Abstract
Background
Spinal cord injury (SCI) presents a major global health challenge, with rising incidence rates and substantial disability. Although progress has been made in understanding SCI’s pathophysiology and early management, there is still a lack of effective treatments to mitigate long-term consequences. This study investigates the potential of sovateltide, a selective endothelin B receptor agonist, in improving clinical outcomes in an acute SCI rat model.
Methods
Thirty male Sprague–Dawley rats underwent sham surgery (group A) or SCI and treated with vehicle (group B) or sovateltide (group C). Clinical tests, including Basso, Beattie, and Bresnahan scoring, inclined plane, and allodynia testing with von Frey hair, were performed at various time points. Statistical analyses assessed treatment effects.
Results
Sovateltide administration significantly improved motor function, reducing neurological deficits and enhancing locomotor recovery compared with vehicle-treated rats, starting from day 7 post injury. Additionally, the allodynic threshold improved, suggesting antinociceptive properties. Notably, the sovateltide group demonstrated sustained recovery, and even reached preinjury performance levels, whereas the vehicle group plateaued.
Conclusions
This study suggests that sovateltide may offer neuroprotective effects, enhancing neurogenesis and angiogenesis. Furthermore, it may possess anti-inflammatory and antinociceptive properties. Future clinical trials are needed to validate these findings, but sovateltide shows promise as a potential therapeutic strategy to improve functional outcomes in SCI. Sovateltide, an endothelin B receptor agonist, exhibits neuroprotective properties, enhancing motor recovery and ameliorating hyperalgesia in a rat SCI model. These findings could pave the way for innovative pharmacological interventions for SCI in clinical settings.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Spinal cord injury (SCI) is a devastating event, often producing severe and permanent disability. According to the Global Burden of Disease Study 2019 [1], in 2019, there were 0.9 million incident cases, with a prevalence of 20.6 million. The burden of SCI has increased over the last decades, with older and male patients being affected more than younger and female individuals. Whereas there has been an advance regarding the pathophysiology, early recognition, and treatment, SCI still produces severe and, in many cases, life-long disability [1, 2].
Current treatment includes stabilization of the patient, and the best medical care to avoid cardiovascular, respiratory, and other medical complications [3, 4]. Surgical decompression and stabilization of the spinal cord can result in better outcomes, but until now, there were no guidelines regarding the timing and method of the intervention [5]. Other approaches (pharmacological, nonpharmacological, and cellular/genetic) targeting secondary inflammation have been tested [6]. Most of them are regarded as neuroprotective and include steroids [7, 8], channel blockers, neurotransmitter agonists and antagonists, and antiapoptotic and antioxidative agents [5, 6, 9,10,11], but they are still under investigation and are not used in clinical practice.
Thus, there is a need for new agents that could reduce the secondary injury and improve the functional outcome. Initial studies in an ischemic stroke model showed that the selective activation of endothelin B (ETB) receptors by the agonist, sovateltide (IRL-1620, PMZ-1620), significantly improved neurological and motor functions while simultaneously reducing infarct volume and oxidative stress damage at both 24 h and 1 week after permanent middle cerebral artery occlusion (MCAO) in rats [12,13,14], possibly due to enhancement of neurogenesis and angiogenesis.
Given that the spinal cord is also a part of the central nervous system (CNS), ETB receptors are also expressed in the spinal cord’s neuronal, neuroglial, and endothelial cells, making them a good target for sovateltide. In this study, we investigated the clinical outcomes of sovalteltide in a rat modal of acute SCI.
Methods
All experiments were performed at the animal laboratory of ELPEN’s Experimental, Educational, and Research Center in Athens, Greece. The Research Ethics Committee of the Veterinary Government Department of Attika approved the study protocol with protocol number 13-06-2018 and 2875/08-06-2018. The study was conducted in accordance with the Declaration of Helsinki.
ELPEN Experimental, Educational, and Research Center provided specialized personnel to facilitate the progress of the experiments and a specialized veterinary surgeon who supervised the experimental process.
Thirty-three male Sprague–Dawley rats, with a mean body weight of 300–350 g, were used for our study. The animals were housed in a standard animal facility with a temperature range between 20 and 26 °C, 50–60% humidity, and adequate ventilation with 12/12-h daylight/darkness. They had free access to food and water throughout the whole process ad libitum. All animals were acclimated to the research facility for 4 days before the initiation of the experiment.
All animals were divided into three groups (11 rats each). The first group included rats that underwent sham surgery (group A, sham-operated) and received vehicle injections (NaCl 0.9%, 1 ml/kg); the surgery was stopped at the stage of laminectomy, and no SCI was introduced. In the two other groups (group B and group C), all rats underwent SCI as described below and received vehicle and sovateltide (IRL-1620, PMZ-1620) injections, respectively. Animals were randomly allocated to each group. The investigator researcher conducting the experimental SCI, as elaborated below, was blinded to the administration of treatment (drug or placebo) and the subsequent clinical assessments. A second researcher, also blinded to the treatment and the nature of surgery (sham-operated or SCI), was in charge of the clinical assessment. Evaluation of the Basso, Beattie and Bresnahan (BBB) rating scale was independently performed by two trained investigators, both blinded to the treatment and the surgery type. Subsequently to the completion of the experiment and after the statistical analysis, the details of the treatment and surgery arms were disclosed.
SCI
To facilitate the SCI, animals were anesthetized. Anesthesia was initiated by exposing the animals to 8% sevoflurane within a confinement chamber. Subsequently, intubation was performed, and the animals were connected to a ventilation system, maintaining a sevoflurane concentration of approximately 2%. Prior to surgery, analgesic treatment was administered at least 1 h in advance, involving a subcutaneous injection of ketoprofen at a dose of 5 mg/kg, corresponding to 0.05 ml/kg of a 10% ketoprofen solution. In addition, prophylactic antibiotics (enrofloxacin, 0.1 ml of a 4 mg/kg Baytril solution subcutaneously) were administered. The choice and use of enrofloxacin as an antibiotic treatment over oxytetracycline (Oxyvet solution) was made because of its smaller effect on the nervous system compared with antibiotics of the tetracycline family [15,16,17]. Additionally, 1 ml/100gr 0.9% NaCl sc was administered preoperatively, and eye ointment was applied, while intraoperatively a warm mat was placed under the rat, and oxygen was provided.
The experimental model of SCI used is based on the direct impact method, involving the release of a rod from a specific height (blunt, nonpenetrating injury). The impact was targeted at the 10th thoracic vertebra after performing a laminectomy. The device used was inspired by the principles of New York University (NYU)/Multicenter Animal Spinal Cord Impactor Study (MASCIS) [18, 19], where a rod’s release resulted in a direct impact on the spinal cord. The force applied to the spinal cord of experimental animals, aiming to achieve a postinjury score on the BBB locomotor rating scale similar to that of studies used for power analysis, was calculated at 150 kdyn (simulating a moderate human SCI) [20]. This force was determined based on the experimental SCI model using the impactor device Infinite Horizon. In the current experimental work, a spinal cord impact device was employed, constructed following the standards and principles of NYU and MASCIS devices [18, 19, 21,22,23,24,25,26,27]. A 10 g rod with a diameter of approximately 2 mm was released from a height of 12.5 mm through a cylindrical tube, resulting in immediate spinal cord impact at the designated level (10th thoracic vertebra). In comparative studies of the two spinal cord impact devices (NYU and MASCIS), it has been observed that the release of a 10 g weight from a height of 12.5 mm corresponds to a force of 225 kdyn (with an impact velocity of 125 mm/s) when evaluating the displacement of the spinal cord and the load of force applied to the spinal cord [28]. The comparison of the two devices in the above experiment was based on the correlation between mechanical deformations and the corresponding pathoanatomical findings. On the other hand, for this specific experiment, the significance lies in the rating achieved by the experimental animals on the locomotor rating scale rather than the mechanical changes induced by the impact. For these reasons, the characteristics of the force (height 12.5 mm, weight 10 g, and rod diameter ~ 2 mm) chosen for the SCI in experimental animals after the weight drop have been calculated based on the literature and with the criterion being the primary endpoint of this study [19, 21,22,23, 25, 27, 29].
