mFOLFOX6 + cetuximab versus FOLFIRI or mFOLFOX6 + bevacizumab [19] and panitumumab [PRIME study: FOLFOX ± panitumumab [9]]). The basis for further efficacy estimations including the all-RAS-wt population were taken from published data [13, 14, 20]. Table 1 gives an overview about the used efficacy data.
Costs
In the economic analysis, we focused on the healthcare payer’s perspective and therefore only direct medical costs were included. Resource use data on length of treatment for mCRC, hospital inpatient stays, outpatient clinic visits for therapy and visits due to side effects were derived from 47 patients in two oncological centres in Vienna (Tables 2 and 3).
Other costs included biomarker test costs, chemotherapy costs and EGFR-inhibitor therapy costs. All costs were based on the reimbursement tariffs of the governing body of the public Viennese hospitals in the year 2013.
Hospital costs were calculated according to current routine practice with 5.05 cycles of treatment administered over 5.2 months. Our estimates based on a sample of 47 patients, 27 men and 20 women, with a median age of 65.4 years (42–83 years), treated with first line EGFR-inhibitor therapy between 2010 and 2014. These patients were selected by incidence for the calculation. Our sample showed a per patient average of 21 days of clinical in-house treatment with a daily cost of 681 €, 1.7 routine outpatient follow-up visits, 0.1 outpatient visits due to adverse events, and 0.89 hospitalisations due to adverse events (Tables 2 and 3).
Chemotherapy costs were adjusted for gender. Calculations were based on therapy costs for an average patient in Austria with a body surface area (BSA) of 1.7 m2 for women and 2.0 m2 for men [21]. The monthly chemotherapy costs for the standard first line chemotherapy including FOLFIRI (5-FU, irinotecan, leucovorin) and FOLFOX (5-FU, oxaliplatin, leucovorin) were calculated (Tables 2 and 3).
Antibody therapy costs of cetuximab and panitumumab primarily depend on the proportion of patients undergoing the treatment (Tables 2 and 3).
Biomarker testing costs of a single KRAS testing amounts to 350 €/test in Austrian laboratories (Clinical Institute of Pathology, Medical University of Vienna).
The testing of further markers of the RAS family and others can be performed by testing each marker individually or by advanced techniques such as gene chip analysis or sequencing of all relevant markers. As estimates for these novel molecular techniques, a one-off payment of 900 € was considered (Clinical Institute of Pathology, Medical University of Vienna).
In the one-way sensitivity analyses (SA), we have varied the average therapy length (−25%, +50%), the antibody costs (±30%) the tumour incidence (±20%) and the outcomes, life years gained (LYG) (±5%). Best-case and worst-case scenarios were also calculated.
Population estimates for Austria
The saving potential per patient was extrapolated to Austria based on the national annual incidence of mCRC. The incidence of cases in need of systemic CRC therapy was estimated at 1100 according to the data from Statistics Austria [5]. These figures were calculated based on the following data:
-
A total incidence of 4722 for new CRC cases/year
-
Metastatic disease initially detected in 15% of these (= 708 cases)
-
Locally advanced CRC occurring in 1912 cases, among these 20% with residual disseminated disease, i.e. 382 patients
Taken into account the incidence in men (~56%) and women (~44%), approximately 616 men and 484 women are diagnosed with metastatic CRC yearly in Austria. European CRC estimates on the rate of mCRC suggest an even higher number of metastatic CRC: about 25% of patients present with stage IV CRC (synchronous metastases) and 50% of patients overall develop liver metastases [22,23,24]. Therefore, our incidence estimates shall be seen as conservative.
Results
The cost analysis is based on the average monthly hospital cost per patient (2864 €), on the average chemotherapy costs per patient for FOLFIRI or FOLFOX (113 € or 100 € for women; 133 € or 111 € for men, respectively) and on the average costs for cetuximab or panitumumab (3230/3274 € for women and 3860/4129 € for men, respectively). The differences between male and female patients are mainly driven by the difference in the body surface area (see Tables 2, 3, 4, 5 and 6 for detailed cost assumptions).
Outcomes in LYG for the different marker scenarios (KRAS, RAS and FM) were calculated according to available efficacy data from published clinical trials (Table 1).
