Abstract
Spinal cord injury (SCI) is known as a debilitating condition which usually occurs due to traumas to the spine. However, the injury could also occur during clinical interventions such as spinal deformity and thoracoabdominal aortic surgeries. Intraoperative cord compression and ischemia are considered the mechanisms of primary injury in this regard. In the current study, we aimed to evaluate the therapeutic effects of minocycline, a promising agent for post-injury treatment, prophylactic administration. In a rat model of SCI through contusion injury, T9 vertebra laminectomy was performed on 40 Sprague–Dawley male rats provided from Pasteur Institute (Tehran, Iran). The reason behind selecting only male rats in our study was the fact that menstrual cycle of female rats affects healing process. Rodents were divided into a sham-operated group, a control group receiving only saline, a minocycline-treated group, and a minocycline pretreated group. Locomotor scaling, behavioral tests for neuropathic pain, and weight changes were evaluated and compared through a 28-days period. At the end of the study, tissue samples were taken to assess neuroinflammatory cytokine and histopathological changes. Minocycline pretreatment was as effective as its post-SCI administration regarding locomotor activity recovery, mechanical pain, and thermal allodynia. Furthermore, spinal cord inflammation and histopathological alterations were both similar in pretreatment and treatment groups indicating substantially better status. None of the treatments could have completely restore or prevent the spinal cord damage. Minocycline pretreatment can show promising therapeutic effects similar to its post-injury administration, inhibiting inflammatory microglial activity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Spinal cord injury (SCI) is known as a debilitating condition which usually occurs due to traumas to the spine. However, the injury could also occur during clinical interventions such as spinal deformity and thoracoabdominal aortic surgeries. Intraoperative cord compression and ischemia are considered the mechanisms of primary injury in this regard. Currently, there are no definite treatments for SCI, thus a wide range of pharmacological and cellular treatments are being studied for this condition. However, considering the common nature of incidents leading to injury, only a few studies have evaluated the potential prophylactic treatments for the condition (Rong et al. 2017).
Minocycline is a synthetic tetracycline derivative which has been shown to enhance the functional recovery and inhibition of neuronal loss after spinal cord injury (Lee et al. 2003). In this regard, different therapeutic mechanisms have been introduced for this drug. It has been suggested that the neuroprotection could be due to inhibition of mitochondrial dysfunction and reactive gliosis (Teng et al. 2004). Furthermore, it has been suggested that minocycline administration could lead to improvements in SCI rodents due to attenuation of inflammatory microglia proliferation and activity, as well as, inhibition of free radicals, e.g., nitric oxide (NO), metalloproteinase enzymes, Ca2+ chelation, and neuronal apoptosis after the injury (Yong et al. 2004; Casha et al. 2012). Minocycline has also been shown as an effective drug in treating CNS ischemia. In this regard, the study by Zheng et al. revealed that this drug can alleviate cerebral ischemia-reperfusion injury by inhibition of caspase-3 and poly (adenosine diphosphate-ribose) polymerase-1 protein (Zheng et al. 2013). Clinical results have also revealed the safety of minocycline administration in SCI patients. The therapeutic efficacy of these agents was only shown to be significant in some specific subgroups of cervical injury. However, controversy in the obtained results and the prior promising findings in SCI and other neurological diseases have led to further research being proposed for investigation of the efficacy of minocycline in SCI (Casha et al. 2012). Minocycline pretreatment has been shown to be effective against central nervous system (CNS) ischemia-reperfusion injuries, anesthesia- and morphine-induced memory impairment (Naderi et al. 2017; Lu et al. 2017; Hamidkhaniha et al. 2019), traumatic brain injury-induced alcohol consumption (Karelina et al. 2018), inhibiting hypertonic saline-induced hypersensitivity (Samour et al. 2017), and transient cerebral ischemia (Park et al. 2015).
Regardless of the modern technical developments and strict cautions taken during operations, spinal cord injury still occurs in up to 14% of cases undergoing thoracoabdominal aorta surgeries leading to devastating functional and sensory complications such as paraplegia and neuropathic pains (Conrad et al. 2008). The changes in blood flow through these surgeries can lead to ischemia which is associated with metabolic distortion, inflammation, and hence injury. In addition, the reestablishment of the flow can lead to ischemia-reperfusion injury linked with increased inflammation (Lopez-Neblina et al. 2005; Bell et al. 2012). Despite the efficacy of intraoperative neuromonitoring in patients undergoing spine deformity correction surgeries, spinal cord injury is considered one of the most important complications in these patients. The injury usually occurs due to incidental cord compression by surgical instruments or surgical site hematoma. In addition, the cord stretch during operation or postoperative anemia and hypotension can also induce neurological deficits in these patients (Rong et al. 2017; Auerbach et al. 2016).