Following the surgical procedure and while the animals were recovering, subcutaneous administration of 5 ml of normal saline and 0.1 ml of Baytril solution was administered. Subsequent administrations of Baytril at 0.1 ml and ketoprofen at 5 mg/kg were continued once daily for the first 7 and 5 days, respectively. Manual bladder emptying was performed three times daily until normal urination was achieved (less than 2 ml of urine during morning emptying for 3 consecutive days). The entire technical protocol, from surgical preparation to postoperative care, including feeding assistance by placing food/water on the cage floor, was according to the guidelines established by both the Federation of European Laboratory Animal Science Associations and Krishna et al. [30, 31]. Intensive monitoring and special care were paid to any signs or symptoms that were indicative of autophagia and self-mutilation/autotomy. Any signs or symptoms of autophagia or autotomy were treated according to established protocols [27].
Sovateltide (previously IRL-1620, PMZ-1620) (N-Succinyl-[Glu9,Ala11,15] endothelin1) (Pharmazz, Inc) was diluted in NaCl 0.9% and was administered intravenously via the tail vein, in doses of 5 μg/kg (volume 1 ml/kg) at 2, 4, and 6 h on after injury (day 0) and the same hours on postsurgical days 1 and 2. The specific dose (mid–high), was chosen based on previous experiments on cerebral ischemia in rats (MCAO model), whereas the selection of multiple doses of sovateltide, as well as the preference for this dosing scheme over a single administration, is based on prior research demonstrating a tachyphylaxis effect against the initial hypotensive response to sovateltide [12,13,14, 32]. Specifically, it was previously shown that IRL-1620 produced a consistent decrease in renal blood flow and increase in cerebral blood flow without any evidence of tachyphylaxis [32]. Dosing of IRL-1620 was based on preliminary studies and publications, and it has been proven to be safe in various dosages both in rodents and humans [12,13,14, 32,33,34,35,36,37].
All clinical tests were performed preoperatively (baseline) and on the 7th, 14th, 21st, and 28th day after surgery. The selection of these particular time intervals for clinical evaluation was guided by the studies used in the power analysis [38] and directed toward observing the behavior of the experimental animals throughout the key stages of SCI pathophysiology, all of which are also pertinent treatment objectives [2].
Clinical Tests
Well-Being
All animals were assessed regarding their well-being. Parameters such as weight, urinary output until normal urinary function was restored, signs of autophagia/autotomy, and food and water consumption were recorded every day until the end of the experiment.
Open-Field/BBB Scoring
The animals were transferred with their cages to a separate room 1 h before the start of each test. Each animal was placed separately in a transparent, plastic plexiglass cage (40 × 40 × 40 cm) and was left for 5 min. With the help of two video cameras, all unconditioned behavior, general activity levels, gross locomotor activity, and exploration habits were observed. Recovery of locomotor function was determined with the BBB locomotor scale [39]. Briefly, the scale ranges from 0 (total paralysis) to 21 (normal locomotion). Stages 1 to 8 are scored for small or large movements of the three joints of the hind limb; a score of 9 indicates weight-bearing status or dorsal stepping, and scores of 10 to 21 indicate progressive improvements in coordinated walking ability [39,40,41,42]. The score for each animal was assigned by two observers (TM, AM) without knowledge of the animal’s treatment condition during each 5-min session of open-field testing.
Inclined Plane
The inclined plane is a behavioral assessment of the animal’s ability to balance on an elevated inclined wooden board. To assess the outcomes of coordinated motor function further, animals were tested using both the conventional incline plane method and its modified version [42, 43]. The apparatus was made according to previous studies [43]. In summary, the apparatus comprised of two rectangular segments (40 × 30 cm) joined by a hinge, wherein one acted as a fixed base and the other as a movable inclined plane. Markings that indicated the different degrees were drawn on one side. A rubber mat featuring minor ridges (~ 0.6 cm tall) was affixed to the surface of the movable plane to enhance traction. The animals relied on their front and rear limbs to stay on the plane. In practice, the angle was systematically increased from a starting point of 10° in 5-degree increments until the rat could not sustain its posture on the inclined plane [43]. The angle at which the animal successfully maintains its stance on the inclined plane for 5 s was recorded [40, 43,44,45]. Horizontal positioning (the rostrocaudal axis of the rat trunk was perpendicular to the longitudinal axis of the plane) [43], in addition to the head-up and head-down positions [42, 44], was assessed. Regarding the two latter positions, head-up primarily evaluates the overall physical condition (mainly based on forelimb activity), and head-down assesses the coordination of the hind limb performance [40, 42].
Sensation/Allodynia Testing—von Frey Hair/Filaments
Pain-related behavior was recorded using various devices applied to the forelimbs, hindlimbs, trunk, and face [46]. The animals were placed individually for 30 min before the experiment in a transparent plastic cage closed over a wire mesh surface, providing access to the plantar surfaces of the hind limbs. All animals were previously conditioned with sugared cereal both before and during the testing, according to Detloff et al. [47]. As Detloff et al. [47] demonstrated previously, the use of sugared cereal as a cognitive distractor during plantar von Frey hair (VFH) testing acts as a stabilization factor to eliminate variability in tactile sensory testing.
Subsequently, the filaments (starting with the 5.18) were gently applied vertically against the center of the plantar surface of the hind limbs until a bend in the fiber was observed, with this pressure being maintained for approximately 1–2 s. A positive response is noted when the limb is withdrawn from the monofilament [47, 48]. Any supraspinally driven attention given to the tactile stimulus, including vocalizing, licking, or guarding the stimulated paw, was also recorded as a positive response [49]. Using VFH (Stoelting Co, Wood Dale, IL) according to the “up-down” method [47, 48], the degree of tactile sensation after SCI was assessed. A set of 10 VFH stimulus applications was collected for each hindlimb [49]. The response threshold refers to the minimum force, measured in the logarithmic VFH scale, required to elicit a plantar withdrawal response in 50% of the applications. A 15-g upper limit is applied to prevent any potential injury.
All rats were killed according to established experimental protocols on the 28th postoperative day after the last clinical tests, and the spinal cord was harvested, freshly frozen, and cut under cryotome for microscopic evaluation. The aim of the kill was to verify the level of SCI and the extent of that; bilateral uniform SCI seen in the microscope was considered a success.
Sample Size Estimation
The power analysis was derived based on previous studies [38, 50, 51] and using the BBB score as the primary outcome. We estimated the sample size using the two-way mixed model analysis of variance (ANOVA) with factors the “intervention” (sham-operated = group A/SCI-vehicle = group B/SCI-sovateltide = group C) and “time” (baseline/presurgery/7 days/14 days/21 days/28 days) and focused on the comparison of group B and C, regardless of the “time” factor. The effect size of the comparison was 0.63 (group, A 21 ± 4; group B, 11.5 ± 4; group C, 16.5 ± 4), and considering power 80% and level of significance 1.7% (Bonferroni correction for three groups), we needed ten laboratory animals per group. Taking into consideration the severity of the experiment and the increased mortality risk occurring either during or following the surgical procedures, we made a projection that the inclusion of three supplementary animals (one per group), would provide adequate contingency measures to ensure the feasibility of conducting the initial statistical analysis, even in the event of unexpected fatalities. Because of the severity and novelty of the experiment, we calculated three more animals (one per group).