Cost analysis
Population cost estimates
The following analyses illustrate the potential cost savings resulting from the application of biomarkers with increasing selection accuracy in patients under “real-life” conditions with an actual mean treatment period of 5.2 months corresponding to a routine schedule over 6 months (Tables 4 and 5).
In Austria, approximately 1100 newly diagnosed disseminated CRC patients (43% female) are in need of treatment per year. Using the observed per patient costs, the total treatment costs at the national level therefore are:
The no-test scenario results in a 5.2 months treatment cost of 37.6 million € for FOLFIRI + cetuximab and almost identical 38.5 million € for FOLFOX + panitumumab. With testing, savings amount to 30.8, 27.3, 23.2 million € for FOLFIRI + cetuximab and 31.3, 27.6, 23.3 million € for FOLFOX + panitumumab for KRAS (35%), RAS (55%), and FM (75%) selection accuracy scenarios, respectively.
In Austria, the potential nationwide savings for a 6-month treatment therefore amount to at least 7 million € for KRAS, 10 million € for the RAS scenario, and about 15 million € for the hypothetical future marker scenario with 75% selection accuracy (Tables 4 and 5).
Cost-effectiveness analysis
We estimated the incremental cost/LYG per patient for the average length of treatment (5.2 months) using the generic ICER formula. Table 6 summarizes the cost and effects of the various scenarios per average length of treatment.
No screening, screening for KRAS, screening for all-RAS and screening for future biomarkers prior to administering an EGFR inhibitor results in an ICER of 212,083 € (SA range: 116,646–1866,332 €) for the no test scenario, 32,251 € (SA range: 30,294–43,488 €) for the KRAS scenario (test selection accuracy: 35%), 19,172 € (SA range: 15,369–28,611 €) for the all RAS scenario (test selection accuracy: 55%), and 12,369 € (SA range: 3865–18,533 €) for a future but achievable biomarker scenario (test selection accuracy: 75%) in comparison to no screening and chemotherapy only.
Sensitivity analyses
Multiple one-way sensitivity analysis were carried out. Related to the cost analysis, the highest contributors to total cost are hospital costs and antibody costs, which were included in the SA. The cost of biomarker tests and chemotherapy, by contrast, contributed very little to the total costs and therefore were not varied. Moreover, based on available data about patients known to harbour a RAS mutation, RAS testing had a sensitivity of 95% and a specificity of 100%. Thus, no sensitivity analysis for marker test bias was needed [25, 26]. We illustrated the effects of varying the incidence (±20%), the treatment duration (−25%, +50%) and the antibody cost (±30%) on the costs per average treatment length and the ICER for the four scenarios (based on the FOLFIRI + cetuximab treatment).
For all four scenarios, a best- and a worst-case calculation was also performed. In conclusion, the ICERs were sensitive to all these changes and spread across a great range, but the magnitude and the conclusion of the base case cost-effectiveness analyses have not changed (Supplementary material, Tables 1–4).
In summary, testing all-RAS is highly effective concerning patient’s outcomes and highly cost saving in the actual therapy selection of mCRC patients. Calculated per LYG, the combination of more biomarkers is very promising as it decreases antibody costs.
Discussion
In most western and northern European countries, KRAS testing is a well-established procedure to determine a negative predictive marker for anti-EGFR strategies in mCRC. KRAS testing selects a population of about 65% of all patients for the administration of cetuximab or panitumumab in addition to general chemotherapy. As shown in our analysis, this selection alone offers high cost savings even in a small country such Austria: 8 million € with just KRAS single biomarker testing for a 5.2-month treatment. All-RAS testing selects (75% selection accuracy). Our CEA showed that both in terms of overall outcomes and costs, RAS testing is superior to no testing or KRAS testing with a potential cost savings of up to about 17 million € when used routinely. Generally, EGFR therapy without pretesting should not be carried out.
Statistically significant changes in efficacy are observed when testing more predictive markers as shown in the PRIME trial after the inclusion of all-RAS testing (OS with 26 vs. 20.2 months, HR 0.78; 95% CI 0.62–0.99). In addition, clinical benefit became evident after analysis of the FIRE-3 and PRIME studies.
