Abstract
Background
Diagnosing brain death in children is challenging. Guidelines recommend using confirmatory testing to provide ancillary information to support the diagnosis. Brain tissue oxygenation (PbtO2) is being increasingly used in the adult neurocritical care for continuous monitoring of the adequacy of brain oxygenation; however, data in pediatrics is limited. Evidence from adult studies suggests that persistent PbtO2 of 0 mmHg is associated with brain death, but this relationship has not yet been demonstrated in children; therefore, we examined our experience with PbtO2 monitoring and brain death in children with acute neurological pathology.
Methods
We retrospectively reviewed patient records from a prospectively maintained database of 85 children who were ventilated for coma due to acute neurological injury and who received intracerebral monitoring.
Results
We identified five children who had suffered brain death while being monitored. PbtO2 had decreased to 0 mmHg in all five children at the time of brain death diagnosis. In contrast, PbtO2 in patients, who did not develop brain death, never decreased to 0 mmHg. We review the benefits and drawbacks of using brain tissue oxygenation as ancillary information in diagnosing brain death in children.
Conclusions
Preliminary data from this study suggest that PbtO2 decreases to 0 mmHg when brain death occurs in children. Further study is needed to determine the limitations, and the sensitivity and specificity of this finding in a larger group of children.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Published guidelines urge caution when diagnosing brain death in children [1–5]. In theory, immature brains have an increased ability to recover from profound insults when compared to adults, and there are reports of children who appeared to regain function after a diagnosis of brain death was made [6–10]. Yet there are also severe shortages of heart-beating cadaveric child organ donors. Therefore, it is important that diagnosis of brain death in children is sensitive, specific, and not unnecessarily delayed. Published guidelines on diagnosing brain death in children vary in their recommendations, reflecting some of the diagnostic uncertainty. Therefore, confirmatory investigations such as electroencephalogram (EEG), or cerebral blood flow (CBF) have been recommended, or indeed are compulsory in many countries [2–5, 11, 12].
However, these ancillary tests are not without their own challenges. There are technical difficulties associated with performing an EEG on small skulls, and the use of EEG in diagnosing brain death is now regarded as less vital than previously. There are case reports of children in whom EEG activity has returned after periods of electrocerebral silence (ECS). Moreover, brain death can occur without ECS [6, 7, 10, 13]. CBF, as measured by single photon emission computed tomography (SPECT) or four vessel cerebral angiography, is currently the confirmatory test of choice in diagnosing brain death in children [3, 13]. When brain death occurs, CBF is expected to be absent, causing cerebral ischemia and irreversible cessation of brain function. Yet rarely, CBF to the cortex may continue after brain death has occurred [14, 15]. However, we are unaware of any cases of return of brain stem function after a clinical diagnosis of brain death with continued CBF. In practice though, SPECT scanning is expensive and requires technical expertise. The scanner is typically located outside of the ICU and patients may not be sufficiently stable for transport. Moreover, when brain death occurs after hours, the definitive diagnosis may be delayed if SPECT is not immediately available. Similarly, formal angiography is not readily available.
Therefore, supporting information from bedside tools used routinely for monitoring brain injured patients would be helpful. Transcranial Doppler (TCDS) is a bedside technique that may be helpful in the diagnosis of brainstem death, but insonation of the intracranial vessels is not always possible due to inadequate transtemporal acoustic windows. Therefore, the sensitivity of this test is only around 80%, with a specificity of around 97% [16]. Also, it requires technical expertise that may not always be available, and there is some inter-observer variation.
Brain tissue oxygen (PbtO2) monitoring has been used for monitoring brain oxygenation in various centers for more than 15 years [17] and use is increasing in general neurocritical care units. Apart from insertion of the catheter, it requires no further technical expertise to manage, so it has become attractive for continuous monitoring. PbtO2 monitoring has now been formally introduced at the level of an option in the current guidelines for the management of severe traumatic brain injury (TBI) in adults [18]. To date, PbtO2 has mostly been reported in the management of adult patients with acute brain injury, for example, secondary to TBI or subarachnoid hemorrhage. However, data are also emerging on the use of PbtO2 in children. Episodes of low brain tissue oxygen tension (PbtO2) are associated with poor outcome [19–22] and PbtO2 appears to decrease in parallel with CBF [22, 23]. We recently published evidence that low PbtO2 is independently associated with poor outcome in children [20]. Little data are available on the relationship between PbtO2 and brain death at present. There are currently only two reports in adult patients, both of which suggest that a PbtO2 of 0 mmHg has a strong correlation with brain death [24, 25]. PbtO2 changes in brain death have not been reported in children. Yet this is important given some of the difficulties described in diagnosing brain death in children. Given that use of PbtO2 appears to becoming more widespread, data on the association between PbtO2 and brain death in children would be useful. Therefore, we examined the changes in PbtO2 in a series of children with acute neurological insults who developed brain death while being monitored.
