Abstract
Introduction
Hyperthermia is common in brain-injured patients and associated with a worse outcome. As brain rather than body temperature reduction, theoretically, is the most important in cerebral protection, there is logic in targeting cooling at the brain. Selective brain cooling can, in theory, be obtained by cooling the skull or by heat loss from the upper airways. In this preliminary safety and efficacy study, we report clinical data from brain-injured patients who because of hyperthermia were treated with intranasal cooling.
Methods
Nine intubated brain-injured patients with hyperthermia were treated using a prototype intranasal balloon system perfused with cold saline. Temperature in the cerebrum, esophagus, and bladder was monitored together with intracranial pressure.
Results
In only two of nine patients, normothermia was reached in the esophagus and in only four of nine patients it was reached in the bladder. When normothermia was reached, the time to normothermia was delayed. In the brain, normothermia was reached in two of five patients after approximately 72 h. Median temperature curves from the first 72 h of cooling showed that normothermia was not reached in any of the three compartments. The temperature in the brain and bladder were on average 0.6 and 0.5 °C higher than in the esophagus. ICP increased with increasing brain temperature. We found no signs of clinical important injury to the nasal mucosa from the cold saline or pressure in the balloons.
Conclusion
In brain-injured patients with hyperthermia, cooling with a prototype intranasal balloon system was clinically inadequate as the effect was delayed and not brain selective.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hyperthermia, body temperature above 37 °C, is common in critically ill neurosurgical patients. Body temperature above 38 °C in the first 72 h after brain injury has been reported in up to 68 % after closed head trauma and is found in up to 70 % after subarachnoid hemorrhage (SAH) [1]. The risk is associated with a prolonged stay in the neurointensive care unit, where body temperature above 38.5 °C is seen in 93 % of those who remained longer than 14 days compared to only 15 % in those who stayed <24 h [2].
Hyperthermia is caused not only by infection but also by endogenous pyrogens released by neuronal injury, as well as by the presence of blood in the brain parenchyma, ventricles, and subarachnoid space [3]. Also, brain-injured patients may have lost the ability to regulate body temperature because of hypothalamic dysfunction [4]. Although hyperthermia is independently associated with a longer neurointensive care unit stay, higher mortality rate, and a worse clinical outcome [4–6], it is difficult to determine whether this is a causative relation or simply coexistence.
Hyperthermia increases the cerebral metabolic rate for oxygen and glucose. In the traumatized brain with impaired autoregulation, this may lead to an increase in cerebral blood flow and blood volume, and a detrimental increase in the intracranial pressure (ICP) [4]. Also, experimental studies suggest that neurotransmitter excitotoxicity and brain inflammation is negatively affected by hyperthermia [4]. Despite a sound physiologic argument for controlling fever in the brain-injured patient and indications from experimental studies, there is, however, no strong clinical evidence that doing so will improve outcome [7, 8]. Furthermore, aside from anoxic encephalopathy from cardiac arrest and perinatal asphyxia [9, 10], there is no substantial clinical evidence to support the use of induced hypothermia in brain-injured patients [7, 8]. Irrespective of the sparse clinical evidence, normothermia is a broadly accepted treatment goal in neurocritical care and is also routinely practiced in our neurointensive care unit [4, 8].
When correction of hyperthermia is attempted, several treatment options are available. The traditional methods of reducing temperature, i.e., anti-pyretics and external body cooling are, however, not particularly effective [2, 4, 11]. Intravascular cooling is effective, but it cools the whole body, and therefore has risks and limitations [4, 11]. As brain temperature rather than body temperature reduction, theoretically, is the most important in cerebral protection, there is logic in targeting cooling at the brain. This would also prevent the systemic complications associated with whole-body hypothermia [7, 8]. Selective brain cooling can in theory be obtained by cooling the skull, and thereby the brain via the venous sinuses and diploic and emissary veins, or by heat loss from the upper airways [12, 13]. The latter regulatory mechanism is normally lost in intubated patients.
Cooling of the head and neck not only reduced tympanic temperature in ten healthy volunteers but also induced prominent peripheral vasoconstriction and a blood pressure increase that could be harmful [14]. In pigs, preferential brain cooling has been demonstrated using nasal balloon catheters perfused with cold saline [15, 16]. However, it is difficult to extrapolate to a clinical setting as pigs have a carotid rete mirabile and humans do not [17]. In rats, which do not have a carotid rete, preferential brain cooling was found when nasal cavities was flushed with oxygen, but the mean temperature decrease was limited [18]. The authors suggest that since the cooling was rapid and evenly distributed it was probably blood mediated and not from simple diffusion [18]. In ten healthy volunteers with intranasal balloon catheters perfused with 20 °C saline for 60 min preferential brain cooling was observed and the treatment was well tolerated [19]. However, the clinical importance of this study is limited as the subjects were healthy volunteers with spontaneous respiration and without brain injury. In intubated brain-injured patients humidified air at room temperature administered through the nostrils did not produce clinical relevant or significant reduction in brain temperature [13]. When combined with head fanning a rapid reduction in brain temperature was observed, but selective brain cooling did not occur [12]. Finally, nasopharyngeal cooling with a perfluorocarbon–oxygen mixture has been demonstrated to be safe and efficient in a preliminary safety study, but cooling was only administered for 1 h and at that time point the cooling was not brain preferential or selective [20]. Jointly, these results imply that cooling of the nasal cavity may be clinical useful in reducing brain temperature but data are limited.