Statistical Analysis
Data were expressed as mean ± standard deviation, and the Shapiro-Wilks test examined the normal distribution of the parameters.
We used the two-way mixed ANOVA model using as factors “the intervention” (comparison between the three groups) and “time” (within group longitudinal comparison) for the analysis of variables using the Bonferroni correction for all pairwise comparisons (post hoc) either between or within groups.
Sensitivity analysis, including the baseline measure of each variable as a covariate was performed in order to assess the robustness of the primary analysis. This was evaluated by using the comparison of percentage change from baseline of variables and analyzed with the one-way ANOVA model (for normal distribution) and Bonferroni test for pairwise comparisons or the Kruskal–Wallis and Mann–Whitney U-tests in case of violation of normality of variables.
Finally, the analysis regarding the correlation of VFH size and response (VFH size-response analysis) between the three groups was done using the Probit regression model. The variable ED-50, meaning 50% of positive answers, was calculated and used for the analysis.
All tests were two sided, and statistical significance was set at p < 0.05. All analyses were carried out using the statistical package SPSS version 21.00 (IBM Corporation, Somers, NY).
Results
Well-Being
Thirty animals (10 in each group) were included in the final analysis. Three animals died during or after surgery due to the severity of the experiment and anesthesia. All animals had a relatively good surgical recovery and achieved feeding milestones, and none of the animals experienced signs of autophagy. Regarding weight gain, there was a statistically significant difference between groups B and C only at 21 days (p = 0.023), whereas there was no difference in all other time points. No drug-related (sovateltide) adverse events were reported.
BBB Score
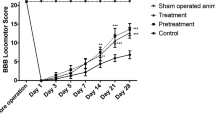
We observed that there are statistically significant differences (F[8, 107] = 261.84 p < 0.005) in the BBB mean scores between the three groups for the five time points, as seen in Fig. 1.
More specifically, there is no statistically significant difference between the three interventions preoperatively (p > 0.99), whereas there is for 7 days (p < 0.005), 14 days (p < 0.005), 21 days (p < 0.005), and 28 days (p < 0.005) for the BBB variable. From the multiple comparisons, a statistically significant difference between group A and groups B and C at 7, 14, 21, and 28 days was observed. More interestingly, there was a statistically significant difference in mean BBB scores between group B and group C at all time points after surgery (7, 14, 21, and 28 days) (p < 0.005), as seen in Table 1. Τhere is also statistically significant difference in percentage changes of mean BBB score from the preoperative assessment to 7-day (p < 0.005), 14-day (p < 0.005), 21-day (p < 0.005), and 28-day (p < 0.005) time points between the three interventions. Again, multiple comparisons showed a statistically significant difference between group B and C at all time points (p < 0.005), as seen in Table 1 and Fig. 2.
In the longitudinal analysis in each group, there was a statistically significant difference in BBB scores between the 7th day and 14th day, but not between the 14th, 21st and 28th day in group B (SCI-vehicle), whereas that difference remains statistically significant across all time points in group C (SCI-sovateltide) (p < 0.005) (Table 1).
Inclined Plane
Inclined plane testing was done with the test subjects positioned vertically (VER), head-up and head-down. Most of the results showed consistency in all their trends.
More specifically, with regards to VER, statistically significant difference was observed between group B and C in 14, 21 and 28 days of the experiment (p value < 0.005) as shown in Table 2, Fig. 3. That difference is also depicted in differences in percentage changes of mean VER angle score from the preoperative assessment to 7-day (p < 0.005), 14-day (p < 0.005), 21-day (p < 0.005), and 28-day (p < 0.005) time points between the three interventions. Multiple comparisons showed a statistically significant difference between group B and C at 14-day, 21-day, and 28-day time points (p < 0.005), as seen in Table 2.
In the longitudinal analysis in each group, there was a statistically significant difference in VER scores across all time points in group C (p < 0.005), and surprisingly there was no difference between the presurgical assessment and day 28 (p = 0.224). In group B, there was not any difference in scores between days 7, 14, 21 and 28 (Table 2).
Similar and consistent results and statistically significant differences were found also in the head-up and head-down inclined plane testing between group B and C in the 14th, 21st, and 28th day of the experiment (p values < 0.005) (Figs. 4 and 5).
VFH Filament
Statistically significant differences were observed between group B and C at the 28th day on the right leg and at the 21st and 28th day on the left (Figs. 6 and 7). There were not any other significant differences between those group at any other time point in both legs (Supplementary Table 1).
Discussion
In this study, sovateltide administration has been shown to improve rats’ functional outcome and reduce hyperalgesia after SCI. Sovateltide is a selective ETB receptor agonist, originally tested for its vasodilatory properties and its ability to enhance the uptake and efficacy of various chemotherapeutic agents [52,53,54].
Endothelins are a group of peptides consisting of 21 amino acids, comprising three isoforms: ET-1, ET-2, and ET-3. In mammals’ normal CNS and spinal cord, ET-1 and ET-3 isoforms are present in vascular endothelial cells and a subset of neurons [55,56,57,58,59]. Studies have demonstrated that ET-1 and ET-3 levels in cerebrospinal fluid and plasma are typically low in an uninjured CNS [60, 61], but significantly rise following traumatic brain injury [62, 63], SCI [64,65,66], and cerebrovascular accidents [67, 68].
Within the mammalian CNS, endothelins exert their physiological effects by activating two receptor subtypes: endothelin A (ETA) receptor (ETAR) and endothelin B receptor (ETBR). ET-1 and ET-2 have a higher affinity for ETAR than ET-3, while all three peptides have similar affinities for ETBR. Research has shown that endothelin receptors are distributed throughout the normal spinal cord in mammals, including humans [69,70,71]. ETAR mRNA is predominantly expressed in vascular smooth muscle cells, whereas ETBR mRNA is found mainly in glial and vascular endothelial cells. Activation of ETAR leads to vasoconstriction, whereas ETBR activation induces vasodilation. Under normal conditions, these receptors regulate cerebral blood flow and influence developmental processes like neuronal migration, proliferation, and apoptosis [14, 72, 73].
Studies have linked elevated ET levels to reduced blood flow in ischemic brain areas after experimental ischemic stroke models [12, 74]. Consequently, researchers have explored various endothelin antagonists for ischemic stroke treatment. While some ETA-specific and nonspecific ETA/B antagonists have shown promise in experimental models, others have not yielded favorable results [12, 33, 75,76,77,78,79]. Most studies have focused on selectively blocking ETA receptors to prevent excessive vasoconstriction, neglecting the impact of selectively activating ETB receptors in stroke models. Early research indicated that inhibiting or lacking endothelin B receptors might disrupt the vasomotor balance, potentially worsening ischemic brain injury [14, 80, 81]. Increased levels of ET-1 in plasma and tissue during ischemic stroke and subarachnoid hemorrhage [12, 82, 83], along with evidence that ETB receptor deficiency worsens outcomes after cerebral ischemia, prompted investigations into the role of ETB receptors in ischemic stroke models [13, 80, 81].
Moreover, recent investigations have also revealed that the intracerebroventricular administration of an ETB receptor agonist in normal rats can enhance vascular endothelial growth factor (VEGF) production and activate VEGF receptors within the brain, hence promote angiogenesis [14, 84, 85]. VEGF exerts its effects by binding to specific tyrosine-kinase receptors, namely VEGFR-1 and VEGFR-2, predominantly located on endothelial cells. During hypoxic conditions such as cerebral ischemia, VEGF expression is induced in neurons, astrocytes, and endothelial cells through hypoxia-inducible factor-1 [86]. Once expressed, VEGF initiates both direct and indirect neuroprotective actions, including apoptosis inhibition, stimulation of neurogenesis and angiogenesis, increased glucose uptake, and activation of antioxidants [87]. VEGF binds to VEGFR-2, activating the extracellular signal-regulated kinase and mitogen-activated protein kinase signaling pathways [88]. ETB receptor antagonism prevents the upregulation of VEGF [89] and downregulates extracellular signal-regulated kinase and mitogen-activated protein kinase pathways [90], thus leading to reduced proliferation, and increased apoptosis.
