Abstract
Background
We investigated whether model-based indices of cerebral autoregulation (CA) are associated with outcomes after pediatric traumatic brain injury.
Methods
This was a retrospective analysis of a prospective clinical database of 56 pediatric patients with traumatic brain injury undergoing intracranial pressure monitoring. CA indices were calculated, including pressure reactivity index (PRx), wavelet pressure reactivity index (wPRx), pulse amplitude index (PAx), and correlation coefficient between intracranial pressure pulse amplitude and cerebral perfusion pressure (RAC). Each CA index was used to compute optimal cerebral perfusion pressure (CPP). Time of CPP below lower limit of autoregulation (LLA) or above upper limit of autoregulation (ULA) were computed for each index. Demographic, physiologic, and neuroimaging data were collected. Primary outcome was determined using Pediatric Glasgow Outcome Scale Extended (GOSE-Peds) at 12 months, with higher scores being suggestive of unfavorable outcome. Univariate and multiple linear regression with guided stepwise variable selection was used to find combinations of risk factors that can best explain the variability of GOSE-Peds scores, and the best fit model was applied to the age strata. We hypothesized that higher GOSE-Peds scores were associated with higher CA values and more time below LLA or above ULA for each index.
Results
At the univariate level, CPP, dose of intracranial hypertension, PRx, PAx, wPRx, RAC, percent time more than ULA derived for PAx, and percent time less than LLA derived for PRx, PAx, wPRx, and RAC were all associated with GOSE-Peds scores. The best subset model selection on all pediatric patients identified that when accounting for CPP, increased dose of intracranial hypertension and percent time less than LLA derived for wPRx were independently associated with higher GOSE-Peds scores. Age stratification of the best fit model identified that in children less than 2 years of age or 8 years of age or more, percent time less than LLA derived for wPRx represented the sole independent predictor of higher GOSE-Peds scores when accounting for CPP and dose of intracranial hypertension. For children 2 years or younger to less than 8 years of age, dose of intracranial hypertension was identified as the sole independent predictor of higher GOSE-Peds scores when accounting for CPP and percent time less than LLA derived for wPRx.
Conclusions
Increased dose of intracranial hypertension, PRx, wPRx, PAx, and RAC values and increased percentage time less than LLA based on PRx, wPRx, PAx, and RAC are associated with higher GOSE-Peds scores, suggestive of unfavorable outcome. Reducing intracranial hypertension and maintaining CPP more than LLA based on wPRx may improve outcomes and warrants prospective investigation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Traumatic brain injury (TBI) is a major cause of morbidity and mortality in children, accounting for up to 280 of 100,000 of hospitalized children [1]. Despite several decades of clinical trials, level I evidence is still lacking to guide clinical management [2]. The challenge of ascertaining high level evidence is understated by the complexity of the disease. Pathophysiologic mechanisms can be heterogeneous, and thus individualized methods of delivering treatment may be necessary toward improving clinical care [3].
After primary insult, a cascade of complex pathophysiologic mechanisms can occur over the ensuing hours and days that place the injured brain at risk for impaired cerebral autoregulation (CA) and resultant ischemic or hyperemic secondary insults [4]. Estimating CA can provide insight into the health of the cerebrovascular system and its ability to maintain cerebral blood flow steadily over a range of cerebral perfusion pressures (CPP). Current pediatric TBI guidelines support maintenance of CPP greater than 40 mmHg, but it remains unclear whether specific children may be at risk for secondary insults with CPP maintained at varying thresholds [5]. Although current guidelines have provided recommendations for developing evidence-based protocols, evidence has been lacking for direct impact on outcome.
Several model-based indices have been developed over the past few years to characterize CA. The cerebrovascular pressure reactivity index (PRx) is a well described model-based index of CA, representing a moving correlation of slow wave fluctuations between arterial blood pressure (ABP) and intracranial pressure (ICP) [6]. When plotting PRx against trends in CPP, the optimal CPP (CPPOpt) can be postulated as the nadir point on the U-shaped curve, at which cerebrovascular pressure reactivity is most efficient. When this process is applied iteratively on moving calculations, continuous CPPOpt values can be observed in real time, offering a promising target for optimizing CPP in a personalized medicine approach [7]. A variety of variant ICP-derived model-based indices of CA have been recently developed, including the wavelet pressure reactivity index (wPRx) [8], pulse amplitude index (PAx) [9], and correlation of ICP pulse amplitude and cerebral perfusion pressure (RAC) [10], each capturing specific elements of the ICP waveform or its coherence to ABP or CPP. The role of these different indices in clinical management, particularly after pediatric TBI still remains unclear with questionable value. In this study, we wished to investigate the association of each of these model-based indices of CA with outcome after pediatric TBI, as well as percent (%) time spent outside thresholds of the lower and upper limits of CA delineated from each of their CPPOpt values.