A clinical benefit of about 3–4.5 months in OS was achieved after the addition of further marker tests to KRAS. Considering the additional RAS mutation analyses from FIRE-3 and PRIME study [27], the sensitivity analysis of the CEA shows even more favourable data as the survival benefit for RAS wt patients would increase from 26 months (PRIME study) to 28 and 33 months respectively.
Although only in its infancy, personalised medicine heavily relies on the biomarker selection of patients. Therefore, increasing accuracy to predict therapy response is expected in the upcoming years. Our simulation demonstrates that this approach is highly effective considering several issues: First the better outcomes and fewer side effects for the patients and second the cost reductions we can achieve by the routine usage of predictive biomarkers.
It is important to prioritise biomarker development for the reasons mentioned above, but also to invest in personalised medicine as a clinical priority in the next decade. Examples such as the use of crizotinib in patients with lung cancer and a EML4-ALK translocation already fulfil these criteria proving that carefully selected and validated biomarker come close to the envisaged target of 55% as the next step [28]. Even higher, prediction by assessing bcr-abl in patients with CML or GIST highly favours the administration of imatinib with positive predictions >90% [29, 30].
Future treatments in mCRC will become more complex: the number of available compounds and combinations with targeted approaches is expected to increase in the next few years. It is estimated that there are 900 drugs under development for oncology in the pipelines of the pharmaceutical industry [31]. This brings oncologists in the favourable position of more therapeutic options, but also raises questions about treatment algorithms, patient selection sustainability and costs.
Patients who benefit from these treatments are supposed to live longer and may receive multiple lines of therapy. In this realistic scenario, patient selection will not only become relevant, it will become a vital issue for the whole healthcare system. As one of the most relevant obstacles in biomarker implementation and personalised-medicine is intratumour heterogeneity which makes a clear statement concerning the tumour genomics landscape from a single tumour biopsy quite challenging [32].
By the implementation of liquid biopsies to detect circulating tumour cells, a big step toward more information about the tumour and tumour heterogeneity is on the horizon.
Recent data, especially in CRC, show that liquid biopsies can be used for initial molecular testing and therapy monitoring, including changes in molecular markers and by this predicting resistance to therapy [33, 34].
Although costs of other standard first-line therapies, for example bevacizumab + chemotherapy, tumour sidedness, localisation of metastases (e.g. liver, lung or peritoneum) or subsequent novel therapies (e.g. trifluridin/tipiracil (Lonsurf), checkpoint inhibitors in MSI-high patient) were not included in the calculation, we do not expect this limitation to have an impact on our conclusions.
Another limitation of the current analysis is that we could not include quality of life adjustment and use QALYs as our outcome measure in the CEA due to the lack of data. Likewise, we could not include broader health care resource use (e.g. general practitioner visits, medication costs) into our calculations either and had to limit the time-frame of our analysis to the standard length of therapy. The cost of the potential further marker test is an estimate and the current cost-effectiveness results would be different and less favourable at a higher cost.
Conclusion
The current analysis confirms that patient selection for mCRC treatment is a valuable goal with benefits for both patients and society. Under these circumstances, the use of predictive biomarkers is an efficient measure to promote precision oncology by concomitantly alleviating the pressure on the healthcare system.
References
Cancer research UK. Worldwide cancer statistics. 2017. http://www.cancerresearchuk.org/health-professional/cancer-statistics/worldwide-cancer#heading-Zero. Accessed 20 July 2017.
National Cancer Institute. Cancer prevalence and cost of care projects. 2017. https://costprojections.cancer.gov/expenditures.html. Accessed 20 July 2017.
Luengo-Fernandez R, Leal J, Gray A, Sullivan R. Economic burden of cancer across the European Union: a population-based cost analysis. Lancet Oncol. 2013;14(12):1165–74.
Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Gut. 2017;66(4):683–91.
Statistik Austria. 2017. http://www.statistik.at/web_de/statistiken/menschen_und_gesellschaft/gesundheit/krebserkrankungen/dickdarm_enddarm/index.html. Accessed 20 July 2017.
Kopetz S, Chang GJ, Overman MJ, Eng C, Sargent DJ, Larson DW, et al. Improved survival in metastatic colorectal cancer is associated with adoption of hepatic resection and improved chemotherapy. J Clin Oncol. 2009;27(22):3677–83.