Methods
Data were retrospectively examined from a prospectively maintained database of patients who received intracranial monitoring in the Red Cross War Memorial Children’s Hospital between June 2006 and December 2008. Ethics approval was granted by the Human Ethics Board of the University of Cape Town. Intracranial monitoring was indicated in patients with TBI if their post-resuscitation Glasgow Coma Scale (GCS) was ≤8 or deteriorated to this level after admission. For patients with other pathologies causing coma with the need for intubation and ventilation, the decision to use intracranial monitoring was made based on the anticipated risk of cerebral ischemia and raised intracranial pressure (ICP).
Intracranial monitoring comprised an intracranial pressure monitor (Codman ICP Express [Codman, Raynham, MA] or Camino [Integra Neurosciences, Plainsboro, NJ]) and PbtO2 monitor (Licox® [Integra Neurosciences, Plainsboro, NJ]). Patients with TBI were managed as previously described [19], based on the current recommendations for the management of severe brain injury in children [26]. PbtO2 monitoring has been used at our hospital since June 2006 for patients who undergo ICP monitoring. PbtO2 catheters were inserted into normal-appearing right frontal white matter if there were no localized lesions, or into the hemisphere with the greater swelling or that contained a focal lesion. Data from patients were collected after a 2-h run-in period to avoid potential artifacts from a stabilizing catheter, and thereafter on an hourly basis. Hourly PbtO2 measurements and the maximum and minimum values in the previous hour were recorded.
Brain death was diagnosed according to standard clinical examination and a local protocol. This required that (1) a definitive diagnosis for cause of coma had been made, (2) that the patient had a head CT scan, which was in keeping with the diagnosis of brain death (for example, showing signs of massive edema, herniation, and extensive hypodensity, or ‘black brain’), and either (3a) two negative brain stem examinations (including apnea testing) with an observation period of twelve hours between, or (3b) one negative brain stem examination and a SPECT scan demonstrating that there was no CBF. The diagnosis of brain death was based on these factors, with results of PbtO2 monitoring playing no role. Brain stem testing was carried out as per the UK guidelines for diagnosing brain death [2, 3] by two senior registrar or consultant level doctors.
SPECT scanning was performed using Tc-99m HMPAO as a radiopharmaceutical and a Philips Axis camera.
Patients who died from cardiovascular instability after only one set of tests confirming brain death and a CT scan consistent with the diagnosis were also included in the analysis. This is because death occurred after a period of cessation of brain stem function in the presence of ongoing cardiac output, as indicated by consistent clinical examination and head CT.
Results
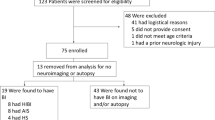
Eighty-five patients received intracranial monitoring at the Red Cross Children’s Hospital between June 2006 and December 2008. Mean age was 6 years (range 5 months to 14 years old) and 75% were male. Eight-one percent were TBI-related, mainly secondary to road traffic accidents. Other causes included meningitis (including tuberculous meningitis), subarachnoid hemorrhage, and Reyes syndrome.
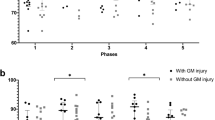
Brain death occurred in five patients while PbtO2 monitoring was in progress. In all cases, PbtO2 deteriorated to 0 mmHg before the clinical diagnosis of brain death was made. The deterioration of PbtO2 to 0 mmHg in patients who developed brain death was characterized also by a failure of response to an increased inspired fraction of oxygen. Therefore, all patients who developed brain death had PbtO2 of 0 mmHg at the time of brain stem testing, and when PbtO2 decreased to 0 mmHg it did not recover in any of the cases. Attempts to increase PbtO2 by increasing the FiO2 are reflected in the high arterial partial pressure of oxygen values at the time of first negative brainstem examination in some of these patients (Table 1). While PbtO2 may be affected by several systemic factors, we did not believe that the decrease in PbtO2 we observed in brain dead patients was related to severe anemia, systemic hypoxia or hypotension (Table 1). Of note, the initial PbtO2 in all of these patients was also low (Table 1), which may reflect the severity of their disease on admission to the ICU.