Methods
Study Design
This is a retrospective analysis from a prospectively collected clinical database. This study was conducted at Phoenix Children’s Hospital (Phoenix, AZ), and was approved by the institutional review board (No: 19-284).
Patients
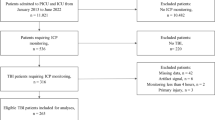
Children and adults ages 14 days to 21 years of age with TBI hospitalized in the Phoenix Children’s Hospital Pediatric intensive care unit from September 2014 to August 2019 who required intraparenchymal ICP monitoring were included. We excluded patients whose ICP monitoring was only intermittently collected through an external ventricular drain. Initial Glasgow Coma Scale (GCS) scores and Pediatric Risk of Mortality III (PRISM3) scores at presentation defined initial measures of clinical severity. Demographic data were collected for this study, including age, sex, and ethnicity, and all patients were managed according to an institutional protocol based on the most up-to-date pediatric TBI guidelines [2, 11] aimed at keeping ICP < 20 mmHg and CPP > 40 mmHg. CPPopt or PRx-guided management was not part of the institutional protocol. The decision to place ICP monitoring was made based on the risk/benefit ratio by the neurosurgeon on call. Reasons for ICP monitoring included initial GCS scores ≤ 8, patients with initial GCS scores > 8 and subsequent progressive neurologic deterioration, or patients for whom sedative and/or paralytic therapy was needed in the acute phase of injury to address other trauma-related complications (e.g., severe intraabdominal bleeding).
The primary aim of this study was to investigate the association of model-based indices of CA and initial clinical and neuroimaging biomarkers of injury severity with functional outcome after pediatric TBI.
Physiologic Data
Neurologic and hemodynamic monitoring that were collected included ICP obtained from an intraparenchymal probe (Codman Microsensor; Integra Life Sciences, Plainsboro Township, NJ) and invasive ABP recorded from a radial arterial line. Data assessed included the period in which both ICP and invasive ABP monitoring were synchronously performed. All continuous physiologic data from all monitoring devices were time synchronized using an intensive care unit monitor (Phillips Intellivue MP70; Phillips Healthcare, Andover, MA), from which waveform data were transferred to a multimodal neurologic monitoring (MMM) device (CNS200; Moberg Intensive Care Unit (ICU). Solutions, Philadelphia, PA). ICP and ABP waveforms were captured at a sampling frequency of 200 Hz. CPP was calculated as the intraparenchymal ICP subtracted from ABP. Intensive Care Monitor Plus (ICM +) software (Cambridge, UK) was used to visualize and process all multimodality monitoring data and calculate model-based indices of CA and the dose of intracranial hypertension (dICH). dICH was calculated using a previously described methodology accounting for cumulative extent and duration identified by the area under the curve above the threshold, ICP > 20 mmHg over a 1-h period, obtaining measurements of mmHg/h [12]. Physiologic data were collected within the first 7 days of monitoring post injury for this study. We implemented artifact reduction by excluding epochs in which there were missing values of either ABP or ICP, as well as epochs in which physiologic values of either ABP and ICP were subphysiologic or supraphysiologic of acceptable ranges, using a normative range for ABP between 25 and 140 mmHg and ICP between > 0 and 90 mmHg.
A variety of CA indices were calculated including the PRx [6], PAx [9], wPRx [8], and RAC [10]. An elevated value for these indices signifies impaired CA. These indices were calculated using moving Pearson correlation coefficients between different physiological parameters: PRx is calculated between ABP and ICP within a 5-min averaging window updated every 60 s; PAx similarly between ABP and ICP pulse amplitude; RAC similarly between ICP pulse amplitude and CPP; and lastly, wPRx was calculated similarly by taking the cosine of the wavelet transform phase shift between ABP and ICP.
To account for immediate clinically actionable biomarkers extracted from CA indices, we evaluated the % time that patients had CPP values below the lower limit of autoregulation (LLA) or above the upper limit of autoregulation (ULA). CPPOpt values were identified using previously described methods [7] by plotting CPP values for all CA indices and identifying the minimum CPP value on a U-shaped curve fit to the data with a multiwindow algorithm (Fig. 1). This multiwindow algorithm used a maximum calculation window of 28,800 s and minimum calculation period of 7200 s, with steps of 600 s. This develops 36 averaged CPPOpt values for 8 h of monitoring. This averaged value is updated every minute, meaning that the computed value in the time trend consists of an average of 36 CPPOpt values. CPPopt values were calculated every minute from CPP and each CA index value taken from the moving 4-h monitoring window. CPPopt values were rejected in cases of an unreliable U-shaped fitting and corrected by using the last reliable U-shaped CPPOpt value. We implemented five curve fitting criteria to keep or remove the curve for building a CPPOpt time series, which included that (1) each CPP bin must represent at least 1% of the total count data, (2) at least 50% of the data in the window must be included in the curve fit, (3) variation in the autoregulation index of 0.2 is mandated, (4) the autoregulatory index range of interest is enforced to be between − 0.3 and 0.6, and (5) the coefficient of determination of the fitted R2 value must be at least 0.0. LLA and ULA were determined for each CA index as the CPP values at which the curve crossed threshold values for impaired cerebrovascular pressure reactivity (PRx, PAx, wPRx = 0.30; RAC = − 0.15) as used or suggested in previous studies [13,14,15,16]. LLA and ULA were determined as the lowest and highest CPP value with such thresholds, respectively. The % time of CPP values below LLA and above ULA were calculated for each patient during their monitoring period.
