Abstract
We aim to harness the natural humoral immune response by various technologies to get novel biomarkers. A complex antibody analysis in sera and in the tumor microenvironment leads to reveal tumor-specific antibodies. More strategies were introduced to select the most effective one to identify potential tumor antigen-binding capacity of the host. Epstein–Barr virus transformation and cloning with limiting dilution assay, magnetic cell sorting and antibody phage display with further methodological improvements were used in epithelial and neuroectodermal cancers. Column-purified sera of patient with melanoma were tested by immunofluorescence assay, while sera of further melanoma patients were processed for membrane-binding enzyme-linked immunosorbent assay. Some supernatants of selected B cell clones and purified antibodies showed considerable cancer cell binding capacity by immunofluorescence FACS analysis and confocal laser microscopy. Our native tumor cell membrane preparations helped to test soluble scFv and patients’ sera for tumor binder antibodies. A complex tumor immunological study was introduced for patients with melanoma (ethical permission: ETT TUKEB 16462-02/2010); peripheral blood (n = 57) and surgically removed primary or metastatic tumors (n = 44) were gathered and processed at cellular immunological level. The technological developments proved to be important steps forward to the next antibody profile analyses at DNA sequence level. Cancer cell binding of patient-derived antibodies and natural immunoglobulin preparations of pooled plasma product intravenous immunoglobulins support the importance of natural human antibodies. Important cancer diagnostics and novel anticancer strategies are going to be built on these tools.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Revealing novel cancer-targeting biomarkers is a great challenge with an urging need worldwide. In cancer types with more pronounced metastatic potential, the question becomes even more exciting, due to the lack of early diagnostics and real effective therapeutics. The outcome in metastatic phases of any cancer types but especially malignant melanomas is extremely poor. Immunotherapy holds great promise recently with new targeted molecules suitable to enhance the cancer hosting immune system’s antitumor activity [1–3]. The majority of therapeutical investigations took advantage of protein-based and HLA-restricted tumor-associated antigens and enhancing cytotoxic T cell responses [4, 5]. These developments have allowed to establish a new treatment paradigm targeting interactions between the tumor cell and its microenvironment [6, 7]. Whereas protective antitumor immunity exists, promalignancy factors might also be found among the immune components [6, 8]. The dichotomy between the humoral and cellular arms of tumor immunity and a Th1/Th2 imbalance occurring in tumor-bearing hosts have great impact [6, 9, 10]. Our tumor immunological hypothesis focuses on the cancer host’s potential antitumor humoral immune response. As specific aim, we harness natural humoral immune response by our novel immunological and molecular genetic techniques developed. In this context, revealing cancer-associated glycolipid and glycoprotein antigens with unique characteristics of abnormal glycosylation on cancerous cells is possible. “Abnormal glycosylation that defines cancer” [11–13] and the importance of anti-ganglioside antibodies have been investigated with “flames of interest” [14–16]. Thanks to the novel technological arsenal to detect these molecules [17, 18] and the detailed biochemical characterization resulting in glycoimmunomics [13, 19], there seems to come up to a “renaissance” in the topic. The hypothesis-based “proof of principle” in the rare type of medullary breast carcinomas (MBC) [17, 20] and investigations with other solid tumors fostered the testing of antibody profile in cancerous patients. Immunological and biochemical assays on various cancerous patients’ antibody response provided essential information and spotlighted the importance of the field [21, 22]. Based on previous studies [17, 23], we have a good chance to define unique GD3 glycosphingolipid-specific antibody fragments from the tumor microenvironment. We suppose that cancerous patients’ comprehensive investigations in terms of antibody profile might reveal more antibodies with important tumor-associated antigen specificities. In our view, autoantibodies derived from patients with autoimmune conditions may be utilized for cancer treatment [24–26]. In addition to these therapeutic modalities, intravenous immunoglobulin (IVIG) is an example of how the autoimmune–cancer relationship generated novel treatment for cancer [27, 28]
The efficacy of IVIG as an inhibitor of tumor spread was shown in experimental murine models of melanoma, sarcoma and carcinoma [29, 30]. Referring to important pioneering studies that resulted in the beneficial effect of IVIG on cancer occurrence [31–33], it is believed that patients’ or healthy controls’ blood might contain natural immunoglobulins with specificity of interest. The tumor infiltrating B cell (TIL-B) phage display technique [34] opened a possibility for epithelial carcinomas or neuroectodermal cancers, to detect novel tumor binder antibody fragments and reveal the antibody repertoire of the cancer-bearing host. We merged this technology development into a broad tumor immunological protocol for patients with melanomas.