In this preliminary safety and efficacy study, we report clinical data from nine brain-injured patients who because of systemic hyperthermia were treated with intranasal cooling with cold saline to obtain normothermia. We examined the effect of cooling on brain temperature and on core temperature determined by temperature probes in the esophagus and bladder. In patients with intracranial pressure (ICP), monitoring the brain temperature’s effect on ICP was also evaluated. The local reaction to the cold balloons in the nasal cavity was evaluated after termination of the cooling.
Materials and Methods
The study was conducted in the neurointensive care unit of the Copenhagen University Hospital, Rigshospitalet, from June 2011 to August 2011. Inclusion criteria were brain injury from trauma, SAH, intraventricular or intraparenchymal hemorrhage, or cerebral infarction requiring intubation and mechanical ventilation. Bladder temperature is routinely monitored in all patients (Mon-a-Term™, Coviden™, Dublin, Ireland), and patients were included if bladder temperature were above 38.5 °C despite cooling with anti-pyretic medication, undressing and in two cases ice packs. Exclusion criteria were ongoing active cooling with other methods, e.g., intravascular cooling or whole-body devise, and anterior skull base fractures.
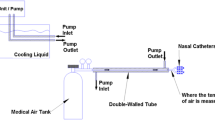
The cooling system was a prototype devise composed of two single-use intranasal balloon catheters (QuickCool Disposable Balloon Catheter, QuickCool AB, Lund, Sweden) perfused with cold isotonic saline from a heat exchanger in a closed circuit system (ComVic, QuickCool AB, Lund, Sweden). The pressure in the balloons was approximately 20–30 mmHg. The flow in the system was set to 200 mL/min and the temperature in the heat exchanger 1 °C. If the patient’s cerebral temperature dropped to 37 °C the temperature in the heat exchanger was changed to 37 °C, and if the patient’s temperature was still dropping the flow in the system was decreased to 100 mL/min. The cooling system was removed when the patient returned to stable normothermia (37 °C for more than 3 h) or if no effect of cooling was seen after 2 days. In patient number five, no effect of cooling was seen after 45 h of cooling wherefore the cooling was stopped. As hyperthermia was still present the next day another attempt of cooling was initiated 27 h after termination of the first attempt.
In the first five patients, a combined temperature and ICP probe (Neurovent-P-Temp, Raumedic AG, Münchberg, Germany) was inserted in the brain parenchyma (dept approximately 3 cm) through a single-use bolt (Bolt kit, Raumedic AG, Münchberg, Germany). As the brain temperature in all these patients matched the temperature in the bladder, it was decided not to measure brain temperature in the last four patients. In patient 9, ICP was measured by our standard parenchymal ICP probe (Codman ICP monitoring system, Codman®, MA, USA) as part of the standard treatment. The esophagus temperature was measured in all patients by a temperature sensor in the esophagus (D-OS4 temperature probe, Exacon Scientific A/S, Roskilde, Denmark).
An ear, nose and throat specialist inspected the nasal and rhinopharyngeal mucosa with a fiberscope after the termination of cooling and removal of the intranasal balloons. She evaluated nasal irritation, bleeding, and signs of infection or skin contact dermatitis.
Data are presented as medians and ranges. Linear regression analysis was performed with accounting for repeated measures. Bland–Altman plots [21] were used to evaluate the difference in temperature between the different compartments with 95 % limits of agreement. Significance was accepted at the 0.05 level. Data analysis was performed by means of SPSS 19 (IBM, New York, NY, USA).
Results
Nine patients were included. Five patients had TBI, two SAH, one intraventricular hemorrhage from an arteriovenous malformation, and one bilateral thalamic infarction after a fourth operation for a pituitary adenoma. The operation was done by a subfrontal approach and the infarct distribution was compatible with an artery of Percheron occlusion. The median age of the patients was 38 years (range 20–66), the median height 180 cm (range 169–190), and the median weight 80 kg (range 70–100). The median cooling period was 5 days (range 2–6).