Example of optimal cerebral perfusion pressure with estimated lower and upper limits of cerebral autoregulation. Example of optimal cerebral perfusion pressure (CPPOpt) values based on the pressure reactivity index (PRx), wavelet pressure reactivity index (wPRx), correlation of ICP pulse amplitude and CPP (RAC), and pulse amplitude index (PAx). Each CPPOpt curve has thresholds (horizontal) for the lower limit of autoregulation (LLA) and upper limit of autoregulation (ULA). These thresholds are set at 0.3 for PRx, wPRx and PAx and − 0.15 for RAC
Analysis of Neuroimaging
Initial computed tomography (CT) scans were reviewed by a neuroradiologist (MK) in their original digital form and graded according to the modified Marshall CT scan classification system [17]. The neuroradiologist was blinded to MMM data or outcome measures at the time of analysis. Volumes of high-density or mixed-density lesions consistent with intracranial hematomas were calculated by creating volumetric three-dimensional rendering using a commercially available region growing segmentation algorithm (Royal Phillips, Best, Netherlands). To account for heterogeneity in total intracranial volume with respect to age and sex, we evaluated the % intracranial hematoma volume in relation to estimated total intracranial volume based on published pediatric normative values [18]. Midline shift was measured in millimeters at the level of maximum deviation.
Outcome Assessment
The primary outcome measure was global functional outcome as measured by the Pediatrics Glasgow Outcome Scale Extended (GOSE-Peds) at 12 months after injury [19]. GOSE-Peds scores range from 1 to 8, with lower scores representing favorable outcome and higher scores representing unfavorable outcome.
Statistical Analysis
Continuous variables were summarized using median values (and interquartile range [IQR]). Categorical variables were summarized as counts and proportions. We performed univariate linear regression to investigate the association of the GOSE-Peds scores with each predictor variable using data from patients for who all predictor data were available. The results were summarized using the slope, corresponding 95% confidence interval, the p value for significance, root mean square error (RMSE), R2 and adjusted R2 values, which are measures of goodness of fit. Demographic predictor variables included age, sex, and GCS and PRISM3 scores at presentation. Neuroimaging predictor variables included initial CT Marshall Scores and midline shift, in addition to the % intracranial hematoma volume in relation to estimated total intracranial volume. Physiologic predictors included ABP, CPP, heart rate (HR), dICH, values for each of the model-based indices of CA, and the % time of CPP either below LLA or above ULA based on each index. To focus this study on the investigation of CA indices and the time below LLA or above ULA for each index, we subdivided those variables as experimental variables and all other predictor variables as classic variables. Multiple linear regression with subset model selection was applied to identify the best subset model of classic variables based on the Akaike information criterion (AIC) [20], in which lower values correspond to better fit. To build the final model when incorporating experimental variables, we forced classic variables identified from subset model selection and entered each experimental variable one at a time because of high Pearson correlation between experimental variables (r = 0.51–0.98). Best fit was investigated using AIC and described using RMSE. After determining the best fit model, we performed age stratification and investigated model performance. Age groups were stratified as < 2 years, ≥ 2 to < 8 years and ≥ 8 years of age. These cutoffs for age stratification were made based on previous literature identifying that there is an age-dependent peak in cerebral blood flow of healthy children between 2 and 8 years of age [21,22,23,24,25], in addition to the incorporation of these age stratification groups in prior pediatric studies incorporating ICP monitoring [26]. In exploratory analyses, we analyzed sex-based differences with measures of cerebral autoregulation by performing Welch two-sample t test analysis to investigate for differences with respect to sex for each index of CA and the % time of CPP either below LLA or above ULA based on each index. We also used univariate linear regression to explore the association of predictor variables with GOSE-Peds with respect to age groups described above. Statistical analyses were performed using Statistical Analysis System (SAS) 9.4 (SAS Institute, Cary, NC).