Likewise, it has been observed that endogenous neurogenesis, involving an increase in the population of neural stem cells in the subventricular zone (SVZ), is part of the reparative process following ischemic stroke [14, 91]. Interestingly, ETB receptors have been identified within the subependymal zone, a neurogenic niche in adult rats, suggesting a potential role in not only regulating the developing CNS but also remodeling the adult brain [92]. ETB agonist administration has been reported to elevate brain-derived neurotrophic factor, glial-derived neurotrophic factor, and neurotrophin-3 in the brains of normal adult rats [93, 94]. Moreover, IRL-1620 has shown to induce cell proliferation and specifically those who are expressing nerve-growth gactor in the SVZ, striatum, and cortex of rats after permanent cerebral ischemia. Notably, the researchers observed migration of these cells from the SVZ to the striatum and cortex, which implies significant neuronal remodeling. The findings emphasize the neurogenic potential of sovateltide in the context of CNS injury (brain and spinal cord) [14].
Activation of ETB receptors through intravenous administration of sovateltide (IRL-1620, PMZ-1620), a potent selective ETB agonist, significantly increased cerebral blood flow in normal rats [12, 32]. Furthermore, functional ETB receptors enhanced neural progenitor cell proliferation and protected against apoptosis in various brain regions. Most investigations scrutinizing the mechanism and effects of sovateltide center around hypoxia models. In a rat model of experimental stroke (MCAO), sovateltide administration demonstrated improved neurological functions, concurrently reducing malondialdehyde levels—a lipid peroxidation indicator—while elevating the levels of potent antioxidants glutathione and superoxide dismutase [13]. Initial studies in an ischemic stroke model demonstrated that selective stimulation of ETB receptors with the agonist IRL-1620 improved neurological and motor functions while reducing infarct volume and provided sustained neuroprotection against oxidative stress at both 24 h and 1 week after permanent MCAO in rats [12,13,14]. Furthermore, the involvement of ET receptors on astrocytes in modulating gap junction permeability and apoptotic signal propagation has been elucidated [95, 96]. Sovateltide treatment demonstrated a mitigating effect on brain edema, while ETB receptor antagonism exacerbated edema, implicating the ETB receptor in gap junction permeability and blood–brain barrier breakdown during subacute ischemia [13]. The ETB receptor, acting as a clearance receptor for ET-1, was associated with increased expression of neuroprotective molecules, potentially contributing to vasoprotective effects [81, 84, 97]. This suggests that ETB receptors may play a pivotal role in CNS plasticity (both brain and spinal cord), and early administration of IRL-1620 could contribute to long-term CNS plastic mechanisms, resulting in improved outcomes in hypoxic and injury scenarios [12,13,14].
Those preclinical studies showed that sovateltide is beneficial in ischemic stroke via activation of ETB receptors and was also tested in the clinical setting. In a phase II trial, sovateltide has proven safe and well-tolerated, improving neurological outcomes in patients with acute ischemic stroke 90 days post-treatment [98].
In SCI studies that tested similar angiogenic and neurogenic agents, such as VEGF and platelet-derived growth factor (PDGF), functional recovery (BBB score) was shown at 28 days compared with placebo [38]. Moreover, other therapeutic agents like rolipram, a phosphodiesterase 4 inhibitor, ganglioside G(M1), and erythropoietin have been found to decrease neuronal sensitivity to myelin inhibitors, increase growth potential and are neuroprotective also in SCI [50, 51], possible via angiogenesis and neurogenesis. Taking the aforementioned into consideration and the possibility that sovateltide is exerting its effects via cell proliferation, similar results are supported by this study and unpublished data [99].
The result of the study depicts the clinical improvement even from early stages (day 7) when compared to placebo. These results are in accordance with previous studies that investigated the clinical effects of sovateltide in ischemic stroke and other vasogenic substances in models of SCI [13, 14, 38, 50, 51, 100, 101]. Although, in the SCI-vehicle group, there was an early motor function improvement between the experiment’s 7th and 14th days (statistically significant), the rats reached a plateau. This observation is seen in many studies and is possibly due to the endogenous repairing mechanisms of the CNS. In contrast, the sovateltide group continued to improve until the end of the experiment, with a longitudinal trajectory that was clinically significant in all time points. This suggests that sovateltide demonstrates a lasting impact, with its effects expected to persist beyond the experimental timeframe.
Our investigation yielded consistent outcomes during the inclined plane test. This assessment provides valuable insights into multiple aspects of their functionality, encompassing motor skills, hindlimb coordination, balance, and overall physical strength. The intriguing aspect of our findings is the absence of any statistically significant difference between the presurgical baseline assessment and the assessment conducted on the 28th day of the experiment, specifically within the sovateltide-treated group, meaning that the subjects reached their pre-SCI status. This noteworthy result strongly indicates that the positive changes in motor function and coordination, while evident within the study’s timeframe, potentially extend beyond the observed period. The clinical observations of this study, however, ceased on day 28, leaving open the possibility that the full extent of improvement facilitated by sovateltide may not have been fully captured. This highlights the need for future investigations to explore the continued progress and sustainability of these effects in the days and weeks following the study’s end point.
The importance of this study lies also in the data presented regarding allodynia testing. It is known that if pain behavior appears in the face, it reflects the reaction to supraspinal mechanisms, because sensory function in the face is regulated by the trigeminal nerve. In thoracic SCI, trunk allodynia reflects at-level neuropathic pain, and allodynia in the hindlimb reflects below-level neuropathic pain [46]. Moreover, it was previously shown that ET-1 has emerged as a potent neuroactive peptide, which plays a role in various pain and pain-related processes through its interaction with its receptors (ETAR and ETBR) [102]. Numerous research investigations have consistently identified ET-1 as a pronociceptive agent, significantly contributing to pain regulation (increase) in numerous pathological conditions [103,104,105,106]. However, it is worth noting that a handful of studies have reported that ET-1 also demonstrates antinociceptive effects. These findings suggest that the effects of ET-1 on nociception may vary depending on receptor-specific actions; different actions on ETA and ETB receptors, potentially resulting in a dual impact of ET-1 on pain perception [107, 108]. There was also a hypothesis that ET-1 exerts its antinociceptive effects through ETBR, potentially through the regulation of both endogenous opioid dependent and independent signaling pathways [109,110,111,112,113]. Mule et al. [114] found that the reduction in nociceptor excitability is facilitated by ET-1 through the specific activation of ETBR in a rat model of neuropathic pain. Various studies suggest that activating peripheral ETB receptors produces an analgesic effect in pathologic conditions in rats [115]. These analgesic/antinociceptive effects of ETB receptor agonism is supported by the data revealing that ETB receptor-deficient mice are hypersensitive to mechanical stimuli [116]. Consequently, it is conceivable that IRL-1620, a selective ETBR agonist, could serve as promising candidate for the treatment of neuropathic pain. This hypothesis is also confirmed by the current study (SCI model of neuropathic pain), as the results depict the change of the allodynic threshold especially at the time point of 4 weeks. A mild inconsistency was observed between the two sides (left and right feet), possibly attributed to the slight angle changes during the weight drop and the small number of the test subjects. Nevertheless, this finding is important, and it can be hypothesized that sovateltide may have also anti-inflammatory and antinociceptive properties that need to be investigated.