In this regard, few studies have investigated prophylactic agents against induction of spinal cord injury; however, no definite protective pretreatment has yet been introduced. In the current study, considering the potential efficacy of minocycline in treatment of SCI, we aimed to investigate its prophylactic effects and their underlying mechanisms in a rat model of spinal cord compression.
Materials and Methods
Subjects
Forty male Sprague–Dawley rats weighing between 220 to 260 g were provided from Pasteur Institute (Tehran, Iran). Kept in a separate housing cage, each rodent had free access to suitable amount of tap water and chow pellets. The room temperature was adjusted on 23 ± 2 °C with a humidity of 50 ± 5% in 12 h day/night cycles. Animal daily weight changes and occurrence of complications were also recorded in this study.
Study Groups
Rodents were randomly divided to 4 groups: a control group receiving placebo (saline 0.9%), minocycline (purchased from sigma, St. Louis, Missouri, United States) pretreatment group which received the injection of a loading dose of 90 mg/kg minocycline 48 h before the operation and two doses of 45 mg/kg every 12 h before surgery in order to reach and maintain sufficient drug levels, the minocycline treatment group which received 90 mg/kg minocycline right after the operation and two doses of 45 mg/kg every 12 h (Lee et al. 2003), and a sham-operated group without any contusion injury, merely receiving the drug vehicle (saline 0.9%) and other postoperative treatments. Drugs were dissolved in physiologic saline and administered intraperitoneally at the time of surgery except pretreatment group, with a volume of 1 ml/kg. The control group consisting of minocycline-administered sham-operated animals was omitted due to the fact that minocycline has not affected sham-operated animals in regard to previous studies having shown its ineffectiveness (Jiang et al. 2009).
Surgery and Postoperative Care
Rodents were anesthetized with a mixture of ketamine (80 mg/kg) and xylazine (10 mg/kg) injection. Thereafter, the rodents were put on a sterile heating pad in prone position. A prophylaxis injection of cefazolin with a dosage of 30 mg/kg was administered to all rodents prior to beginning of the surgery. After T9 vertebra complete laminectomy, a spinal cord contusion injury was induced by compression of the cord with an aneurysmal clip (YASARGIL® Aneurysm clip system, Titanium mini clips FT712T; closing force, 110 g [1.08 N]; 4.7 mm blade length; 3.8 mm maximum opening diameter) for 60 s. After the procedure, the wounds were sutured, animals received intraperitoneal injection of buprenorphine (0.1 mg/kg), and were kept in a 35 °C incubator until becoming conscious. In accordance with previous studies, for the postoperative care, the rodents received normal saline (2 ml), cefazolin (20 mg/kg), and buprenorphine (0.1 mg/kg) for 7 consecutive days. In addition, each rodent’s bladder was voided manually twice a day until its complete functional rehabilitation.
Locomotor Assessment
The hind limb locomotor activity of the subjects was assessed on days 0, before the surgery, and 1, 3, 5, 7, 14, 21, and 28 after the SCI. The Basso, Beattie, Bresnahan (BBB) rating scale was used, with a scoring system ranging from 0 to 21 based on weight bearing, limb and joint movements, and coordination of individual joints (0 indicates no motor function, while 21 represents full motor ability) (Basso et al. 1995). In this study, investigators were unaware of the groups observed and scored locomotor activity of each hind limb of rats in an open field for 10 min. After averaging the scores of both hind limbs in the rats, the results were reported as the locomotor score of the subjects at each session.
Neuropathic Pain Assessment
Tactile Sensation
The withdrawal thresholds of each rat’s hind paws were assessed prior to surgical manipulation (day 0) and then again on days 7, 14, 21, and 28. The simplified up-down (SUDO) method of von Frey testing was used for the assessment. After the accommodation of rats with an elevated wire mesh as testing environment, the series of von Frey hairs were applied from below the customized platform to the central region of the plantar surface of the rat hind paw in ascending order beginning with the lowest hair (0.23 g). Using von Frey hairs with the hairs in the range of 0.23–60 g, the hind paw 50% withdrawal threshold was determined and expressed in grams by a blinded experimenter (Afshari et al. 2018).