Results
Patient Characteristics
Demographic and classic physiologic data are summarized in Table 1, and CA data are summarized in Table 2. Age-stratified demographic and classic physiologic data and CA data are summarized in Supplements 1 and 2, respectively. Seventy-two children were identified with TBI who underwent intraparenchymal ICP monitoring with MMM, and 16 were excluded from final analysis because of missing data, allowing for multivariable analysis of 56 children. Fourteen of 16 children (87.5%) were excluded because of a lack of initial CT head imaging available for analysis (e.g., performed at other hospitals), and 2 children (12.5%) were excluded for insurmountable technical challenges in signal processing for predictor variable calculation. Ages ranged from 14 days to 20 years of age (7.0 ± 11.5 years [median ± IQR]). S ixteen (28.6%) patients were girls. Initial GCS scores ranged from 3 to 15 (6.0 ± 4.0). Twenty-one children (37.5%) underwent decompressive craniectomies. We did not observe significant differences with respect to sex for any CA index or % time of CPP either < or > LLA for each index (Supplement 3).
Association with GOSE-Peds Scores
Table 3 summarizes the association of predictor variables with GOSE-Peds scores at 12 months post injury, and Supplement 4 summarizes such associations stratified to age. Because of the presence of high skewness in the distribution of the scores of dICH, we dichotomized the variable using the median value (194.8 mmHg/h; 25 patients < median value) to be able to implement it in multivariable model development. At the univariate level, age or sex were not associated with GOSE-Peds scores. Regarding neuroimaging, midline shift and % intracranial hematoma volume in relation to estimated total intracranial volume were not associated with GOSE-Peds scores. In terms of injury severity measures, we did not observe that initial GCS, PRISM3, or CT Marshall Scores were associated with GOSE-Peds scores. When performing univariate linear regression in terms of physiologic variables, we observed that increased dICH (slope = 1.52, p = 0.0059, R2 = 13.2%, RMSE = 1.97) and lower median CPP (slope = − 0.06, p = 0.0023, R2 = 16.0%, RMSE = 1.94) values were associated with higher GOSE-Peds scores. We observed that higher median PRx (slope = 3.47, p < 0.0001, R2 = 25.2%, RMSE = 1.83), PAx (slope = 3.59, p = 0.0001, R2 = 24.0%, RMSE = 1.84), wPRx (slope = 3.62, p = 0.0001, R2 = 24.1%, RMSE = 1.84), and RAC (slope = 2.84, p = 0.0013, R2 = 17.6%, RMSE = 1.92) values were associated with higher GOSE-Peds scores. Median ABP and HR were not associated with GOSE-Peds scores. Increased % time < LLA based on PRx (slope = 0.04, p = 0.0001; R2 = 23.6%, RMSE = 1.85), PAx (slope = 0.06, p < 0.0001, R2 = 34.2%, RMSE = 1.72), wPRx (slope = 0.05, p < 0.0001, R2 = 36.5%, RMSE = 1.68), and RAC (slope = 0.06, p < 0.0001, R2 = 35.3%, RMSE = 1.70) were all associated with higher GOSE-Peds scores. Decreased % time > ULA based on PAx (slope = − 0.21, p = 0.0207; R2 = 9.5%, RMSE = 2.01) was associated with increased GOSE-Peds scores, although % time > ULA for PRx, wPRx, and RAC were not associated with GOSE-Peds scores. Among classic variables, best subset model selection identified GCS, dICH, and median CPP as most predictive of GOSE-Peds scores (Table 4; AIC = 72.72, R2 = 26.0%, adjusted R2 = 22%, RMSE = 1.85), with median CPP and dICH being independently associated with GOSE-Peds scores and GCS not achieving significance. After forcing GCS, dICH, and median CPP in the multivariable model and entering each experimental variable at a time (because of high correlation between experimental variables), the three best fit models, from lowest to highest AIC values, included % time < LLA for RAC (AIC = 57.0, R2 = 46%, adjusted R2 = 42%, RMSE 1.59), % time < LLA for PAx (AIC = 59.6, R2 = 44%, adjusted R2 = 39%, RMSE 1.63), and % time < LLA for wPRx (AIC = 60.7, R2 = 43%, adjusted R2 = 38%, RMSE 1.64), respectively. Given high correlation between median CPP and % time < LLA for RAC (r = − 0.87) and % time < LLA for PAx (r = − 0.87), the final model was built to include median CPP, dICH, and % time < LLA for wPRx (Table 5; AIC = 59.2, R2 = 42%, Adjusted R2 = 38%, RMSE 1.64). In the final model, increased dICH (slope = 0.97, p = 0.0409) and % time < LLA for wPRx (slope = 0.05, p = 0.0001) were independently associated with GOSE-Peds, whereas median CPP did not achieve significance (slope = 0.03, p = 0.2781). The effect size, described using the standardized slope, was higher for % < LLA for wPRx (0.68), as compared with both dICH (0.23) and GCS (0.18). When evaluating the performance of this model within each stratified age group, we observed that increased % time < LLA for wPRx was independently associated with increased GOSE-Peds scores when adjusting for median CPP and dICH for children < 2 years of age (slope = 0.07, p = 0.0185, R2 = 90%, RMSE = 1.11) and for children ≥ 8 to ≤ 21 years of age (slope = 0.05, p = 0.0028; R2 = 33%, RMSE = 1.45), whereas dICH and median CPP did not approach significance. For patients ≥ 2 to < 8 years of age, we observed that higher dICH (slope = 2.67, p = 0.0116; R2 = 46%, RMSE = 1.71) was independently associated with increased GOSE-Peds scores, whereas median CPP and % time < LLA for wPRx did not achieve significance.