This study is not without limitations. As in every experimental study there, is a translational gap between rats and humans, and those results must not be interpreted in clinical settings without further investigation and clinical trials. Despite rats being larger than mice, they still represent a different species relative to humans. Consequently, long-distance axon regeneration, a very important concept of the pathophysiology and recovery in human SCI, cannot be directly extrapolated from rat models. Notably, findings from rodent studies that document enhanced axonal growth, such as through axons spanning the lesion site, may lead to potential misconceptions. This is attributed to the difference in the volumes of gray matter requiring reinnervation between humans and rats. The recuperative process following SCI in humans is slower compared to rats. Human spontaneous recovery is recognized to continue evolving without reaching a plateau until 6–12 months post injury. Conversely, the same process in rats typically reaches a plateau around ∼6–8 weeks after injury [117]. Whereas contusion injuries, induced by the impact of an accelerating rod on the spinal cord, are regarded as reasonably faithful representations of human spinal cord impact injuries [118], certain assumptions (simplified representation of the spinal cord and omission of cerebrospinal fluid flow) were made, that may affect the impact force, the spinal cord displacement, and clinical outcomes. Noteworthy is the observation that rats exhibit strain-specific recovery patterns from spinal cord injuries [119], and discernible variations in functional outcomes and tissue preservation exist even among substrains [120]. The origins of these differences, albeit not fully comprehended, appear to have a genetic basis. Additionally, there are known and evident differences in the anatomy of spinal fiber tracts between humans and rats [121] and even among rat substrains [122]. Consequently, careful consideration of substrain selection becomes imperative for the accurate interpretation of outcomes in experimental models of SCI. In this study, the selection of the Spraque-Dawley strain was driven by the literature as most of the contusion models use the specific strain due to its reproducibility and validation. Moreover, Sprague–Dawley rats have a more potent endogenous neurogenetic and angiogenetic ability after injury than humans and can be enhanced more by therapeutic agents. Confounding factors that may have been introduced during the study design or execution of the experiment could include the subjectivity of BBB scoring (measurement bias), as this needs experience and is operator dependent. Nevertheless, the two investigators who were involved in the scoring had vast experience and a very strong interrater reliability (κ = 0.91). Because of the careful design of the study, there was no selection or treatment bias. Another limitation of the study is the lack of immunofluorescent investigation of the spinal cord at the end of the experiment, as the primary end point was the motor function (BBB score) and not the immunohistochemistry analysis. This could provide more information regarding the mechanism of action and the pathophysiology.
This study supports the role of sovateltide in SCI showing promising results. Ongoing phase II clinical trial comparing the safety and efficacy of sovateltide therapy along with standard supportive care in patients with acute SCI will help to elaborate the role of ETBRs as an attractive pharmacological tool for SCI treatment and would establish a new ETBRs based spinal cord regenerative strategy to increase neuronal survival, regeneration, and function after injury [99, 123]. Sovateltide, along with targeted activation of ETB receptors, has yielded valuable insights into the pathophysiology of nervous system injuries, extending beyond ischemia to encompass trauma and inflammation. This knowledge encourages further exploration of sovateltide's potential applications in diverse CNS disorders, including autoimmune conditions and degenerative diseases. Investigating the molecular pathways involved in inflammation-associated disorders of the CNS could unveil novel therapeutic avenues for sovateltide. Additionally, examining its impact on common pathways shared among various neuroinflammatory conditions may provide a broader perspective, potentially enhancing its clinical utility and expanding its role in future treatments for a spectrum of CNS diseases.
Conclusions
The present study demonstrates that ETB receptor activation by sovateltide may be a novel neuroprotective therapy in treating SCI. Selective ETB receptor agonist, sovateltide, significantly decreases neurological deficit and motor impairment, and ameliorates hyperalgesia by lowering the allodynic threshold following a rat model of SCI. More studies are needed to shed light into the mechanism of neuroprotection by the ETB receptor agonism.
References
Ding W, Hu S, Wang P, et al. Spinal cord injury: the global incidence, prevalence, and disability from the global burden of disease study 2019. Spine. 2022;47:1532–40.
Sekhon LH, Fehlings MG. Epidemiology, demographics, and pathophysiology of acute spinal cord injury. Spine. 2001;26:S2-12.
Management of acute spinal cord injuries in an intensive care unit or other monitored setting. Neurosurgery 2002;50:S51–S7.
Jia X, Kowalski RG, Sciubba DM, Geocadin RG. Critical care of traumatic spinal cord injury. J Intensive Care Med. 2013;28:12–23.
Fehlings MG, Perrin RG. The role and timing of early decompression for cervical spinal cord injury: update with a review of recent clinical evidence. Injury. 2005;36(Suppl 2):B13-26.
Anjum A, Yazid MD, Fauzi Daud M, et al. Spinal cord injury: pathophysiology, multimolecular interactions, and underlying recovery mechanisms. Int J Mol Sci. 2020;21:7533.
Breslin K, Agrawal D. The use of methylprednisolone in acute spinal cord injury: a review of the evidence, controversies, and recommendations. Pediatr Emerg Care 2012;28:1238–45; quiz 46–8.
Hugenholtz H, Cass DE, Dvorak MF, et al. High-dose methylprednisolone for acute closed spinal cord injury—only a treatment option. Can J Neurol Sci. 2002;29:227–35.
Pitts LH, Ross A, Chase GA, Faden AI. Treatment with thyrotropin-releasing hormone (TRH) in patients with traumatic spinal cord injuries. J Neurotrauma. 1995;12:235–43.
Casha S, Zygun D, McGowan MD, Bains I, Yong VW, Hurlbert RJ. Results of a phase II placebo-controlled randomized trial of minocycline in acute spinal cord injury. Brain. 2012;135:1224–36.
Inada T, Takahashi H, Yamazaki M, et al. Multicenter prospective nonrandomized controlled clinical trial to prove neurotherapeutic effects of granulocyte colony-stimulating factor for acute spinal cord injury: analyses of follow-up cases after at least 1 year. Spine. 2014;39:213–9.
Leonard MG, Briyal S, Gulati A. Endothelin B receptor agonist, IRL-1620, reduces neurological damage following permanent middle cerebral artery occlusion in rats. Brain Res. 2011;1420:48–58.
Leonard MG, Briyal S, Gulati A. Endothelin B receptor agonist, IRL-1620, provides long-term neuroprotection in cerebral ischemia in rats. Brain Res. 2012;1464:14–23.
Leonard MG, Gulati A. Endothelin B receptor agonist, IRL-1620, enhances angiogenesis and neurogenesis following cerebral ischemia in rats. Brain Res. 2013;1528:28–41.
Festoff BW, Ameenuddin S, Arnold PM, Wong A, Santacruz KS, Citron BA. Minocycline neuroprotects, reduces microgliosis, and inhibits caspase protease expression early after spinal cord injury. J Neurochem. 2006;97:1314–26.
Kobayashi K, Imagama S, Ohgomori T, et al. Minocycline selectively inhibits M1 polarization of microglia. Cell Death Dis. 2013;4: e525.
Yong VW, Wells J, Giuliani F, Casha S, Power C, Metz LM. The promise of minocycline in neurology. Lancet Neurol. 2004;3:744–51.
Gruner JA. A monitored contusion model of spinal cord injury in the rat. J Neurotrauma 1992;9:123–6; discussion 6–8.
Vijayaprakash KM, Sridharan N. An experimental spinal cord injury rat model using customized impact device: a cost-effective approach. J Pharmacol Pharmacother. 2013;4:211–3.
Scheff SW, Rabchevsky AG, Fugaccia I, Main JA, Lumpp JE Jr. Experimental modeling of spinal cord injury: characterization of a force-defined injury device. J Neurotrauma. 2003;20:179–93.
Rabchevsky AG, Fugaccia I, Fletcher-Turner A, Blades DA, Mattson MP, Scheff SW. Basic fibroblast growth factor (bFGF) enhances tissue sparing and functional recovery following moderate spinal cord injury. J Neurotrauma. 1999;16:817–30.