In one patient, an initial brain stem examination elicited only a corneal reflex with no gag or pupillary reflexes and no response to central pain. PbtO2 remained at 0.8 mmHg during this examination. This patient was retested 1 h later, after PbtO2 had decreased to 0 mmHg, and the complete brain stem examination was negative. Of note, brain temperature in this patient progressively decreased during this period; at the time of the second clinical examination, it was 2 °C lower than core body temperature. Four hours later, the patient died of cardiovascular instability.
Other patients (non-brain dead)
By contrast, PbtO2 was never 0 mmHg in any patient who did not develop brain death. PbtO2 did not deteriorate to 0 mmHg in any of our other patients who were monitored during this period (n = 80), even when infarction occurred and the PbtO2 catheter was located within the infarcted area. For these other 80 patients, initial PbtO2 was 17.3 (range 1.8–64.5) mmHg and the lowest PbtO2 was 11.5 (range 0.2–29.1) mmHg. Of these patients, five experienced an episode where PbtO2 was less than 2 mmHg, and three experienced PbtO2 less than 1 mmHg, but in all patients these episodes were transient and responded promptly to intervention. None of these patients experienced a PbtO2 of 0 mmHg. The lowest PbtO2 recorded in a patient who survived was 0.2 mmHg. This episode, occurred for less than 10 min, was not associated with absent brainstem reflexes, and like episodes of very low PbtO2 in other patients, responded promptly to intervention. Episodes of low PbtO2 in general though, were associated with poor outcome as is described in our other reports [20].
Discussion
In this retrospective case series, PbtO2 decreased to 0 mmHg in all children who developed brain death. By contrast, although episodes of low PbtO2 occurred in other patients, it did not deteriorate to 0 mmHg in any of our other patients who did not develop brain death. These preliminary results suggest that PbtO2 of 0 mmHg is associated with brain death in children, similar to that in adults, and may be useful corroborative information in the diagnosis of brain death in children.
These findings are limited by the sample size; therefore, these results should be regarded as preliminary. Brain death is not a common occurrence in most single centers; and PbtO2 monitoring is not yet widely used. In fact, in the two adult case series, which also studied the association between PbtO2 and brain death, refer to only 6 and 11 patients [24, 25].
The actual area of brain tissue monitored by the PbtO2 monitor is small, and conceivably it may not reflect global brain tissue oxygen tension. However, when PbtO2 is measured in normal-appearing white matter the value appears to reflect global brain oxygenation [23]. Therefore, probe placement is critical, and confirmation of probe position using repeat CT head is an important part of the procedure.
Another potential source of error is in the diagnosis of brain death. A limitation of any retrospective study is that local criteria for a diagnosis may differ from that used at other centers. In the case of diagnosing brain death, there are no internationally recognized criteria, but instead a number of partially conflicting local guidelines [2, 4, 12]. The Red Cross Children’s Hospital uses a pragmatic approach, which is flexible and satisfies the criteria that irreversible cessation of brain stem function has occurred (see “Methods” section). This approach could be criticized because it is not the same as other standards, but it would be highly unlikely that any of the patients in this series were not brain dead. Therefore, it is likely that the deterioration of PbtO2 to 0 mmHg in these patients reflects a pattern that is seen after brain death ensues, particularly when one also considers the data from the adult studies. However, whether the decrease of PbtO2 to 0 mmHg reliably occurs with brain death, with a sufficiently high sensitivity and specificity for it to be used as an ancillary investigation for brain death, requires further study.
The use of PbtO2 monitoring in supporting the diagnosis of brain death has several advantages. First, PbtO2 is being increasingly used in the management of TBI and subarachnoid hemorrhage in adults in many centers, and experience in children is increasing [20, 28, 29]. The monitoring is continuous and is sensitive to changes in CBF and tissue oxygenation [30–32]. When cerebral perfusion pressure and CBF decrease, PbtO2 consistently decreases. Current methods used to support or confirm the diagnosis of brain death require technical expertise or transfer of the patient out of the neurocritical care unit. Second, the time period needed to confirm brain death may be shorter. This may help to partially decrease parent’s anxiety and suffering as well as minimizing ischemic damage to transplantable organs.