Discussion
TBI is a serious neurologic condition that places the brain at risk of a significant pathophysiologic cascade of events which alters CA leading to additional secondary insults and further subsequent damage. In this study, we investigated the association of global functional outcome with patient characteristics, initial neuroimaging findings, physiologic vital signs, and ICP-derived model-based indices of CA. This is the first study to investigate wPRx, PAx and RAC after pediatric TBI, and the first pediatric TBI study to investigate the association of global functional outcome with time below or above thresholds of LLA and ULA for ICP-derived indices of CA. At the univariate level, we observed that higher values of PRx, PAx, wPRx and RAC, indicative of worsened CA, are associated with unfavorable outcome. Additionally, greater % time below LLA based on all these indices were associated with unfavorable outcome. When accounting for each of these CA indices as well as clinical and neuroimaging markers of injury severity, we observed that increased dICH and % time < LLA based on wPRx are most predictive of unfavorable outcomes, when accounting for GCS. An improvement in adjusted R2 from 22% in the classic variable model that included median CPP and dICH to 38% with incorporation of % time < LLA for wPRx in the final model suggested that the latter model can explain more variability of GOSE-Peds scores.
Our findings are consistent with the growing of body of literature investigating multimodal physiologic monitoring of CA after pediatric and adult TBI. After pediatric TBI, elevated PRx values have been associated with increased risk for mortality [27], and duration and magnitude of negative deviations between CPP and CPPOpt values have been associated with unfavorable outcome [28]. Existing studies after adult TBI suggest that % time below LLA derived from PRx-based CPPopt curves is associated with unfavorable outcome [13, 14] and prospective work evaluating the feasibility, safety and physiologic implications of CPPopt-guided management is underway in adult patients with severe TBI (CPPopt-Guided Therapy; Assessment of Target Effectiveness [COGiTATE]]; clinicaltrials.gov identifier NCT02982122) [29]. This work has not been done in children and there is an ongoing need to investigate the potential impact that these CA indices may have on innovative therapeutic protocols in pediatric TBI management.
PRx and PRx-derived CPPOpt values have been previously described as statistical constructs that provide patient risk stratification. Limitations do exist however, in the ability to evaluate PRx as a marker for CA. PRx assumes cerebral blood volume (CBV) as a surrogate for cerebral blood flow. It relies on adequate transmission of CBV into ICP, which may not apply efficiently in situations of high brain compliance such as after decompressive craniectomy or in infants with open anterior fontanelles. It relies on an assumption that variability of ICP data is purely due to extracranial sources (e.g., ABP). PRx values near zero could reflect working CA or incoherent variability between ABP and ICP. To address these limitations, variant ICP-derived CA indices have been developed. WPRx investigates wavelet transform phase shifts between ABP and ICP, deriving values when there is strong coherence [8]. PAx relates ABP to ICP pulse amplitude, accounting situations of high brain compliance [9]. RAC relates ICP pulse amplitude to CPP, accounting for situations of changing brain compliance that may relate to intracranial sources [10]. While our study affirmed PRx as a marker for injury severity, it also demonstrated significance with elevated wPRx, PAx and RAC. While PRx, PAx wPRx and RAC offer risk stratification, their immediate values are not clinically actionable.
Much interest surrounding CPPOpt-guided therapy involves targeting CPP management toward either CPPOpt values or maintenance of values above LLA or below ULA. In our study, we demonstrated that greater % time < LLA for PRx, PAx, wPRx and RAC were associated with unfavorable outcome, with % time < LLA for wPRx being independently predictive of unfavorable outcomes in a best fit model accounting for GCS and dICH (Fig. 2). This suggests that maintenance of CPP values within these targeted thresholds may lead to improved outcome in children. When we performed age-based stratification of the best fit model, we identified that % time of CPP < LLA for wPRx carried independent predictive value for children < 2 years of age or ≥ 8 years of age, whereas dICH carried independent predictive value for children ≥ 2 to < 8 years of age. This finding may relate to age-related changes in cerebral blood flow for children that have been previously described. Multiple studies using perfusion imaging or transcranial Doppler ultrasound techniques show that cerebral blood flow peaks at approximately 2–4 years of age and then stabilizes at around 8 years of age [21,22,23,24,25]. It is possible that targeting CPP values greater than ICP-derived LLA targets may carry increased relevance at pediatric ages where CBF is relatively stable and decreasing the burden of intracranial hypertension remains paramount for children ≥ 2 to < 8 years of age while changes in cerebral blood flow are dynamic. We did not observe an association of decreased % time above ULA to unfavorable outcomes with respect to PRx, wPRx and RAC, whereas decreased time above ULA for PAx was associated with worsened outcomes. Thresholds for upper limits for CPP are not provided in recent pediatric TBI guidelines [2]. Our findings did not suggest ULA for any CA index as a target for which higher CPP values confer increased risk for worsened outcomes, though further work is needed to investigate whether CPP values at such levels can lead to secondary insults such as hematoma enlargement, intracranial hypertension, or hyperemic injury.