Rabchevsky AG, Sullivan PG, Fugaccia I, Scheff SW. Creatine diet supplement for spinal cord injury: influences on functional recovery and tissue sparing in rats. J Neurotrauma. 2003;20:659–69.
Rabchevsky AG, Fugaccia I, Sullivan PG, Scheff SW. Cyclosporin A treatment following spinal cord injury to the rat: behavioral effects and stereological assessment of tissue sparing. J Neurotrauma. 2001;18:513–22.
Basso DM, Beattie MS, Bresnahan JC, et al. MASCIS evaluation of open field locomotor scores: effects of experience and teamwork on reliability. Multicenter Animal Spinal Cord Injury Study. J Neurotrauma. 1996;13:343–59.
Agrawal G, Kerr C, Thakor NV, All AH. Characterization of graded multicenter animal spinal cord injury study contusion spinal cord injury using somatosensory-evoked potentials. Spine. 2010;35:1122–7.
Young W. Spinal cord contusion models. Prog Brain Res. 2002;137:231–55.
Young W. MASCIS spinal cord contusion model. In: Chen J, Xu ZC, Xu X-M, Zhang JH, editors. Animal models of acute neurological injuries. Totowa, NJ: Humana Press; 2009. p. 411–21.
Khuyagbaatar B, Kim K, Kim YH. Conversion equation between the drop height in the New York University impactor and the impact force in the infinite horizon impactor in the contusion spinal cord injury model. J Neurotrauma. 2015;32:1987–93.
Park JH, Kim JH, Oh SK, et al. Analysis of equivalent parameters of two spinal cord injury devices: the New York University impactor versus the Infinite Horizon impactor. Spine J. 2016;16:1392–403.
Krishna V, Andrews H, Jin X, et al. A contusion model of severe spinal cord injury in rats. J Vis Exp JoVE. 2013. https://doi.org/10.3791/50111-v.
FELASA working group on revision of guidelines for health monitoring of rodents and rabbits, Mahler Convenor M, et al. FELASA recommendations for the health monitoring of mouse, rat, hamster, guinea pig and rabbit colonies in breeding and experimental units. Lab Anim. 2014;48:178–92.
Leonard MG, Gulati A. Repeated administration of ET(B) receptor agonist, IRL-1620, produces tachyphylaxis only to its hypotensive effect. Pharmacol Res. 2009;60:402–10.
Briyal S, Gulati A. Endothelin-A receptor antagonist BQ123 potentiates acetaminophen induced hypothermia and reduces infarction following focal cerebral ischemia in rats. Eur J Pharmacol. 2010;644:73–9.
Lavhale MS, Briyal S, Parikh N, Gulati A. Endothelin modulates the cardiovascular effects of clonidine in the rat. Pharmacol Res. 2010;62:489–99.
Briyal S, Ranjan AK, Hornick MG, Puppala AK, Luu T, Gulati A. Anti-apoptotic activity of ETB receptor agonist, IRL-1620, protects neural cells in rats with cerebral ischemia. Sci Rep. 2019;9:10439.
Ranjan AK, Briyal S, Gulati A. Sovateltide (IRL-1620) activates neuronal differentiation and prevents mitochondrial dysfunction in adult mammalian brains following stroke. Sci Rep. 2020;10:12737.
Gulati A, Hornick M, Briyal S, Lavhale MJPr. A Novel Neuroregenerative Approach Using ET B Receptor Agonist, IRL-1620, to Treat CNS Disorders. 2018; 67.
Chehrehasa F, Cobcroft M, Young YW, Mackay-Sim A, Goss B. An acute growth factor treatment that preserves function after spinal cord contusion injury. J Neurotrauma. 2014;31:1807–13.
Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995;12:1–21.
Konya D, Liao WL, Choi H, et al. Functional recovery in T13–L1 hemisected rats resulting from peripheral nerve rerouting: role of central neuroplasticity. Regen Med. 2008;3:309–27.
Ropper AE, Zeng X, Anderson JE, et al. An efficient device to experimentally model compression injury of mammalian spinal cord. Exp Neurol. 2015;271:515–23.
Teng YD, Choi H, Onario RC, et al. Minocycline inhibits contusion-triggered mitochondrial cytochrome c release and mitigates functional deficits after spinal cord injury. Proc Natl Acad Sci USA. 2004;101:3071–6.
Rivlin AS, Tator CH. Objective clinical assessment of motor function after experimental spinal cord injury in the rat. J Neurosurg. 1977;47:577–81.
Gale K, Kerasidis H, Wrathall JR. Spinal cord contusion in the rat: behavioral analysis of functional neurologic impairment. Exp Neurol. 1985;88:123–34.
Kerasidis H, Wrathall JR, Gale K. Behavioral assessment of functional deficit in rats with contusive spinal cord injury. J Neurosci Methods. 1987;20:167–79.
Nakae A, Nakai K, Yano K, Hosokawa K, Shibata M, Mashimo T. The animal model of spinal cord injury as an experimental pain model. J Biomed Biotechnol. 2011;2011: 939023.
Detloff MR, Fisher LC, Deibert RJ, Basso DM. Acute and chronic tactile sensory testing after spinal cord injury in rats. J Visual Exp: JoVE. 2012. https://doi.org/10.3791/3247.
Detloff MR, Clark LM, Hutchinson KJ, Kloos AD, Fisher LC, Basso DM. Validity of acute and chronic tactile sensory testing after spinal cord injury in rats. Exp Neurol. 2010;225:366–76.
Detloff MR, Wade RE Jr, Houle JD. Chronic at- and below-level pain after moderate unilateral cervical spinal cord contusion in rats. J Neurotrauma. 2013;30:884–90.
Costa LM, Pereira JE, Filipe VM, et al. Rolipram promotes functional recovery after contusive thoracic spinal cord injury in rats. Behav Brain Res. 2013;243:66–73.
Marcon RM, Cristante AF, de Barros TEF, Ferreira R, Dos Santos GB. Effects of ganglioside G(M1) and erythropoietin on spinal cord lesions in rats: functional and histological evaluations. Clinics (Sao Paulo). 2016;71:351–60.
Rajeshkumar NV, Matwyshyn G, Gulati A. IRL-1620, a tumor selective vasodilator, augments the uptake and efficacy of chemotherapeutic agents in prostate tumor rats. Prostate. 2007;67:701–13.
Rai A, Rajeshkumar NV, Gulati A. Effect of the ET(B) receptor agonist, IRL-1620, on paclitaxel plasma pharmacokinetics of breast tumor rats. Exp Biol Med (Maywood). 2006;231:1120–2.
Rajeshkumar NV, Rai A, Gulati A. Endothelin B receptor agonist, IRL 1620, enhances the anti-tumor efficacy of paclitaxel in breast tumor rats. Breast Cancer Res Treat. 2005;94:237–47.
Barnes K, Turner AJ. The endothelin system and endothelin-converting enzyme in the brain: molecular and cellular studies. Neurochem Res. 1997;22:1033–40.
Giaid A, Gibson SJ, Ibrahim BN, et al. Endothelin 1, an endothelium-derived peptide, is expressed in neurons of the human spinal cord and dorsal root ganglia. Proc Natl Acad Sci USA. 1989;86:7634–8.
Lee ME, de la Monte SM, Ng SC, Bloch KD, Quertermous T. Expression of the potent vasoconstrictor endothelin in the human central nervous system. J Clin Investig. 1990;86:141–7.
Yanagisawa M, Kurihara H, Kimura S, et al. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988;332:411–5.
MacCumber MW, Ross CA, Snyder SH. Endothelin in brain: receptors, mitogenesis, and biosynthesis in glial cells. Proc Natl Acad Sci USA. 1990;87:2359–63.