Thermal Allodynia
Tail-flick latency (TFL) was measured using a Tail-Flick Analgesia Meter (IITC life science model 33T, Los Angeles, California, USA) at day 0 before the surgery and days 7, 14, 21, and 28 following the induction. The dorsal part of each rat’s tail was placed to a beaming heat source, and TFL was documented as soon as the first movement of the rat’s tail from the painful thermal incitement was observed. The procedure was repeated for five times on each rat, and the average was reported as the mean withdrawal threshold. The strength of the incitement was adjusted with a cut-off time of 8 s to prevent injury of the tail (Farsi et al. 2015).
Histopathological Scoring
At the end of the study, all rodents were anesthetized with ketamine (100 mg/kg) and xylazine (10 mg/kg) and then intracardially perfused with 150 ml of phosphate buffer saline (PBS, pH 7.4). The perfusion was followed by 250 ml formalin-acid picric mixture (4% paraformaldehyde, 0.4% picric acid in 0.16 M phosphate buffer, pH 7.4) in half of the subjects, while in the other half, the spinal cord was dissected and kept inside liquid nitrogen in – 70 °C freezer, until the tissues were sent for the assessment of inflammatory cytokines content.
Afterwards, the spinal cord sections of the first half were stained with hematoxylin and eosin (H & E) for histopathological assessment. The staining procedure was performed based on the following protocol: the specimens were primarily deparaffinized at 70 °C for 20 min and xylene solution for 3 min and then rehydrated in two changes of absolute alcohol, 5 min each. For staining nuclei, slides were rinsed in hematoxylin solution for 15 min and then washed in running tap water for 5 min. After bluing in 0.2% ammonia water and then washing in tap water for 5 min, the slides were counterstained in eosin-phloxine for 1 min, and then samples were rinsed in 90, 96, and 100% ethanol for 2 min. Afterwards, slides were embedded in xylene and mounting medium. At last, a blinded, professional pathologist assessed the specimens based on four factors including immune cells infiltration and inflammation, hemorrhage, axonal vacuolation, and cyst formation to acquire a quantitative analysis and histopathological scoring of the spinal cord damage (Afshari et al. 2018).
Elisa
In order to measure the levels of two inflammatory mediators, tumor necrosis factor-alpha (TNF-α), Interleukin-6 (IL-6), and Interleukin-1β (IL-1β), the spinal cord specimens were homogenized in lysis buffer (Peng et al. 2006). Afterwards, the samples were centrifuged for 20 min at 13000 rpm rate at 4 °C. In order to determine levels of the mediators, enzyme-linked immunosorbent assay (ELISA; Abcam, Cambridge, UK) was used.
Nitrite Assay
Nitrite levels of the hippocampus and plasma were measured according to our previous studies (Haj-Mirzaian et al. 2018; Amiri et al. 2016). Based on the Griess reaction, the colorimetric assay was done to assess the nitrite concentration. Briefly, 100 μL of samples were mixed with 100 μL Griess reagent following 10 min of incubation at the room temperature, absorbance was measured at 540 nm in an automated plate reader. Nitrite concentration was evaluated by reference to standard curve of sodium nitrite (Sigma, USA) and normalized to the mg protein of each sample.
Statistical Analysis
Analysis was performed using SPSS version 24. The p value below 0.05 was regarded as significant. General linear model (GLM) repeated measures analysis followed by Tukey’s post hoc test was used to compare locomotor activity and neuropathic pain assessment between the groups during the study. Additionally, analysis of variance (ANOVA) test followed by Tukey’s post hoc test was utilized to assess differences of histopathological scorings and aforementioned mediators of the spinal cord tissue between the groups. Also, the sample size was calculated with G*Power software version 3, considering the power of the study reaching 0.8 and α = 0.05.
Ethical Considerations
We certify that this investigation obeyed all the applicable ethics of using animals in line with institutional and governmental concerns. This study was in accordance with the Declaration of Helsinki and its consecutive revisions. The protocol was approved by the Ethical Committee of AJA University of Medical Sciences (Reference number: 91000425).