Burden of time below the lower limit of autoregulation. a A 3-year-old boy with traumatic brain injury experiences recurrent epochs in which cerebral perfusion pressure (gray line) drops below the lower limit of autoregulation (LLA) derived from the wavelet pressure reactivity index (wPRx). b He experiences malignant cerebral edema over the right hemisphere with midline shift evident on subsequent neuroimaging. At 12 months post injury, he has an unfavorable outcome with a Glasgow Outcome Scale Extended-Pediatrics score of 5. ICP intracranial pressure, CPP cerebral perfusion pressure, ULA upper limit of autoregulation, mmHg millimeters of mercury
Although CPPOpt-guided therapy represents a pragmatic, empirical therapeutic approach to clinical care, it may not fully account for the underlying pathophysiology representative of elevated PRx, PAx, RAC or wPRx values. In this study, we observed significant effect of dose of intracranial hypertension, consistent with findings in other adult studies which have also demonstrated that ICP tolerability may be associated with CA status [12, 30]. In silico mechanistic simulations of blood pressure, intracranial PaCO2 and CA have demonstrated that shifts in either ABP or ICP (without corresponding change in ABP) during intact CA can change PRx-based CPPopt values [31]. These mechanistic studies also demonstrate that positive PRx values can occur during intact CA. In our study, we observed that % time < LLA based on wPRx was independently associated with poor outcome when adjusting for dose of intracranial hypertension and CPP. Pathophysiologic mechanisms beyond insufficient CPP or intracranial hypertension may exist that are representative of elevated PRx, PAx or wPRx values contributing toward worsened outcome. Multivariate compartmental models, such as the Ursino Model, provide a mechanistic approach to investigating cerebral physiology in relation to ABP, ICP, CA, CBV, partial pressure of carbon dioxide (PaCO2), and cerebral blood flow [32, 33]. The parameters we investigated in our study do not account for physiologic factors such as PaCO2 or other factors influencing cerebral dynamics such as cerebral metabolic demand. This represents a limitation to extrapolating fully encompassing mechanistic insights from our analysis. Complementary time series approaches that account for both model-based indices of CA and multiparametric models of cerebral dynamics may help extract other modifiable biomarkers beyond CPP that can improve care for patients who are high-risk.
Conclusions
Our study was limited by its retrospective nature, single site data collection, and a relatively small sample size with wide age range of children. Changes in cerebral hemodynamics represent dynamic processes and because this is a retrospective analysis, we are limited in appraising how physiologic changes occur in real time. The method of artifact reduction utilized in this study may not be the most optimal method for removing the truest artifact and maintaining analysis of the most physiologically accurate data. Automated tools such as median absolute deviation may be of value in future multicenter work to improve generalizability and applicability to multicenter studies. Larger, multicenter prospective work is needed to determine whether CPPOpt-guided therapy can improve outcome for pediatric patients with TBI, and whether other modifiable biomarkers can be identified beyond CPP that contribute toward elevated CA indices and unfavorable outcome.
References
Hawley CA, Ward AB, Long J, Owen DW, et al. Prevalence of traumatic brain injury amongst children admitted to hospital in one health district: a population based study. Injury. 2003;354:256–60.
Kochanek PM, Tasker RC, Carney N, Totten AM, et al. Guidelines for the management of pediatric severe traumatic brain injury, third edition: update of the brain trauma foundation guidelines. Pediatr Crit Care Med. 2019;20(3S Suppl 1):S1–82.
Maas AIR, Menon DK, Adelson PD, Andelic N, et al. Traumatic brain injury: integrated approaches to improve prevention, clinical care, and research. Lancet Neurol. 2017;16:987–1048.
Donnelly J, Budohoski KP, Smielewski P, Czosnyka M. Regulation of the cerebral circulation: bedside assessment and clinical implications. Crit Care. 2016;20(1):129.
Mehta A, Kochanek PM, Tyler-Kabara E, Adelson PD, et al. Relationship of intracranial pressure and cerebral perfusion pressure with outcome in young children after severe traumatic brain injury. Dev Neurosci. 2010;32(5–6):413–9.
Steiner LA, Czosnyka M, Piechnik SK, Smielewski P, et al. Continuous monitoring of cerebrovascular pressure reactivity allows determination of optimal cerebral perfusion pressure in patients with traumatic brain injury. Crit Care Med. 2002;30:7338.