Suzuki N, Matsumoto H, Kitada C, Masaki T, Fujino M. A sensitive sandwich-enzyme immunoassay for human endothelin. J Immunol Methods. 1989;118:245–50.
Yoshizawa T, Shinmi O, Giaid A, et al. Endothelin: a novel peptide in the posterior pituitary system. Science (New York, NY). 1990;247:462–4.
Hama H, Kasuya Y, Sakurai T, et al. Role of endothelin-1 in astrocyte responses after acute brain damage. J Neurosci Res. 1997;47:590–602.
Siren AL, Knerlich F, Schilling L, Kamrowski-Kruck H, Hahn A, Ehrenreich H. Differential glial and vascular expression of endothelins and their receptors in rat brain after neurotrauma. Neurochem Res. 2000;25:957–69.
McKenzie AL, Hall JJ, Aihara N, Fukuda K, Noble LJ. Immunolocalization of endothelin in the traumatized spinal cord: relationship to blood-spinal cord barrier breakdown. J Neurotrauma. 1995;12:257–68.
Salzman SK, Acosta R, Beck G, Madden J, Boxer B, Ohlstein EH. Spinal endothelin content is elevated after moderate local trauma in the rat to levels associated with locomotor dysfunction after intrathecal injection. J Neurotrauma. 1996;13:93–101.
Uesugi M, Kasuya Y, Hama H, et al. Endogenous endothelin-1 initiates astrocytic growth after spinal cord injury. Brain Res. 1996;728:255–9.
Lampl Y, Fleminger G, Gilad R, Galron R, Sarova-Pinhas I, Sokolovsky M. Endothelin in cerebrospinal fluid and plasma of patients in the early stage of ischemic stroke. Stroke. 1997;28:1951–5.
Peters CM, Rogers SD, Pomonis JD, et al. Endothelin receptor expression in the normal and injured spinal cord: potential involvement in injury-induced ischemia and gliosis. Exp Neurol. 2003;180:1–13.
Davenport AP, Nunez DJ, Hall JA, Kaumann AJ, Brown MJ. Autoradiographical localization of binding sites for porcine [125I]endothelin-1 in humans, pigs, and rats: functional relevance in humans. J Cardiovasc Pharmacol. 1989;13(Suppl 5):S166–70.
Kar S, Chabot JG, Quirion R. Quantitative autoradiographic localisation of [125I]endothelin-1 binding sites in spinal cord and dorsal root ganglia of the rat. Neurosci Lett. 1991;133:117–20.
Niwa M, Kawaguchi T, Himeno A, et al. Specific binding sites for 125I-endothelin-1 in the porcine and human spinal cord. Eur J Pharmacol. 1992;225:281–9.
Ehrenreich H, Nau TR, Dembowski C, et al. Endothelin b receptor deficiency is associated with an increased rate of neuronal apoptosis in the dentate gyrus. Neuroscience. 2000;95:993–1001.
Vidovic M, Chen MM, Lu QY, et al. Deficiency in endothelin receptor B reduces proliferation of neuronal progenitors and increases apoptosis in postnatal rat cerebellum. Cell Mol Neurobiol. 2008;28:1129–38.
Patel TR, Galbraith SL, McAuley MA, Doherty AM, Graham DI, McCulloch J. Therapeutic potential of endothelin receptor antagonists in experimental stroke. J Cardiovasc Pharmacol. 1995;26(Suppl 3):S412–5.
Barone FC, Ohlstein EH, Hunter AJ, et al. Selective antagonism of endothelin-A-receptors improves outcome in both head trauma and focal stroke in rat. J Cardiovasc Pharmacol. 2000;36:S357–61.
Barone FC, White RF, Elliott JD, Feuerstein GZ, Ohlstein EH. The endothelin receptor antagonist SB 217242 reduces cerebral focal ischemic brain injury. J Cardiovasc Pharmacol. 1995;26(Suppl 3):S404–7.
Gupta YK, Briyal S, Sharma U, Jagannathan NR, Gulati A. Effect of endothelin antagonist (TAK-044) on cerebral ischemic volume, oxidative stress markers and neurobehavioral parameters in the middle cerebral artery occlusion model of stroke in rats. Life Sci. 2005;77:15–27.
Zhang RL, Zhang C, Zhang L, et al. Synergistic effect of an endothelin type A receptor antagonist, S-0139, with rtPA on the neuroprotection after embolic stroke. Stroke. 2008;39:2830–6.
Zhang Y, Belayev L, Zhao W, Irving EA, Busto R, Ginsberg MD. A selective endothelin ET(A) receptor antagonist, SB 234551, improves cerebral perfusion following permanent focal cerebral ischemia in rats. Brain Res. 2005;1045:150–6.
Chuquet J, Benchenane K, Toutain J, MacKenzie ET, Roussel S, Touzani O. Selective blockade of endothelin-B receptors exacerbates ischemic brain damage in the rat. Stroke. 2002;33:3019–25.
Ehrenreich H, Oldenburg J, Hasselblatt M, et al. Endothelin B receptor-deficient rats as a subtraction model to study the cerebral endothelin system. Neuroscience. 1999;91:1067–75.
Asano T, Ikegaki I, Satoh S, et al. Endothelin: a potential modulator of cerebral vasospasm. Eur J Pharmacol. 1990;190:365–72.
Viossat I, Duverger D, Chapelat M, Pirotzky E, Chabrier PE, Braquet P. Elevated tissue endothelin content during focal cerebral ischemia in the rat. J Cardiovasc Pharmacol. 1993;22(Suppl 8):S306–9.
Koyama Y, Nagae R, Tokuyama S, Tanaka K. I.c.v. administration of an endothelin ET(B) receptor agonist stimulates vascular endothelial growth factor-A production and activates vascular endothelial growth factor receptors in rat brain. Neuroscience. 2011;192:689–98.
Koyama Y, Maebara Y, Hayashi M, Nagae R, Tokuyama S, Michinaga S. Endothelins reciprocally regulate VEGF-A and angiopoietin-1 production in cultured rat astrocytes: implications on astrocytic proliferation. Glia. 2012;60:1954–63.
Breier G, Risau W. The role of vascular endothelial growth factor in blood vessel formation. Trends Cell Biol. 1996;6:454–6.
Góra-Kupilas K, Jośko J. The neuroprotective function of vascular endothelial growth factor (VEGF). Folia Neuropathol. 2005;43:31–9.
Nowacka MM, Obuchowicz E. Vascular endothelial growth factor (VEGF) and its role in the central nervous system: a new element in the neurotrophic hypothesis of antidepressant drug action. Neuropeptides. 2012;46:1–10.
Spinella F, Rosanò L, Di Castro V, et al. Endothelin-1 and endothelin-3 promote invasive behavior via hypoxia-inducible factor-1alpha in human melanoma cells. Cancer Res. 2007;67:1725–34.
Paolillo M, Russo MA, Curti D, Lanni C, Schinelli S. Endothelin B receptor antagonists block proliferation and induce apoptosis in glioma cells. Pharmacol Res. 2010;61:306–15.
Minger SL, Ekonomou A, Carta EM, Chinoy A, Perry RH, Ballard CG. Endogenous neurogenesis in the human brain following cerebral infarction. Regen Med. 2007;2:69–74.
Blomstrand F, Giaume C, Hansson E, Rönnbäck L. Distinct pharmacological properties of ET-1 and ET-3 on astroglial gap junctions and Ca2+signaling. Am J Physiol Cell Physiol. 1999;277:C616–27.
Lin JH, Weigel H, Cotrina ML, et al. Gap-junction-mediated propagation and amplification of cell injury. Nat Neurosci. 1998;1:494–500.