Results
Locomotion
BBB Locomotor Scale
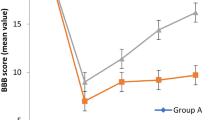
Investigation of the Basso, Beattie, Bresnahan (BBB) locomotor scale through the 28-day period of the study revealed that the locomotor activity significantly improved through time in all injured animals except the control group (p value < 0.001). In addition, all sham-operated animals, having their cords intact, received a complete locomotion score (mean 21, SD 0) throughout the study. Results of the two-way repeated measure ANOVA showed that both minocycline treatment (mean difference 2.02, S.E 0.27, p value < 0.001) and pretreatment (mean difference 2.57, S.E 0.27, p value < 0.001) led to substantially better locomotion compared to saline treated animals. However, these treatments could not have restored the locomotor ability to the same level as sham-operated animals (mean difference with minocycline pretreatment 13.16, mean difference with minocycline treatment 13.71, S.E both 0.27, p value both < 0.001). In addition, there was statistically no significant difference in BBB locomotor score between the pretreatment and treatment group (mean difference 0.55, S.E 0.27, p value = 0.193) (Fig. 1).
Line chart of BBB locomotor scores of rodents treated and pretreated with minocycline, the saline receiving control group, and the sham-operated animals. Minocycline 180 mg/kg treatment and pretreatment similarly (p value = 0.193) improved rodents’ locomotor score in a significant manner compared to the saline receiving rodents (control group) through the 28-day course of the study (p value < 0.001). However, none of them was able to recover the BBB locomotor score to the same level as sham-operated animals (p value < 0.001). The data are presented as mean ± SD (10 rats per group). **p < 0.01 and ***p < 0.001 compared to the control group on the same day
Pain Assessment
Thermal Allodynia
In the current study, thermal allodynia was assessed via the tail-flick latency test. Our results showed that the response threshold, which was significantly decreased after SCI, increased in a time dependent manner in all injured rodents except the control group (p value < 0.001). Furthermore are results indicated animals receiving minocycline pretreatment and treatment experienced statistically similar (mean difference 0.10, S.E 0.06, p value = 0.42) variations in their TFL test. However, both minocycline pretreatment (mean difference 0.46, S.E 0.06, p value < 0.001) and treatment (mean difference 0.57, S.E 0.06, p value < 0.001) compared with the saline-treated rodents showed a substantially comparable improvement in thermal allodynia. In addition, none of the applied interventions were able to completely reverse the SCI-induced thermal allodynia to the same level as sham-operated rodents (mean difference with minocycline pretreatment 1.07, mean difference with minocycline treatment 0.96, S.E both 0.06, p value both < 0.001) (Fig. 2).
Line chart of mean tail-flick latencies of rats treated and pretreated with minocycline, the saline receiving control group, and the sham-operated animals. Minocycline 180 mg/kg treatment and pretreatment similarly (p value = 0.42) improved rodents’ TFL in a significant manner compared the saline receiving rodents (control group) through the 28-day course of the study (p value < 0.001). However, none of them was able to recover the mean TFL to the same level as sham-operated animals (p value < 0.001). The data are presented as mean ± SD (10 rats per group). *p < 0.05, **p < 0.01, and ***p < 0.001 compared to the control group on the same day
Tactile Sensation
Von Frey (VF) monofilaments were used in order to evaluate tactile sensation and mechanical hyperalgesia in the studied animals. Results indicated that the 50% withdrawal threshold significantly decreased in animals with spinal cord injury (p value < 0.001). However, results of the repeated measure ANOVA indicated significant recovery of mechanical hyperalgesia in minocycline pretreatment (mean difference 13.82, S.E 1.43, p value < 0.001) and treatment (mean difference 11.34, S.E 1.43, p value < 0.001) groups compared to the saline receiving control group. Interestingly, no significant difference was observed regarding the tactile sensation recovery between the minocycline pretreatment and treatment groups (mean difference 2.48, S.E 1.43, p value = 0.32). Furthermore, none of the applied interventions were able to completely reverse the SCI-induced mechanical hyperalgesia to the same level as sham-operated rodents (mean difference with minocycline pretreatment 18.51, mean difference with minocycline treatment 20.99, S.E both 1.43, p value both < 0.001) (Fig. 3).