Liu X, Maurits NM, Aries MJH, Czosynka M, Ercole A, Donnelly J, et al. Monitoring of optimal cerebral perfusion pressure in traumatic brain injured patients using a multi-window weighting algorithm. J Neurotrauma. 2017;34:3081–8.
Liu X, Donnelly J, Czosnyka M, Aries MJH, et al. Cerebrovascular pressure reactivity monitoring using wavelet analysis in traumatic brain injury patients: a retrospective study. PLoS Med. 2017;14(7):e1002348.
Zeiler FA, Donnelly J, Calviello L, Lee JK, et al. Validation of pressure reactivity and pulse amplitude indices against the lower limit of autoregulation, part I: experimental intracranial hypertension. J Neurotrauma. 2018;35(23):2803–11.
Zeiler FA, Donnelly J, Menon DK, Smielewski P, et al. A description of a new continuous physiological index in traumatic brain injury using the correlation between pulse amplitude of intracranial pressure and cerebral perfusion pressure. J Neurotrauma. 2018;35:963–74.
Kochanek PM, Carney N, Adelson PD, Ashwal S, et al. Guidelines for the acute management of severe traumatic brain injury in infants, children and adolescents—second edition. Pediatr Crit Care Med. 2012;13(Suppl 1):S1–82.
Lazardis C, Desantis SM, Smielewski P, Menon DK, Hutchinson P, Pickard JD, et al. Patient-specific thresholds of intracranial pressure in severe traumatic brain injury. J Neurosurg. 2014;120(4):893–900.
Zeiler FA, Donnelly J, Smielewski P, Menon DK, Hutchinson J, Czosynka M. Critical thresholds of intracranial pressure-derived continuous cerebrovascular reactivity indices for outcome prediction in noncraniectomized patients with traumatic brain injury. J Neurotrauma. 2018;35(10):1107–15.
Donnelly J, Czosnyka M, Adams H, Robba C, et al. Individualizing thresholds of cerebral perfusion pressure using estimated limits of autoregulation. Crit Care Med. 2017;45(9):1464–71.
Czosnyka M, Czosynka Z, Smelewski P. Pressure reactivity index: journey through the past 20 years. Acta Neurochir. 2019;519:2063–5.
Appavu B, Foldes S, Burrows BT, Jacobson A, Abruzzo T, Boerwinkle V, Willyerd A, Mangum T, Gunnala V, Marku I, Adelson PD. Multimodal assessment of cerebral autoregulation and autonomic function after pediatric cerebral arteriovenous malformation rupture. Neurocrit Care. 2021;34(2):537–46.
Hiler M, Czosnyka M, Hutchinson P, Balestreri M, et al. Predictive value of initial computerized tomography scan, intracranial pressure, and state of autoregulation in patients with traumatic brain injury. J Neurosurg. 2006;104:731–7.
Sgouros S, Goldin JH, Hockley AD, Wake MJC, Natarajan K. Intracranial volume change in childhood. J Neurosurg. 1999;91:610–6.
Beers SR, Wisniewski SR, Garcia-Filion P, Tian Y, Hahner T, Berger RP, et al. Validity of a pediatric version of the glagow outcome scale—extended. J Neurotrauma. 2012;29(6):1126–39.
Portet S. A primer on model selection using the Akaike information criterion. Infect Dis Model. 2020;5:111–28.
Suzuki K. The changes of regional cerebral blood flow with advancing age in normal children. Nagoya Med J. 1990;34:159–70.
Bode H, Wais U. Age dependence of flow velocities in basal arteries. Arch Dis Child. 1998;63(6):606–11.
Wintermark M, Cotting D, Roulet E, van Melle G, Meuli R, Maeder P, et al. Brain perfusion in children: evolution with age assessed by quantitative perfusion computed tomography. Pediatrics. 2004;113(6):1642–52.
Chiron C, Raynaud C, Maziere B, et al. Changes in regional cerebral blood flow during brain maturation in children and adolescents. J Nucl Med. 1992;33:696–703.
Vavilala MS, Kincaid MS, Muangman SL, Suz P, Roet I, Lam AM. Gender differences in cerebral blood flow velocity and autoregulation between the anterior and posterior circulations in healthy children. Pediatr Res. 2005;58:574–8.
Woods KS, Horvat CM, Kantawala S, Simon DW, Rakkar J, Kochanek PK, et al. Intracranial and cerebral perfusion pressure thresholds associated with in hospital mortality across pediatric neurocritical care. Pediatr Crit Care Med. 2021;22(2):135–46.
Brady KM, Shaffner DH, Lee JK, Easley RB, et al. Continuous monitoring of cerebrovascular pressure reactivity after traumatic brain injury in children. Pediatrics. 2009;124(6):e1205–12.
Lewis PM, Czosnyka M, Carter BG, Rosenfeld JV, et al. Cerebrovascular pressure reactivity in children with traumatic brain injury. Pediatr Crit Care Med. 2015;16(8):739–49.