Li F, Irie K, Anwer MS, Fisher M. Delayed triphenyltetrazolium chloride staining remains useful for evaluating cerebral infarct volume in a rat stroke model. J Cereb Blood Flow Metabol. 1997;17:1132–5.
Gulati A, Agrawal N, Vibha D, et al. Safety and efficacy of sovateltide (IRL-1620) in a multicenter randomized controlled clinical trial in patients with acute cerebral ischemic stroke. CNS Drugs. 2021;35:85–104.
Ranjan AK, Gulati A. Sovateltide Mediated Endothelin B Receptors Agonism and Curbing Neurological Disorders. Int J Mol Sci. 2022;23(6):3146. https://doi.org/10.3390/ijms23063146.
Bhalla S, Leonard MG, Briyal S, Gulati A. Distinct alteration in brain endothelin A and B receptor characteristics following focal cerebral ischemia in rats. Drug Res. 2016;66:189–95.
Briyal S, Ranjan AK, Hornick MG, Puppala AK, Luu T, Gulati A. Anti-apoptotic activity of ET(B) receptor agonist, IRL-1620, protects neural cells in rats with cerebral ischemia. Sci Rep. 2019;9:10439.
Khodorova A, Montmayeur JP, Strichartz G. Endothelin receptors and pain. J Pain. 2009;10:4–28.
Ferreira SH, Romitelli M, de Nucci G. Endothelin-1 participation in overt and inflammatory pain. J Cardiovasc Pharmacol. 1989;13(Suppl 5):S220–2.
Gokin AP, Fareed MU, Pan HL, Hans G, Strichartz GR, Davar G. Local injection of endothelin-1 produces pain-like behavior and excitation of nociceptors in rats. J Neurosci. 2001;21:5358–66.
Piovezan AP, D’Orleans-Juste P, Souza GE, Rae GA. Endothelin-1-induced ET(A) receptor-mediated nociception, hyperalgesia and oedema in the mouse hind-paw: modulation by simultaneous ET(B) receptor activation. Br J Pharmacol. 2000;129:961–8.
Werner MF, Trevisani M, Campi B, Andre E, Geppetti P, Rae GA. Contribution of peripheral endothelin ETA and ETB receptors in neuropathic pain induced by spinal nerve ligation in rats. Eur J Pain (London, England). 2010;14:911–7.
Khodorova A, Navarro B, Jouaville LS, et al. Endothelin-B receptor activation triggers an endogenous analgesic cascade at sites of peripheral injury. Nat Med. 2003;9:1055–61.
Khodorova A, Zou S, Ren K, Dubner R, Davar G, Strichartz G. Dual roles for endothelin-B receptors in modulating adjuvant-induced inflammatory hyperalgesia in Rats. Open Pain J. 2009;2:30–40.
Khodorova A, Fareed MU, Gokin A, Strichartz GR, Davar G. Local injection of a selective endothelin-B receptor agonist inhibits endothelin-1-induced pain-like behavior and excitation of nociceptors in a naloxone-sensitive manner. J Neurosci. 2002;22:7788–96.
Quang PN, Schmidt BL. Peripheral endothelin B receptor agonist-induced antinociception involves endogenous opioids in mice. Pain. 2010;149:254–62.
Quang PN, Schmidt BL. Endothelin-A receptor antagonism attenuates carcinoma-induced pain through opioids in mice. J Pain. 2010;11:663–71.
Zheng JH, Walters ET, Song XJ. Dissociation of dorsal root ganglion neurons induces hyperexcitability that is maintained by increased responsiveness to cAMP and cGMP. J Neurophysiol. 2007;97:15–25.
Chen G, Tanabe K, Yanagidate F, et al. Intrathecal endothelin-1 has antinociceptive effects in rat model of postoperative pain. Eur J Pharmacol. 2012;697:40–6.
Mule NK, Singh JN, Shah KU, Gulati A, Sharma SS. Endothelin-1 decreases excitability of the dorsal root ganglion neurons via ET(B) receptor. Mol Neurobiol. 2018;55:4297–310.
Millecamps M, Laferrière A, Ragavendran VJ, Stone LS, Coderre TJ. Role of peripheral endothelin receptors in an animal model of complex regional pain syndrome type 1 (CRPS-I). Pain. 2010;151:174–83.
Berti-Mattera LN, Gariepy CE, Burke RM, Hall AK. Reduced expression of endothelin B receptors and mechanical hyperalgesia in experimental chronic diabetes. Exp Neurol. 2006;201:399–406.
Kjell J, Olson L. Rat models of spinal cord injury: from pathology to potential therapies. Dis Model Mech. 2016;9:1125–37.
Metz GA, Curt A, van de Meent H, Klusman I, Schwab ME, Dietz V. Validation of the weight-drop contusion model in rats: a comparative study of human spinal cord injury. J Neurotrauma. 2000;17:1–17.
Mills CD, Hains BC, Johnson KM, Hulsebosch CE. Strain and model differences in behavioral outcomes after spinal cord injury in rat. J Neurotrauma. 2001;18:743–56.
Kjell J, Sandor K, Josephson A, Svensson CI, Abrams MB. Rat substrains differ in the magnitude of spontaneous locomotor recovery and in the development of mechanical hypersensitivity after experimental spinal cord injury. J Neurotrauma. 2013;30:1805–11.
Watson C, Paxinos G, Kayalioglu G. The spinal cord: a Christopher and Dana Reeve Foundation text and atlas. Cambridge: Academic Press; 2009.
Clark FM, Proudfit H. Anatomical evidence for genetic differences in the innervation of the rat spinal cord by noradrenergic locus coeruleus neurons. Brain Res. 1992;591:44–53.
Pharmazz I. PMZ-1620 (Sovateltide) in Patients of Acute Spinal Cord Injury. https://classic.clinicaltrials.gov/show/NCT04054414; 2019.
Castañeda MM, Cubilla MA, López-Vicchi MM, Suburo AM. Endothelinergic cells in the subependymal region of mice. Brain Res. 2010;1321:20–30. https://doi.org/10.1016/j.brainres.2010.01.056.
Koyama Y, Tsujikawa K, Matsuda T, Baba A. Intracerebroventricular administration of an endothelin ETB receptor agonist increases expressions of GDNF and BDNF in rat brain. Eur J Neurosci. 2003;18:887–94.
Koyama Y, Baba A, Matsuda TJN. Endothelins stimulate the expression of neurotrophin-3 in rat brain and rat cultured astrocytes. Neuroscience. 2005;136:425–33.
Funding
Funding for the whole experimental procedures, study materials, laboratory animals, and equipment for the experiment was provided by the Experimental, Educational and Research Center ELPEN, Pikermi, Attica, Greece. There was no grant awarded directly or indirectly to any of the authors.
Author information
Authors and Affiliations
Contributions
TM contributed to conceptualization; TM and AM contributed to methodology; TM and AM performed validation; TM, AM, AG, EK, GG, NI and TX performed formal analysis; TM, AM and AG contributed to investigation; TM contributed to writing—original draft preparation; TM contributed to writing—review and editing; TM, AM, AG, EK, GG, NI and TX performed visualization; TM and AG contributed to supervision; GG, NI and TX contributed to project administration. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Ethical Approval/Informed Consent
We confirm adherence to ethical guidelines and indicate ethical approvals (IRB). The Research Ethics Committee of the Veterinary Government Department of Attika approved the study protocol with protocol number 13–06-2018 and 2875/08–06-2018. The study was conducted in accordance with the Declaration of Helsinki.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mavridis, T., Mavridi, A., Karampela, E. et al. Sovateltide (ILR-1620) Improves Motor Function and Reduces Hyperalgesia in a Rat Model of Spinal Cord Injury. Neurocrit Care 41, 455–468 (2024). https://doi.org/10.1007/s12028-024-01950-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-024-01950-2