At the initiation of treatment, the median temperature in the esophagus was 38.7 °C (range 37.8–40.4), in the cerebrum it was 38.7 °C (range 38.3–39.0) and in the bladder it was 38.9 (range 38.2–40.3). In only two patients, the temperature in the esophagus reached a stable level of 37 °C and this was observed after 40 and 43 h of cooling, respectively. In the five patients with temperature probes in the brain parenchyma, a stable temperature level of 37 °C was reached in two patients after 101 and 42 h of cooling, respectively. In the bladder, a stable temperature level of 37 °C was reached in four patients after, respectively, 101, 42, 98, and 78 h of cooling. Median temperature curves from the first 72 h of cooling are presented in Figs. 1, 2, and 3. As indicated by the three linear regression lines a temperature level of 37 °C was not reached in any of the three compartments within 72 h.
Figure 4 is a Bland–Altman scatterplot with the mean of the temperatures in the cerebrum and esophagus for each time point on the horizontal axis and the difference of the two measurements on the vertical axis. The three horizontal reference lines indicate the mean difference and 95 % limits of agreement. The temperature in the cerebrum was on average 0.6 °C (SD = 0.70) higher than in the esophagus and showed no systematic variation with the mean of the two measurements. Figure 5 is a similar plot with temperature in the bladder and esophagus, and the temperature in the bladder was on average 0.5 °C (SD = 0.61) higher than in the esophagus, but with a systematic variation indicating a higher difference the higher the mean temperature. With hypothermia, determined from the mean temperature values, the temperature in the bladder was lower than in the esophagus.
In Fig. 6, the ICP values are plotted against the temperature in the cerebrum. Though, only around 10 % of the variation in ICP can be explained by the linear model (R 2 = 0.105, p < 0.0001), the linear regression line may indicate that the ICP increases with increasing cerebral temperature.
Intracranial pressure values plotted against the temperature in the cerebrum. The linear regression line indicates that the intracranial pressure (ICP) increased with increasing brain temperature though the linear model implies that only a smaller part of the variation in ICP could be explained by the cerebral temperature (R 2 = 0.105, p < 0.0001)
After removal of the cooling system, the nasal and rhinopharyngeal mucosa was inspected using a fiberscope and in none of the patients the cold balloons had caused necrosis or atrophy.
Discussion
This is the first clinical study of the effect of intranasal cooling with a balloon system using cold saline in brain-injured patients. Hyperthermia is a common problem in brain-injured patient [1, 2], and the clinical rationale of this study was based on experimental data and clinical data from patients who were cooled by upper respiratory tract airflow [12, 13, 15, 16, 18, 19].
The patients in this study were representative of patients generally admitted to our neurointensive care unit regarding demographic and physiologic data and reason for brain injury. This makes direct extrapolation of the results to future patients easy.
In this study, we did not observe selective brain cooling as the median temperature curves showed temperature reductions in all the three compartments examined. Similar findings have been done by others [12].
Using Bland–Altman scatterplots [21], we found higher average temperatures in the brain and bladder compared to the esophagus. In the brain/esophagus, this difference was unaffected by the mean of the two measurements, but in the bladder/esophagus the difference increased with increasing mean temperatures. Although the literature is limited, and we have not found other studies comparing brain parenchyma temperature with temperature in the esophagus, this study seems to confirm the general finding that brain temperature is higher than core temperature [22]. The possible relationship between temperature in the brain parenchyma and the bladder, which we observed in the first five patients, has not previously been demonstrated; in patients with middle cerebral artery strokes, contradictory results have been published [23]. We have no obvious explanation for this controversy, but a higher temperature in the infarcted hemisphere compared to the contralateral unaffected hemisphere has been demonstrated [23], and none of the patients in this study had large ischemic infarcts, which might contribute to the diversity. The fact that the Bland–Altman plot for bladder/esophagus temperature implies a temperature-dependent proportional bias, but the brain/esophagus plot does not, raises questions whether bladder temperature for the last four patients without cerebral temperature measurements is sufficiently representative of cerebral temperature. The present data cannot answer that question, which suggest that the findings in this study should be interpreted with caution and supported by more data before general conclusions are made.
When ICP was plotted against brain temperature, we found that ICP increased with increasing brain temperature though the linear model could explain only around 10 % of the variation in ICP. Although theoretic, this may support aggressive treatment of hyperthermia in patients where elevated ICP is a concern.
After the removal of the intranasal balloons, we found no signs of clinical important injury to the nasal or rhinopharyngeal mucosa from the cold saline or pressure in the balloons. This observation is important if further development of an intranasal balloon cooling system should be initiated in the future.
The prototype cooling device was generally easy to use regarding application and nursing procedures compared to the whole body devices we have previously tried.