However, many questions remain regarding the use of PbtO2 monitoring in the confirmation of brain death in children. First, as discussed above, in the few cases where CBF does not fall to 0 ml/min despite brain death [22, 23], it is possible that PbtO2 may also fail to decrease to 0 mmHg. A larger sample of patients with results of both CBF and PbtO2 is needed to confirm or refute this hypothesis, as brain death with continued CBF is thought to occur in less than 10% of clinically brain dead patients 24 h after loss of brainstem reflexes [15]. In this circumstance, PbtO2 may be a surrogate marker of CBF, with a similar sensitivity for the diagnosis of brain death. Another possibility is that PbtO2 may not fall to 0 mmHg due to residual cellular oxygen after cellular death. This is unlikely, particularly in view of the fast aerobic metabolism of neurones and glial cells, and because brain death is usually associated with progressive ischemia and anaerobic glycolysis. However, more data are needed to clarify this. Second, there is a possibility that poor probe placement could lead to an erroneous measurement of PbtO2 of 0 mmHg, for example, if the probe tip is within an isolated infarct. Such results have been observed by Smith et al. [24], but have never been observed in this institution. This problem would be excluded by routine head CT scan following probe placement, as is performed when PbtO2 is monitored and so should not present any diagnostic uncertainty. Third, the effects of a transient decrease of PbtO2 to 0 mmHg for less than 30 min as described by Smith et al. [24] is not known in children. In our sample, all patients with a PbtO2 of 0 mmHg for any length of time remained at 0 mmHg and did not respond to intervention. However, it may be possible that some patients might experience a transient PbtO2 of 0 mmHg without developing brain death. Given that brain infarction is a function both of the depth of ischemia and the duration thereof, it would be reasonable to presume that this situation would not persist for long without irreversible brain injury occurring. Indeed, in our cohort only three patients who did not develop brain death had a PbtO2 of less than 1 mmHg, and in each case this was for less than 15 min. All of our patients who had a PbtO2 of 0 mmHg also experienced brain death. It is possible that a brief episode of 0 mmHg may not be associated with brain death; however, further study is needed to determine the minimum length of time of PbtO2 = 0 mmHg that is associated with brain death.
Conclusions
In this small case series, PbtO2 of 0 mmHg shows a strong association with brain death in children, consistent with evidence in adult studies [24, 25]. In addition to its clinical utility in managing patients at risk of hypoxic/ischemic injury, PbtO2 monitoring has the potential to support the diagnosis of brain death in severely brain injured children. However, further study is required to answer several remaining questions regarding such practice, and in particular to provide information on the sensitivity and specificity of PbtO2 data in the diagnosis of brain death in children. Use of PbtO2 monitoring may help to add clarity to the challenge of diagnosing brain death in children and help elucidate some of the mechanisms of irreversible injury to the brain.
References
President’s Commission. Guidelines for the determination of death. Report of the medical consultants on the diagnosis of death to the President’s Commission for the Study of Ethical Problems in Medicine and Biomedical and Behavioral Research. JAMA 1981; 246(19):2184–6.
Academy of Medical Royal Colleges. A code of practice for the diagnosis and confirmation of death. London: Academy of the Medical Royal Colleges; 2008.
Academy of Medical Royal Colleges. A code of practice for the diagnosis of brain stem death. London: Department of health; 1998.
Australian and New Zealand Intensive Care Society. Recommendations on brain death and organ donation. 2nd ed. Melbourne: Australian and New Zealand Intensive Care Society; 1998.
Task Force for the Determination of Brain Death in Children. Guidelines for the determination of brain death in children. Task Force for the Determination of Brain Death in Children. Arch Neurol. 1987;44(6):587–8.
Green JB, Lauber A. Return of EEG activity after electrocerebral silence: two case reports. J Neurol Neurosurg Psychiatry. 1972;35(1):103–7.
Montes JE, Conn AW. Near-drowning: an unusual case. Can Anaesth Soc J. 1980;27(2):172–4.
Shemie SD, Pollack MM, Morioka M, Bonner S. Diagnosis of brain death in children. Lancet Neurol. 2007;6(1):87–92.
Baines PB. Diagnosis and management of brain death in children. Curr Paediatr. 2005;15(4):301–7.
Kohrman MH, Spivack BS. Brain death in infants: sensitivity and specificity of current criteria. Pediatr Neurol. 1990;6(1):47–50.
Wijdicks EF. Brain death worldwide: accepted fact but no global consensus in diagnostic criteria. Neurology. 2002;58(1):20–5.
Bell MDD, Moss E, Murphy PG. Brainstem death testing in the UK—time for reappraisal? Br J Anaesth. 2004;92(5):633–40.