Materials and methods
Patients and samples
Minor samples (taken by punch biopsy) from surgically removed cancerous tissues of patients with melanomas and some patients with colorectal and breast cancers were obtained by Ethycal permission, received from Ministry of Human Resources in Hungary, Hungarian Medical Research Council (ETT TUKEB 16462-02/2010). Patients signed their formal consent for the cancerous tissue and peripheral blood specimen for the study. Further results according to the program goals are still summarized (two manuscripts in preparation). Primary melanoma [(nodular melanoma (NM) or superficial spreading melanoma (SSN)] and metastatic tissues (n = 44 and peripheral blood of the patients (n = 57) have been investigated by various immunological and molecular techniques, and are at time being under further processing and/or evaluation.
Cell lines
Melanoma and colorectal cancer cell lines (SK-Mel28, HCT 116) were purchased from American Type Cell Collections (ATCC), while two others (M1/9 melanoma and IDC Tu2) were developed in the previous Department of Tumor Immunology at National Institute of Oncology [35] and by a previous Department and Institution of the first author, respectively. Cells were maintained in steady-state culture conditions in RPMI 1640 (supplemented with 5 or 10 % FBS and antibiotics penicillin/streptomycin). After cultures were grown to confluent, cells were harvested by EDTA 0.02 % (Sigma), washed and checked for viability (by trypan blue (0.4 %) exclusion) before processing to any cellular immunological assays or using as controls.
Ficoll-Uromiro density gradient separation
Ficoll density gradient centrifugation was used to obtain peripheral blood mononuclear cells for further Epstein–Barr virus transformation and/or molecular techniques, and also, frozen vials were stored. Sera of native blood were collected and tested for tumor antigen binding and also kept for further enzyme-linked immunosorbent assay (ELISA) and other techniques indicated in the protocol of the complex panel assay for immunoglobulin repertoire analysis.
Epstein–Barr virus transformation and cloning
Epstein–Barr virus transformation and cloning with limiting dilution assay (LDA) were performed in selected cases, according to previous techniques [20].
Magnetic cell sorting (MACS)
Using super-paramagnetic microbeads coupled with specific antibodies leads to effective separation of cells of interest. Out of a large scale of technology improvements provided, we used the technology of Miltenyi. In some cases with colorectal carcinomas, negative or positive selection with anti-CD45-coated microbeads or anti-CEA-coated microbeads were used with the magnetic separator and reagent kit provided (Miltenyi Biotech).
Antibody phage display technology
Total RNA was prepared from B cells in the tissue and peripheral blood RNA extraction with column kit (RNeasy Qiagen and Total RNA isolation Kit, Macherey–Nagel, Germany) or standard method [36]. cDNA was reverse transcribed from tumor and/or peripheral blood B cells, and antibody variable region genes were amplified according to the technique developed earlier [17, 20, 34].
Column purification for immunoglobulin enrichment
Column purification for immunoglobulin enrichment was performed according to the previously developed and published technique [37].