Mariotto AB, Yabroff KR, Shao Y, Feuer EJ, Brown ML. Projections of the cost of cancer care in the United States: 2010–2020. J Natl Cancer Inst. 2011;103(2):117–28.
Amado RG, Wolf M, Peeters M, Van Cutsem E, Siena S, Freeman DJ, et al. Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26(10):1626–34.
Douillard JY, Siena S, Cassidy J, Tabernero J, Burkes R, Barugel M, et al. Randomized, phase III trial of panitumumab with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) versus FOLFOX4 alone as first-line treatment in patients with previously untreated metastatic colorectal cancer: the PRIME study. J Clin Oncol. 2010;28(31):4697–705.
Tabernero J, Van Cutsem E, Diaz-Rubio E, Cervantes A, Humblet Y, Andre T, et al. Phase II trial of cetuximab in combination with fluorouracil, leucovorin, and oxaliplatin in the first-line treatment of metastatic colorectal cancer. J Clin Oncol. 2007;25(33):5225–32.
Van Cutsem E, Kohne CH, Lang I, Folprecht G, Nowacki MP, Cascinu S, et al. Cetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: updated analysis of overall survival according to tumor KRAS and BRAF mutation status. J Clin Oncol. 2011;29(15):2011–9.
Qiu LX, Mao C, Zhang J, Zhu XD, Liao RY, Xue K, et al. Predictive and prognostic value of KRAS mutations in metastatic colorectal cancer patients treated with cetuximab: a meta-analysis of 22 studies. Eur J Cancer. 2010;46(15):2781–7.
Stintzing S, Jung A, Rossius L, Modest D, Fischer von Weikersthal L, Decker T, et al., editors. Mutations within the EGFR signaling pathway: Influence on efficacy in FIRE-3—A randomized phase III study of FOLFIRI plus cetuximab or bevacizumab as first-line treatment for wild-type (WT) KRAS (exon 2) metastatic colorectal cancer (mCRC) patients. Proceedings of ASCO GI. J Clin Oncol. 2014;32(Suppl 3):445 (abstr).
Kaczirek K, Ciuleanu TE, Vrbanec D, Marton E, Messinger D, Liegl-Atzwanger B, et al. FOLFOX4 plus cetuximab for patients with previously untreated metastatic colorectal cancer according to tumor RAS and BRAF mutation status: updated analysis of the CECOG/CORE 1.2.002 study. Clin Colorectal Cancer. 2015;14(2):91–8.
Bokemeyer C, Kohne CH, Ciardiello F, Lenz HJ, Heinemann V, Klinkhardt U, et al. FOLFOX4 plus cetuximab treatment and RAS mutations in colorectal cancer. Eur J Cancer. 2015;51(10):1243–52.
Lange A, Prenzler A, Frank M, Kirstein M, Vogel A, von der Schulenburg JM. A systematic review of cost-effectiveness of monoclonal antibodies for metastatic colorectal cancer. Eur J Cancer. 2013;50(1):40.
Graham CN, Hechmati G, Hjelmgren J, de Liege F, Lanier J, Knox H, et al. Cost-effectiveness analysis of panitumumab plus mFOLFOX6 compared with bevacizumab plus mFOLFOX6 for first-line treatment of patients with wild-type RAS metastatic colorectal cancer. Eur J Cancer. 2014;50(16):2791–801.
Heinemann V, von Weikersthal LF, Decker T, Kiani A, Vehling-Kaiser U, Al-Batran SE, et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (FIRE-3): a randomised, open-label, phase 3 trial. Lancet Oncol. 2014;15(10):1065–75.
Venook AP, Niedzwiecki D, Lenz H‑J, Innocenti F, Mahoney MR, O’Neil BH, et al., editors. CALGB/SWOG 80405: Phase III trial of irinotecan/5-FU/leucovorin (FOLFIRI) or oxaliplatin/5-FU/leucovorin (mFOLFOX6) with bevacizumab (BV) or cetuximab (CET) for patients (pts) with KRAS wild-type (wt) untreated metastatic adenocarcinoma of the colon or rectum (MCRC)2014: J Clin Oncol 32:5s, 2014 (suppl; abstr LBA3)
Lenz H, Niedzwiecki D, Innocenti F, editors. CALGB/SWOG 80405: Phase III trial of irinotecan/5-FU/leucovorin (FOLFIRI) or oxaliplatin/5-FU/leucovorin (mFOLFOX6) with bevacizumab (BV) or cetuximab (CET) for patients (pts) with expanded ras analyses untreated metastatic adenocarcinoma of the colon or rectum (mCRC). ESMO Conference, Abstract 501O; 2014.