Conclusion
In brain-injured intubated patients with hyperthermia, cooling with an intranasal balloon system perfused with cold saline is clinically inadequate as the effect is delayed and not brain selective. However, the cold balloons were well tolerated, which is important if one should wish to further develop the system. We confirmed that brain temperature is higher than core temperature, as measured in the esophagus, but observed a relationship between brain and bladder temperature, which has not previously been described. ICP raised with increasing brain temperature, which may support aggressive treatment of hyperthermia in patients were ICP is a concern.
References
Albrecht RF, Wass CT, Lanier WL. Occurrence of potentially detrimental temperature alterations in hospitalized patients at risk for brain injury. Mayo Clin Proc. 1998;73:629–35.
Kilpatrick MM, Lowry DW, Firlik AD, Yonas H, Marion DW. Hyperthermia in the neurosurgical intensive care unit. Neurosurgery. 2000;47:850–5.
Georgilis K, Plomaritoglou A, Dafni U, Bassiakos Y, Vemmos K. Aetiology of fever in patients with acute stroke. J Intern Med. 1999;246:203–9.
Cairns CJ, Andrews PJ. Management of hyperthermia in traumatic brain injury. Curr Opin Crit Care. 2002;8:106–10.
Diringer MN, Reaven NL, Funk SE, Uman GC. Elevated body temperature independently contributes to increased length of stay in neurologic intensive care unit patients. Crit Care Med. 2004;32:1489–95.
Li J, Jiang JY. Chinese Head Trauma Data Bank: effect of hyperthermia on the outcome of acute head trauma patients. J Neurotrauma. 2012;29:96–100.
Grande PO, Reinstrup P, Romner B. Active cooling in traumatic brain-injured patients: a questionable therapy? Acta Anaesthesiol Scand. 2009;53:1233–8.
Polderman KH. Induced hypothermia and fever control for prevention and treatment of neurological injuries. Lancet. 2008;371:1955–69.
Arrich J, Holzer M, Herkner H, Mullner M. Hypothermia for neuroprotection in adults after cardiopulmonary resuscitation. Cochrane Database Syst Rev. 2009;4:CD004128.
Jacobs S, Hunt R, Tarnow-Mordi W, Inder T, Davis P. Cooling for newborns with hypoxic ischaemic encephalopathy. Cochrane Database Syst Rev. 2007;4:CD003311.
Badjatia N. Hyperthermia and fever control in brain injury. Crit Care Med. 2009;37:S250–7.
Harris BA, Andrews PJ, Murray GD. Enhanced upper respiratory tract airflow and head fanning reduce brain temperature in brain-injured, mechanically ventilated patients: a randomized, crossover, factorial trial. Br J Anaesth. 2007;98:93–9.
Andrews PJ, Harris B, Murray GD. Randomized controlled trial of effects of the airflow through the upper respiratory tract of intubated brain-injured patients on brain temperature and selective brain cooling. Br J Anaesth. 2005;94:330–5.
Koehn J, Kollmar R, Cimpianu CL, et al. Head and neck cooling decreases tympanic and skin temperature, but significantly increases blood pressure. Stroke. 2012;43:2142–8.
Covaciu L, Allers M, Lunderquist A, Rubertsson S. Intranasal cooling with or without intravenous cold fluids during and after cardiac arrest in pigs. Acta Anaesthesiol Scand. 2010;54:494–501.
Covaciu L, Allers M, Enblad P, Lunderquist A, Wieloch T, Rubertsson S. Intranasal selective brain cooling in pigs. Resuscitation. 2008;76:83–8.
Harris B, Andrews P. Intranasal selective brain cooling in pigs. Resuscitation. 2008;78:102–3.
Einer-Jensen N, Khorooshi MH. Cooling of the brain through oxygen flushing of the nasal cavities in intubated rats: an alternative model for treatment of brain injury. Exp Brain Res. 2000;130:244–7.
Covaciu L, Weis J, Bengtsson C, et al. Brain temperature in volunteers subjected to intranasal cooling. Intensive Care Med. 2011;37:1277–84.
Abou-Chebl A, Sung G, Barbut D, Torbey M. Local brain temperature reduction through intranasal cooling with the RhinoChill device: preliminary safety data in brain-injured patients. Stroke. 2011;42:2164–9.
Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–10.
Mcilvoy L. Comparison of brain temperature to core temperature: a review of the literature. J Neurosci Nurs. 2004;36(1):23–31.
Schwab S, Spranger M, Aschoff A, Steiner T, Hacke W. Brain temperature monitoring and modulation in patients with severe MCA infarction. Neurology. 1997;48:762–7.
Acknowledgments
Quickcool AB provided the cooling device and the esophagus and cerebral temperature probes. The company also afforded technical assistance and funding for a research nurse.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Springborg, J.B., Springborg, K.K. & Romner, B. First Clinical Experience with Intranasal Cooling for Hyperthermia in Brain-Injured Patients. Neurocrit Care 18, 400–405 (2013). https://doi.org/10.1007/s12028-012-9806-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-012-9806-x