Immunofluorescence assay with FACS analysis and confocal laser microscopy
Cancerous cell suspensions were reacted with antibody containing supernatants of B cell cultures originated from colorectal cancer, breast cancer or melanomas. Column-purified fractions of immunoglobulins from sera of a melanoma patient were also tested in different ways. Indirect immunofluorescence (IF) assay was set up using fluorescein isothiocyanate (FITC)-labeled antihuman IgG or IgGAM, and TRITC-labeled IgM from Sigma and Dako. Antibody fragments from human origin developed by phage display were visualised with the help of the vector tags and antimouse Fab`2 IgG PE or antimouse IgG Fab`2FITC/Dako. The CyFlow SL-Green, Partec (Munster, Germany), cytofluorimeter was used. Forward and side scatter dot plots and immunohistological curves were evaluated for antigen expression intensity and percent of positive cells with data analyzing software FloMax (Partec) or (BD FACS Diva software at sorting by BD).
Immunofluorescence: confocal laser microscopy
Mainly, chamber slide technique (LAB-TEK Brand Products, Nalgen Nunc, Naperville, US) or cytospins made from the cell lines were used and fixed in 4 % paraformaldehyde (PFA) PBS (15 min, 20 °C). After blocking (3 % BSA PBS), IF labeling was performed. After labeling reaction with first and second antibodies [antihuman IgG FITC, antimouse IgG FITC (Fab’) 2 (DAKO)] or biotinylated rabbit antimouse IgG [or IgG (Fab`)2] (1:100) and streptavidin-FITC (Vector Laboratories, Burlingame, CA, USA), a short propidium iodide nuclear staining was done. IF confocal laser microscopy (Nikon Eclipse E600, Nikon Model C1-Lu3, Tokyo, Japan) was used for detection.
Tumor immunological panel assay
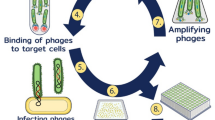
Various types of breast carcinomas and melanomas were processed for cellular and molecular studies. In selected cases, peripheral blood mononuclear cells and sera were processed and stored. Immune reactivity was tested against cancerous cells and tissues. This novel panel assay according to the protocol (Fig. 1) has been offered to the patients, and tissue and blood samples gathered and processed for the investigations indicated.
Results
Novel strategy to reveal the host’s antitumor humoral immune response
A protocol was set up to investigate patients with various epithelial or neuroectodermal cancers to reveal the host’s humoral immune response toward essential tumor-associated antigens (Fig. 1). The protocol was based on our previous technical developments and initial experiments related to various tumors of epithelial and neuroectodermal origin. Supernatants of EBV-transformed, cloned or long-term-cultured B cells gave positive reactions with cancer cells, evidencing the presence of potentially tumor binder antibodies. Tumor binder antibodies could be revealed by IF assay in various cancers (Fig. 2). Supernatants of EBV-transformed B cell clones originated from breast carcinomas and long-term-cultured immune B cells showed considerable tumor-binding capacity. Tumor cell binding could be detected in HCT116 colorectal cancer cells (CRC) (Fig. 2) and in other cancer types (data shown elsewhere, manuscript in preparation to Journal for ImmunoTherapy of Cancer, 2014/2015).
Technological improvements to select human antibodies with glycolipid-based cancer-associated antigen specificities
EBV transformation and limiting dilution assay-based selection of tumor binder antibodies
Epstein–Barr virus-transformed cultures were set up from peripheral blood B lymphocytes and from Ficoll-Uromiro density gradient-enriched peripheral blood mononuclear fractions (PBMN) obtained of lymph node melanoma metastases. Supernatants of propagated cultures are under testing against “tumor membrane ghost”—ELISA technique, standardized previously.
Affinity column-purified antibodies from patients’ sera
Pooled sera taken from a patient with malignant melanoma with his/her scientifically explained formal consent were purified on a Sepharose G column and eluted and protein concentrations were defined. The IgG1 antibody subtype preparations gave positive reaction with melanoma cell lines in indirect IF FACS (Fig. 3a–f) and chamber slides of SK-Mel 28 in IF confocal laser microscopy (Fig. 3g, h). The fact that strong melanoma binding capacity could be detected with purified IgG originated from melanoma patient’s sera supports the notion to continue this type of research in an extensive and more quantitative form.