BMG. Österreichischer Ernährungsbericht 2012. 2012. http://www.bmg.gv.at/cms/home/attachments/4/5/3/CH1048/CMS1348749794860/oeb12.pdf. Accessed 20 July 2017.
Adam R, De Gramont A, Figueras J, Guthrie A, Kokudo N, Kunstlinger F, et al. The oncosurgery approach to managing liver metastases from colorectal cancer: a multidisciplinary international consensus. Oncologist. 2012;17(10):1225–39.
Nordlinger B, Van Cutsem E, Rougier P, Kohne CH, Ychou M, Sobrero A, et al. Does chemotherapy prior to liver resection increase the potential for cure in patients with metastatic colorectal cancer? A report from the European Colorectal Metastases Treatment Group. Eur J Cancer. 2007;43(14):2037–45.
Van Cutsem E, Nordlinger B, Adam R, Kohne CH, Pozzo C, Poston G, et al. Towards a pan-European consensus on the treatment of patients with colorectal liver metastases. Eur J Cancer. 2006;42(14):2212–21.
Jimeno A, Messersmith WA, Hirsch FR, Franklin WA, Eckhardt SG. KRAS mutations and sensitivity to epidermal growth factor receptor inhibitors in colorectal cancer: practical application of patient selection. J Clin Oncol. 2009;27(7):1130–6.
Tol J, Dijkstra JR, Vink-Borger ME, Nagtegaal ID, Punt CJ, Van Krieken JH, et al. High sensitivity of both sequencing and real-time PCR analysis of KRAS mutations in colorectal cancer tissue. J Cell Mol Med. 2009;14(8):2122–31.
Douillard JY, Oliner KS, Siena S, Tabernero J, Burkes R, Barugel M, et al. Panitumumab-FOLFOX4 treatment and RAS mutations in colorectal cancer. N Engl J Med. 2013;369(11):1023–34.
Shaw AT, Kim DW, Nakagawa K, Seto T, Crino L, Ahn MJ, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med. 2013;368(25):2385–94.
Demetri GD, von Mehren M, Blanke CD, Van den Abbeele AD, Eisenberg B, Roberts PJ, et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med. 2002;347(7):472–80.
Druker BJ, Talpaz M, Resta DJ, Peng B, Buchdunger E, Ford JM, et al. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N Engl J Med. 2001;344(14):1031–7.
Medco Health Solutions. Drug trend report. 2011. http://www.drugtrendreport.com/2011-report. Accessed 10 Sept 2013.
Gerlinger M, Rowan AJ, Horswell S, Larkin J, Endesfelder D, Gronroos E, et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med. 2012;366(10):883–92.
Russo M, Siravegna G, Blaszkowsky LS, Corti G, Crisafulli G, Ahronian LG, et al. Tumor heterogeneity and lesion-specific response to targeted therapy in colorectal cancer. Cancer Discov. 2016;6(2):147–53.
Siravegna G, Mussolin B, Buscarino M, Corti G, Cassingena A, Crisafulli G, et al. Clonal evolution and resistance to EGFR blockade in the blood of colorectal cancer patients. Nat Med. 2015;21(7):827.
Acknowledgements
The data were partly presented at WCGC (World Congress on Gastrointestinal Cancers) in Barcelona in 2015.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
D. Niedersüß-Beke, J. Simon, M. Schiffinger, and R.M. Mader declare that they have no competing interests.
Caption Electronic Supplementary Material
Rights and permissions
About this article
Cite this article
Niedersüß-Beke, D., Simon, J., Schiffinger, M. et al. Economic analysis of biomarker-based anti-EGFR therapies in metastatic colorectal cancer in the Austrian context. memo 11, 322–329 (2018). https://doi.org/10.1007/s12254-018-0440-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12254-018-0440-y