Line chart of von Frey filaments test scores of rodents treated and pretreated with minocycline, the saline receiving control group, and the sham-operated animals. Minocycline treatment and pretreatment (180 mg/kg) similarly (p value = 0.32) improved rodents’ VFT in a significant manner compared the saline receiving rodents (control group) through the 28-day course of the study (p value < 0.001). However, none of them was able to recover the VFT score to the same level as sham-operated animals (p value < 0.001). The data are presented as mean ± SD (10 rats per group). **p < 0.01 and ***p < 0.001 compared to the control group on the same day
Inflammatory Cytokines Assessment
Spinal cord tissue levels of four proinflammatory cytokines, i.e., nitrite oxide, TNF-α, and Interleukin 6 and 1-β, were evaluated at the end of the study. Comparison of mean cytokine levels indicated significant difference of mean TNF-α (F (3,36) = 78.17, p value < 0.001), IL-6 (F (3,36) = 148.4, p value < 0.001), Interleukin 1-β (F (3,36) = 89.97, p value < 0.001), and nitrite oxide level (F (3,36) = 16.31, p value < 0.001) between all study groups. Tukey’s post hoc test revealed that proinflammatory cytokine levels were significantly lower than the saline-treated control animals in both minocycline pretreatment and treatment groups as well as nitrite oxide level (p value < 0.001). However, these groups had still significantly higher cytokine levels in addition to nitrite oxide level than sham-operated rodents (p value < 0.001) (Table 1).
Body Weight
After surgery, all SCI-induced animals suffered a significant weight loss with the mean of 13.34 ± 4.76 g after 1 week. Moreover, repeated measure ANOVA reveled that through the 28 days, the control group experienced a significant weight loss of 64 g (p < 0.001), while rodents prophylaxis treated with minocycline experienced a non-significant mean weight gain of 5.76 ± 3.57 g, and post-treated animals had a non-significant weight gain of 7.05 ± 4.16 g. In addition, statistical analysis showed a significant mean weight gain of 50.5 ± 13.7 in sham-operated animals after 28 days (p < 0.001). Repeated measure ANOVA for all the studied rodents showed that weight changes during the 28 days was not statistically significant in pretreatment in comparison to post-treatment (p > 0.05); however, weight loss in minocycline-treated rodents was significantly less than the control group (p < 0.05, Fig. 4).
Measured body weights of animals through 28 days. Following the surgery, the control group experienced a significant weight loss of 64 g (p < 0.001), while pretreatment group as well as post-treated animals had a non-significant weight gain. Significant mean weight gain of 50.5 ± 13.7 was seen in sham-operated animals after 28 days (p < 0.001). Weight loss in minocycline-treated rodents was significantly less than the control group (p < 0.05). *p < 0.05, **p < 0.01, and ***p < 0.001 compared to the control group on the same day
Histopathological Assessment
Evaluating the spinal cord tissue hemorrhage and edema, invasion of inflammatory cells, vacuolation in neurons, and cyst formation, cumulative histopathologic scores of 0–12 were assigned to subjects by an expert pathologist where higher scores indicate more severe damage (Fig. 5). In this regard, data analysis revealed a significant difference between the study groups (F (3,36) = 19, p value < 0.001). According to the performed Tukey’s post hoc test, the histopathological scores were significantly lower in minocycline treatment and pretreatment groups compared to saline-treated control animals (p value < 0.001). However, all minocycline receiving groups had a significantly higher score compared to the sham-operated rodents (p value < 0.001) (Table 2).
Representative histopathology of spinal cord sections at the injury site 28 days after injury. Sections were examined by H&E staining and the presence of inflammatory cells, hemorrhage, tissue degeneration, and the extent of cyst formation were quantified. Images represent samples from the a saline receiving control group, b sham group, c pretreatment with minocycline 180 mg/kg, and d treatment with minocycline 180 mg/kg, H&E slides with 40X magnification. In image a significant cyst formation, immune cell infiltration, and vacuolization are visible in the spinal cord tissue. Part b represents a rather intact spinal cord at T9–10 level. Image c and d represent rather small cysts with minor vacuolization and immune cell infiltration compared to the control group
Discussion
In the current study, the efficacy of minocycline prophylactic treatment was evaluated and compared to its post-injury treatment in a rat model of spinal cord compression. Interestingly, our results showed that minocycline pretreatment was as effective as its post-SCI administration regarding functional recovery, SCI sensory complication, spinal cord inflammation, and histopathological alterations.