Testing of affinity column-purified immunoglobulin fractions originated from melanoma patient’s sera by indirect immunofluorescence FACS analyses gave positive reactions in higher concentrated fractions (IF positivity: 15.9 and 43.8 %) (a, e) compared to negative control values (IF: 3.4 %, 5.0 % respectively) (b, d, f) and lower concentrations (c) that was proved by confocal laser microscopy (g, h)
Tumor infiltrating B cell single-chain Fv antibody fragments
The TIL-B antibody fragment phage display technology involving the “tumor membrane ghost”—ELISA technique and the novel native tumor cell membrane preparation technique developed and standardized proved to be an effective technology both for epithelial and for neuroectodermal cancers. The technique is useful to reveal tumor binder antibody variable regions and gave evidence that immunoglobulin repertoire of patients with breast carcinomas and also melanomas (Fig. 4a) containing antibodies specific for the normally less immunogenic sialylated glycolipids is associated with cancerous tissues (Fig. 4b, c). It is hereby suggested that the technology has the potential to reveal those antibody specificities that characterize abnormal glycosylation-based tumor antigenic epitopes. The ScFv antibody technology that was previously developed according to the references has been performed in selected cases of the present patient cases of melanomas. Data are under evaluation and preparation for the Journal of Autoimmunity, 2014.
a Generation and testing for antigen specificity of single-chain Fv immunoglobulin fragments from tumor infiltrating B lymphocytes. b Selected ScFv G anti-GD3 antibody fragment gave positive reaction with SK-Mel 28 melanoma cell line in chamber slide culture–indirect immunofluorescence assay and confocal laser microscopy. c Selected ScFv G anti-GD3 antibody fragment gave positive reaction with invasive ductal breast carcinoma primary cultures (IDC TU2 cell line) in an immunofluorescence cytospin assay and confocal laser microscopy
Characteristic features of disialylated glycosphingolipids in malignant melanomas: antibody profile analysis
Immunofluorescence FACS analysis on various cancerous cells
The author’s main interest became focused on the ganglioside profile of various carcinomas, and how patients’ humoral immune response follows the increased expression of these tumor-associated antigens. In this respect, the measured strong disialylated glycosphingolipid antigen positivity on melanomas and breast carcinomas and on tissue sections serve as a perfect tool to evaluate the antibody profile of patients, investigating either the peripheral blood or the tumor microenvironment (Fig. 5).
Immunohistochemistry on cancerous tissue microarrays
After successfully overcoming some technical obstacles appearing when glycolipid antigens are tested, we managed to standardize the process, to be able to evaluate patients’ stored formalin-fixed paraffin-embedded cancerous tissue materials. Immunohistochemistry staining, when using sensitive detection systems resulted in unambiguous positive reaction in cancerous tissues and negative reaction in controls (data will be published elsewhere, manuscript ready for submission to “Blood”, 2014).
Intravenous immunoglobulins, containing natural antibodies influence proliferation of cancer cell
Significant antiproliferative characteristics of relatively high IVIG concentration treatment were measured in 2-(4,5-dimethyl-2-thiazolyl)-3,5-diphenyl-2H-tetrazolium bromide MTT cell proliferation assay with invasive tough cancers like melanoma (SK-Mel 28, M1/9). The reduction in malignant cell growth was comparable to that of selected positive controls and selected other immune modulators (peptides and alpha interferon) tested (Table 1). IVIG preparations’ characteristic cancer cell binding capacity postulates that a substantial amount of potentially antitumor antibodies is available there.
Discussion
The previously developed biotechnological processes together with further technical improvements and the present new strategy proved to be of great help to reveal novel biomarkers at the tissue and the sera level. All that provided this way a technological background we may readily use for antibody profile analysis of the patients. The more so, as this kind of complex panel assay for diagnostics has not yet been conducted in the clinic. However, our results show that it seems to hide yet great potentials for cancer research, especially its immunological aspects and chances for revealing its relations with autoimmunity also. The broad potentials of the technology may be mostly proved by the fact that not only in the case of melanoma, but also in breast cancer, neuroblastoma and even colorectal carcinoma, we could make important related achievements through TIL-B phage display or Epstein–Barr virus transformation. The power of antibody profile analysis seems to be well supported by an affinity column study of melanoma patient’s sera that showed a considerable tumor binding capacity, when tested in different ways against allogen melanoma cells and tissue sections.