Heran MK, Heran NS, Shemie SD. A review of ancillary tests in evaluating brain death. Can J Neurol Sci. 2008;35(4):409–19.
Blend MJ, Pavel DG, Hughes JR, et al. Normal cerebral radionuclide angiogram in a child with electrocerebral silence. Neuropediatrics. 1986;17(3):168–70.
Drake B, Ashwal S, Schneider S. Determination of cerebral death in the pediatric intensive care unit. Pediatrics. 1986;78(1):107–12.
Monterio LM, Bollen CW, van Huffelen AC, Ackerstaff RGA, Jansen NJG, van Vught AJ. Transcranial Doppler ultrasonography to confirm brain death: a meta analysis. Intensive Care Med. 2006;32:1937–44.
Maas AI, Fleckenstein W, de Jong DA, et al. Monitoring cerebral oxygenation: Experimental studies and preliminary clinical results of continuous monitoring of cerebrospinal fluid and brain tissue oxygen tension. Acta Neurochir Suppl (Wien). 1993;59:50–7.
Guidelines for the management of severe traumatic brain injury. J Neurotrauma 2007; 24(suppl 1): chapter X. doi: 10.1089/neu.2007.9999.
Figaji AA, Fieggen AG, Argent AC, Leroux PD, Peter JC. Does adherence to treatment targets in children with severe traumatic brain injury avoid brain hypoxia? A brain tissue oxygenation study. Neurosurgery. 2008;63(1):83–91.
Figaji AA, Zwane E, Thompson C, et al. Brain tissue oxygen tension monitoring in pediatric severe traumatic brain injury: part 1: relationship with outcome 2009 (epub before print).
Stiefel MF, Udoetuk JD, Spiotta AM, Gracias VH, Goldberg A, Maloney-Wilensky E, et al. Conventional neurocritical care and cerebral oxygenation after traumatic brain injury. J Neurosurg. 2006;105(4):568–75.
Valadka AB, Gopinath SP, Contant CF, Uzura M, Robertson CS. Relationship of brain tissue PO2 to outcome after severe head injury. Crit Care Med. 1998;26(9):1576–81.
Scheufler KM, Röhrborn HJ, Zentner J. Does tissue oxygen-tension reliably reflect cerebral oxygen delivery and consumption? Anesth Analg. 2002;95:1042–8.
Smith ML, Counelis GJ, Maloney-Wilensky E, Stiefel MF, Donley K, LeRoux PD. Brain tissue oxygen tension in clinical brain death: a case series. Neurol Res. 2007;29(7):755–9.
Palmer S, Bader MK. Brain tissue oxygenation in brain death. Neurocrit Care. 2005;1(2):17–22.
Adelson PD, Bratton SL, Carney NA, et al. Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents. Chap. 4. Resuscitation of blood pressure and oxygenation and prehospital brain-specific therapies for the severe pediatric traumatic brain injury patient. Pediatr Crit Care Med. 2003;4:S65–7.
Lang EW, Mulvey JM, Mudaliar Y, Dorsch NWC. Direct cerebral oxygenation monitoring—a systematic review of recent publications. Neurosurg Rev. 2007;30(2):1437–2320.
Stiefel MF, Udoetuk JD, Storm PB, et al. Brain tissue oxygen monitoring in pediatric patients with severe traumatic brain injury. J Neurosurg. 2006;105:281–6.
Narotam PK, Burjonrappa SC, Raynor SC, et al. Cerebral oxygenation in major pediatric trauma: its relevance to trauma severity and outcome. J Pediatr Surg. 2006;41:505–13.
Valadka AB, Hlatky R, Furuya Y, et al. Brain tissue PO2: correlation with cerebral blood flow. Acta Neurochir Suppl. 2002;81:299–301.
Scheufler KM, Lehnert A, Rohrborn HJ, et al. Individual value of brain tissue oxygen pressure, microvascular oxygen saturation, cytochrome redox level, and energy metabolites in detecting critically reduced cerebral energy state during acute changes in global cerebral perfusion. J Neurosurg Anesthesiol. 2004;16:210–9.
Doppenberg EM, Watson JC, Broaddus WC, et al. Intraoperative monitoring of substrate delivery during aneurysm and hematoma surgery: initial experience in 16 patients. J Neurosurg. 1997;87:809–16.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Figaji, A.A., Kent, S.J. Brain Tissue Oxygenation in Children Diagnosed With Brain Death. Neurocrit Care 12, 56–61 (2010). https://doi.org/10.1007/s12028-009-9298-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-009-9298-5