The obtained results of the current research were in line with previous studies indicating therapeutic effects of minocycline in spinal cord injury (Shultz and Zhong 2017). It was shown that minocycline substantially inhibits the inflammatory and neurodegenerative responses to the spinal cord mechanical injury, i.e., targeting the secondary mechanisms of injury. The inhibition of these mechanisms was revealed by significantly attenuated proinflammatory cytokines, NO, TNF-α, IL-1β, and IL-6 in the cord tissue. The attenuation of the aforementioned agents can be indicative of reduced inflammatory microglial activity. According to previous studies, inhibiting inflammation and reactive microglial activity reduces histopathological indicators of spinal cord damage where cyst formation, neuronal vacuolization, and gliosis are regarded as some of the major injury hallmarks (Afshari et al. 2018; Qiao et al. 2010; Gonzalez et al. 2003; Najafi et al. 2013).
In this regard, significantly lower histopathological score represented attenuated neuronal tissue damage which is in accordance with the obtained functional and sensory results. However, the applied treatment was not able to completely reverse the pathological course of the injury, strengthening the assumption regarding the complexity of secondary damage mechanisms.
In accordance with the results of the current research, Naderi et al. have shown that minocycline pretreatment can inhibit inflammatory microglial activity, neuronal apoptosis, and also play an antioxidative role in cerebral ischemia-reperfusion, leading to reduced learning and memory deficits. In this regard, TNF-α, NO, IL-6, and IL-1β have been introduced as four major markers which their attenuation is associated with minocycline pretreatment therapeutic effects (Naderi et al. 2017). Furthermore, minocycline pretreatment is also shown to counter anesthesia induced neuronal damage by increasing CNS neurogenesis and inhibiting injury. However, similar to the current study, minocycline pretreatment was not capable of restoring the injured tissue to its previously normal status (Lu et al. 2017; Giri et al. 2018).
Pretreatment with minocycline has shown promising results in relieving the neuropathic pain in rodents’ sciatic injury models. Similar to obtained results of the current study, it has been shown that minocycline administration prior to nerve injury affects the neuronal-glial interactions by preventing the upregulation of pronociceptive cytokines such as IL-1β in the spinal cord and dorsal root ganglions (Rojewska et al. 2014). Furthermore, in vitro investigations have revealed that minocycline pretreatment can attenuate neuronal apoptosis by preventing microglial activation and also inhibiting the production of tumor necrosis factor-alpha from activated microglia (Seki et al. 2013). In addition, it has been demonstrated that minocycline pretreatment can substantially prevent microglial activity leading to inhibition of traumatic brain injury-induced alcohol consumption (Karelina et al. 2018) and prevention of hypertonic saline-induced hypersensitivity (Samour et al. 2017). In accordance with the aforementioned studies, our results indicated that minocycline pretreatment significantly prevented the production of proinflammatory and neurotoxic cytokines, TNF-α, NO, and IL-1β,which also acts as a significant pronociceptive agent, leading to significantly attenuated post-injury spinal cord tissue damage and functional and sensory complications. Although we could hypothesize the possible underlying mechanism of minocycline on healing process, and despite of insignificantly lower level of inflammatory cytokines in the pretreatment group, this study could not find any pathological or behavioral advantage of pretreatment group over the treatment group. Thus, results of the present study encourage more research regarding minocycline pretreatment and its possible effects on neuroinflammation especially with different and possibly more prolonged pretreatment period.
It has been indicated that delayed microglial activation could occur in rodents receiving acute doses of minocycline for its neuroprotective properties (Li et al. 2018). In this regard, administration of multiple minocycline doses before and during the first week after injury might result in relatively better therapeutic effects considering the estimated 7-day duration for acute phase of secondary injury before the initiation of glial scar formation and neuroregeneration period (Li et al. 2018; Kanno et al. 2012). However, being a limitation of the current study, long-term drug administration was not performed as a major scope. Hence, considering the potential effect of continued minocycline administration in SCI, the authors suggest that further and detailed investigations in this regard can be of a great value.
Conclusion
According to the obtained results of this study, it could be concluded that minocycline pretreatment can show promising therapeutic effects similar to its post-injury administration, inhibiting inflammatory microglial activity.