The present antibody profile analysis involves very new techniques that also enable to enhance the great capacity of the immune system. Lacking these strategies would never reveal the kind of results we obtained in the field. All that enabled a new arm of the diagnostical protocol, related to tumor-cell-surface-associated novel TAA of glycolipid or glicoprotein origin. Those potentials for diagnostic relevancy became most obvious, when successfully setting up and standardizing the detection of glycosphingolipid-based structures, namely also disialylated glycosphingolipids.
Primary and secondary tumor tissue sections were tested, and also tissue microarrays of IHC-proved melanoma were prepared, to test cancer cells in its tumor microenvironment surroundings. The great power of human antibody repertoire of the peripheral blood could be further well proved with the previous and present intraveous immunoglobulin-related data. Not only could the binding of IVIG preparation be stated, but also a considerable amount of functional analysis could be done with IVIG. All that well supports the pioneering data [24, 25, 33] in this respect. Relatively high levels of IVIG had considerable anti-proliferative effect on various tumor cells. It seems that the beneficial anti-proliferative effect is not only bound to melanoma, an especially tough type among all tumors, but also other important ones like pancreatic carcinoma or prostate cancer (experimental data available, not shown due to page limits). The present methodological flowchart and immunological protocol have provided a possibility to investigate even further, namely to harness the antitumor potentials for additional, clinically useful developments.
A complex panel assay being set up this way and shown here became essential to reveal novel aspects about the host’s anti-tumor immune response.
Our specific aim is to show how the patients’ clinical follow-up can be compared to that of the antibody profile. This guides toward a new field of tumor immunology, which would give an impact to find out more important aspects of clinically relevant, tumor immunological pathways and important relations to autoimmunity and its importance in potential anticancer strategy developments.
Abbreviations
- ATCC:
-
American Type Cell Collections
- EBV:
-
Epstein–Barr virus
- GD3:
-
Ganglioside 3 type disialylated glycosphingolipids
- ELISA:
-
Enzyme-linked immunosorbent assay
- FDA:
-
Food and Drug Administration
- JITC:
-
Journal for ImmunoTherapy of Cancer
- HJLCT:
-
Harry J Loyd Charitable Trust
- HSA:
-
Human serum albumin
- IDC:
-
Invasive ductal carcinoma of the breast
- IF:
-
Immunofluorescence
- IHC:
-
Immunohistochemistry
- alphaIFN:
-
Interferon alpha
- IVIG:
-
Intracenous immunoglobulin
- LDA:
-
Limiting dilution assay
- MACS:
-
Magnetic cell sorting
- MBC:
-
Medullary breast carcinoma
- MTT:
-
2-(4,5-Dimethyl-2-thiazolyl)-3,5-diphenyl-2H-tetrazolium bromide
- ETT-TUKEB:
-
Ministry of Human Resources in Hungary, Hungarian Medical Research Council
- NM:
-
Nodular melanoma
- PFA:
-
Paraformaldehyde
- PBMC:
-
Peripheral blood mononuclear cells
- SSM:
-
Superficial spreading melanoma
- TAA:
-
Tumor-associated antigen
- TIL-B:
-
Tumor infiltrating B cells
References
Ascierto PA, Marincola FM. What have we learned from cancer immunotherapy in the last 3 years? J Transl Med. 2014;12(1):141. doi:10.1186/1479-5876-12-141.
Fox BA, Schendel DJ, Butterfield LH, Aamdal S, Allison JP, Ascierto PA, et al. Defining the critical hurdles in cancer immunotherapy. J Transl Med. 2011;9(1):214. doi:10.1186/1479-5876-9-214.