Zeiler FA, Ercole A, Czosnyka M, Smielewski P, et al. Continuous cerebrovascular reactivity monitoring in moderate/severe traumatic brain injury: a narrative review of advances in neurocritical care. Brit J Anesth. 2020;124(4):440–53.
Akerlund CA, Donnelly J, Zeiler FA, Helbok R, Holst A, Cabeleira M, et al. Impact of duration and magnitude of raised intracranial pressure on outcome after severe traumatic brain injury: a CENTER-TBI high resolution group study. PLOS ONE. 2020;15(12):e0243427.
Akhondi-Asl A, Vonberg FW, Au CC, Tasker RC. Meaning of intracranial pressure-to-blood pressure fisher-transformed Pearson correlation-derived optimal cerebral perfusion pressure: testing empiric utility in a mechanistic model. Crit Care Med. 2018;46(12):e1160–6.
Ursino M. A mathematical study of human intracranial hydrodynamics. Part 1—the cerebrospinal fluid pulse pressure. Ann Biomed Eng. 1988;16:379–401.
Ursino M. A mathematical study of human intracranial hydrodynamics. Part 2—simulation of clinical tests. Ann Biomed Eng. 1988;16:403–16.
Funding
This work was funded in part by the United States Department of Defense Congressionally Directed Medical Research Programs Epilepsy Research Program (W81XWH-19-1-0514).
Author information
Authors and Affiliations
Contributions
BA, MT, SF, and PDA contributed to conception and design of this study. BA, MT, SF, BB, MK, AJ, and PDA contributed to acquisition and analysis of data and drafting a significant portion of the article and figures. All authors approved the final draft of this manuscript.
Corresponding author
Ethics declarations
Conflicts of Interest
Dr. Appavu previously received a completed research grant from Moberg ICU Solutions, outside the scope of this work. All other authors do not have relevant conflicts of interest.
Ethical Approval/Informed Consent
This study was performed under all ethical research guidelines at Phoenix Children’s Hospital, and the institutional review board (#19-284) at Phoenix Children’s Hospital approved this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is related to the Invited Commentary available at https://springerlink.bibliotecabuap.elogim.com/article/10.1007/s12028-021-01307-z.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplement 1
Age-stratified demographic and classic physiologic data. n, count; %, percent; CT, computed tomography; IQR, interquartile ratio; PRISM, Pediatric Risk of Mortality; mm, millimeters; mmHg, millimeters of mercury; ICP, intracranial pressure; CPP, cerebral perfusion pressure; ABP, arterial blood pressure; HR, heart rate; bpm, beats per minute; GOSE-Peds, Glasgow Outcome Scale Extended-Pediatrics. (DOCX 15 KB)
Supplement 2
Age-stratified characteristics of model-based indices of cerebral autoregulation. n, count; IQR, interquartile ratio; mmHg, millimeters of mercury; %, percent; PRx, pressure reactivity index; PAx, pulse amplitude index; wPRx, wavelet pressure reactivity index; RAC, correlation coefficient of intracranial pressure pulse amplitude and cerebral perfusion pressure; CPPOpt, optimal cerebral perfusion pressure; LLA, lower limit of autoregulation; ULA, upper limit of autoregulation. (DOCX 18 KB)
Supplement 3
Sex-based differences in model-based indices of cerebral autoregulation. SD, standard deviation; PRx, pressure reactivity index; PAx, pulse amplitude index; wPRx, wavelet pressure reactivity index; RAC; correlation coefficient of intracranial pressure pulse amplitude and cerebral perfusion pressure; %, percent; LLA, lower limit of autoregulation; ULA, upper limit of autoregulation. (DOCX 14 KB)
Supplement 4
Age-stratified univariate analysis of the association of demographic, neuroimaging and physiologic data with GOSE-Peds scores, 12 months post injury. Bold represent significant variables as related to global functional outcome determined by GOSE-Peds, Glasgow Outcome Scale Extended-Pediatrics (GOSE-Peds). RMSE, root mean square error; GCS, Glasgow Coma Scale; PRISM, pediatric risk of mortality; CT, computed tomography; dICH, dose of intracranial hypertension; CPP, cerebral perfusion pressure; ABP, arterial blood pressure; HR, heart rate; mmHg, millimeters of mercury; bpm, beats per minute; PRx, pressure reactivity index; PAx, pulse amplitude index; wPRx, wavelet pressure reactivity index; RAC; correlation coefficient of intracranial pressure pulse amplitude and cerebral perfusion pressure; %, percent; LLA, lower limit of autoregulation; ULA, upper limit of autoregulation. (DOCX 30 KB)
Rights and permissions
About this article
Cite this article
Appavu, B., Temkit, M., Foldes, S. et al. Association of Outcomes with Model-Based Indices of Cerebral Autoregulation After Pediatric Traumatic Brain Injury. Neurocrit Care 35, 640–650 (2021). https://doi.org/10.1007/s12028-021-01279-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-021-01279-0