References
Afshari K, Dehdashtian A, Haddadi N-S, Haj-Mirzaian A, Iranmehr A, Ebrahimi MA, Tavangar SM, Faghir-Ghanesefat H, Mohammadi F, Rahimi N, Javidan AN, Dehpour AR (2018) Anti-inflammatory effects of metformin improve the neuropathic pain and locomotor activity in spinal cord injured rats: introduction of an alternative therapy. Spinal Cord 56(11):1032–1041
Amiri S, Haj-Mirzaian A, Amini-Khoei H, Shirzadian A, Rahimi-Balaei M, Razmi A, Bergen H, Rastegar M, Kordjazy N, Haj-Mirzaian A, Ejtemai-Mehr S, Dehpour AR (2016) Lithium attenuates the proconvulsant effect of adolescent social isolation stress via involvement of the nitrergic system. Epilepsy Behav 61:6–13
Auerbach JD, Kean K, Milby AH, Paonessa KJ, Dormans JP, Newton PO et al (2016) Delayed postoperative neurologic deficits in spinal deformity surgery. Spine 41(3):E131–E1E8
Basso DM, Beattie MS, Bresnahan JC (1995) A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma 12(1):1–21
Bell MT, Puskas F, Smith PD, Agoston VA, Fullerton DA, Meng X, Weyant MJ, Reece TB (2012) Attenuation of spinal cord ischemia-reperfusion injury by specific α-2a receptor activation with dexmedetomidine. J Vasc Surg 56(5):1398–1402
Casha S, Zygun D, McGowan MD, Bains I, Yong VW, John HR (2012) Results of a phase II placebo-controlled randomized trial of minocycline in acute spinal cord injury. Brain 135(4):1224–1236
Conrad MF, Jason YY, Chung TK, Davison JK, Cambria RP (2008) Spinal cord complications after thoracic aortic surgery: long-term survival and functional status varies with deficit severity. J Vasc Surg 48(1):47–53
Farsi L, Afshari K, Keshavarz M, NaghibZadeh M, Memari F, Norouzi-Javidan A (2015) Postinjury treatment with magnesium sulfate attenuates neuropathic pains following spinal cord injury in male rats. Behav Pharmacol 26(3):315–320
Giri PK, Lu Y, Lei S, Li W, Zheng J, Lu H, Chen X, Liu Y, Zhang P (2018) Pretreatment with minocycline improves neurogenesis and behavior performance after midazolam exposure in neonatal rats. Neuroreport 29(3):153–159
Gonzalez R, Glaser J, Liu MT, Lane TE, Keirstead HS (2003) Reducing inflammation decreases secondary degeneration and functional deficit after spinal cord injury. Exp Neurol 184(1):456–463
Haj-Mirzaian A, Amiri S, Amini-Khoei H, Haj-Mirzaian A, Hashemiaghdam A, Ramezanzadeh K, Ghesmati M, Afshari K, Dehpour AR (2018) Involvement of NO/NMDA-R pathway in the behavioral despair induced by amphetamine withdrawal. Brain Res Bull 139:81–90
Hamidkhaniha S, Bashiri H, Omidi A, Hosseini-Chegeni A, Tavangar SM, Sabouri S et al (2019) Effect of pretreatment with intracerebroventricular injection of minocycline on morphine-induced memory impairment in passive avoidance test: role of P-CREB and c-Fos expression in the dorsal hippocampus and basolateral amygdala regions. Clin Exp Pharmacol Physiol
Jiang W, Desjardins P, Butterworth RF (2009) Cerebral inflammation contributes to encephalopathy and brain edema in acute liver failure: protective effect of minocycline. J Neurochem 109(2):485–493
Kanno H, Ozawa H, Sekiguchi A, Yamaya S, Tateda S, Yahata K, Itoi E (2012) The role of mTOR signaling pathway in spinal cord injury. Cell Cycle 11(17):3175–3179
Karelina K, Nicholson S, Weil ZM (2018) Minocycline blocks traumatic brain injury-induced alcohol consumption and nucleus accumbens inflammation in adolescent male mice. Brain Behav Immun 69:532–539
Lee SM, Yune TY, Kim SJ, Park DW, Lee YK, Kim YC, Oh YJ, Markelonis GJ, Oh TH (2003) Minocycline reduces cell death and improves functional recovery after traumatic spinal cord injury in the rat. J Neurotrauma 20(10):1017–1027
Li W, Chai Q, Zhang H, Ma J, Xu C, Dong J, Wei X, Wang Z, Zhang K (2018) High doses of minocycline may induce delayed activation of microglia in aged rats and thus cannot prevent postoperative cognitive dysfunction. J Int Med Res 46(4):1404–1413
Lopez-Neblina F, Toledo AH, Toledo-Pereyra LH (2005) Molecular biology of apoptosis in ischemia and reperfusion. J Investig Surg 18(6):335–350
Lu Y, Giri P, Lei S, Zheng J, Li W, Wang N et al (2017) Pretreatment with minocycline restores neurogenesis in the subventricular zone and subgranular zone of the hippocampus after ketamine exposure in neonatal rats. Neuroscience 352:144–154
Naderi Y, Sabetkasaei M, Parvardeh S, Moini ZT (2017) Neuroprotective effects of pretreatment with minocycline on memory impairment following cerebral ischemia in rats. Behav Pharmacol 28(2):214–222
Najafi A, Mojtahedzadeh M, Ahmadi A, Ramezani M, Shariatmoharari R, Hazrati E (2013) Rapidly changing tachyarrhythmia in acute stroke. Basic Clin Neurosci 4(2):169–171
Park SI, Park SK, Jang KS, Han YM, Kim CH, Oh SJ (2015) Preischemic neuroprotective effect of minocycline and sodium ozagrel on transient cerebral ischemic rat model. Brain Res 1599:85–92
Peng XM, Zhou ZG, Glorioso JC, Fink DJ, Mata M (2006) Tumor necrosis factor–α contributes to below-level neuropathic pain after spinal cord injury. Ann Neurol 59(5):843–851
Qiao F, Atkinson C, Kindy MS, Shunmugavel A, Morgan BP, Song H, Tomlinson S (2010) The alternative and terminal pathways of complement mediate post-traumatic spinal cord inflammation and injury. Am J Pathol 177(6):3061–3070
Rojewska E, Makuch W, Przewlocka B, Mika J (2014) Minocycline prevents dynorphin-induced neurotoxicity during neuropathic pain in rats. Neuropharmacology 86:301–310
Rong H, Zhao Z, Feng J, Lei Y, Wu H, Sun R et al (2017) The effects of dexmedetomidine pretreatment on the pro-and anti-inflammation systems after spinal cord injury in rats. Brain Behav Immun 64:195–207
Samour MS, Nagi SS, Shortland PJ, Mahns DA (2017) Minocycline prevents muscular pain hypersensitivity and cutaneous allodynia produced by repeated intramuscular injections of hypertonic saline in healthy human participants. J Pain 18(8):994–1005
Seki Y, Kato TA, Monji A, Mizoguchi Y, Horikawa H, Sato-Kasai M, Yoshiga D, Kanba S (2013) Pretreatment of aripiprazole and minocycline, but not haloperidol, suppresses oligodendrocyte damage from interferon-γ-stimulated microglia in co-culture model. Schizophr Res 151(1–3):20–28
Shultz RB, Zhong Y (2017) Minocycline targets multiple secondary injury mechanisms in traumatic spinal cord injury. Neural Regen Res 12(5):702–713
Teng YD, Choi H, Onario RC, Zhu S, Desilets FC, Lan S et al (2004) Minocycline inhibits contusion-triggered mitochondrial cytochrome c release and mitigates functional deficits after spinal cord injury. Proc Natl Acad Sci 101(9):3071–3076
Yong VW, Wells J, Giuliani F, Casha S, Power C, Metz LM (2004) The promise of minocycline in neurology. Lancet Neurol 3(12):744–751
Zheng Y, Xu L, Yin J, Zhong Z, Fan H, Li X, Chang Q (2013) Effect of minocycline on cerebral ischemia-reperfusion injury. Neural Regen Res 8(10):900–908
Acknowledgments
The authors would like to acknowledge all those who assisted us during the performance of the current study. Specially, those in the Department of Pharmacology and Brain and Spinal Cord Injury Research Center of Tehran University of Medical Sciences, Tehran, Iran, for their kind assistance in providing laboratory facilities for this research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Khashiar Afshary and Mohsen Chamanara had similar contribution in this manuscript and should be considered as co-first authors.
Rights and permissions
About this article
Cite this article
Afshary, K., Chamanara, M., Talari, B. et al. Therapeutic Effects of Minocycline Pretreatment in the Locomotor and Sensory Complications of Spinal Cord Injury in an Animal Model. J Mol Neurosci 70, 1064–1072 (2020). https://doi.org/10.1007/s12031-020-01509-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-020-01509-8