Shurin MR, Umansky V, Malyguine A, Hurwitz AA, Apte RN, Whiteside T, et al. Cellular and molecular pathways in the tumor immunoenvironment: 3rd Cancer Immunotherapy and Immunomonitoring (CITIM) meeting, 22–25 April 2013, Krakow, Poland. Cancer Immunol Immunother. 2014;63(1):73–80.
Pedicord VA, Montalvo W, Leiner IM, Allison JP. Single dose of anti-CTLA-4 enhances CD8+ T-cell memory formation, function, and maintenance. Proc Natl Acad Sci USA. 2011;108(1):266–71. doi:10.1073/pnas.1016791108.
Rosenberg SA. IL-2: the first effective immunotherapy for human cancer. J Immunol. 2014;192(12):5451–8. doi:10.4049/jimmunol.1490019.
Witz IP. Yin-Yang activities and vicious cycles in the tumor microenvironment. Cancer Res. 2010;68:9–13.
Ferrone S, Whiteside TL. Histocompatibility antigens, tumor microenvironment and escape mechanisms utilized by tumor cells, chap. 2. In: Yefenof E, editor. Innate and adaptive immunity in the tumor microenvironment. New York: Springer; 2008. p. 35–51.
Maman S, Witz IP. The metastatic microenvironment, chap. 2. In: Shurin MR, Umansky V, Shurin MR, Malyguine A, editors. The tumor immunoenvironment. Dordrecht: Springer; 2013. p. 15–38.
Malyguine A, Umansky V, Shurin MR. Role of the immunological environment in cancer initiation, development and progression, chap. 1. In: Shurin MR, Umansky V, Shurin MR, Malyguine A, editors. The tumor immunoenvironment. New York: Springer; 2011. p. 1–12.
Shurin MR, Lu L, Kalinski P, Stewart-Akels AM, Lotze MT. Th1/Th2 balance in cancer, transplantation and pregnancy. Springer Semin Immunpathol. 1999;21:339–59.
Hakomori S, Kannagi R. Glycosphingolipids as tumor-associated and differentiation markers. JNCI. 1983;71:231–51.
Hakomori S. Glycosylation defining cancer malignancy: new wine in an old bottle. Proc Natl Acad Sci USA. 2002;99:10231–3.
Varki NM, Varki A. Diversity in cell surface sialic acid presentations: implications for biology and disease. Lab Invest. 2007;87(9):851–7.
Ravindranath MH, Ravindranath DMH. Human antiganglioside autoantibodies, chap. 37. In: Shoenfeld Y, Gerschwin ME, Meroni PL, editors. Autoantibodies, 2nd ed. Amsterdam: Elsevier; 2007. p. 277–83.
Livingston PO, Wong GY, Adluri S, Tao Y, Padavan M, Parente R, et al. Improved survival in stage III melanoma patients with GM2 antibodies: a randomized trial of adjuvant vaccination with GM2 ganglioside. J Clin Oncol. 1994;12:1036–44.
Irie RF, Matsuki T, Morton DL. Human monoclonal antibody to ganglioside GM2 for melanoma. Lancet. 1989;1(8641):786–7.
Kotlan B, Simsa P, Teillaud JL, Fridman WH, Toth J, McKnight M, et al. Novel ganglioside antigen identified by B cells in human medullary breast carcinomas: the proof of principle concerning the tumor-infiltrating B lymphocytes. J Immunol. 2005;175:2278–85.
Daniotti JL, Vilcaes AA, Demichelis VT, Ruggiero FM, Rodriguez-Walker M. Glycosylation of glycolipids in cancer: basis for development of novel therapeutic approaches. Front Oncol. 2013;3:306–18. doi:10.3389/fonc.2013.00306.
Ravindranath MH, Yesowitch P, Sumobay C, Morton D. Glycoimmunomics of human cancer: current concepts and future perspectives. Future Oncol. 2007;3:201–14.
Kotlan B, Gruel N, Zafrani B, Füredi G, Foldi J, Petranyi GG, et al. Immunoglobulin variable regions usage by B-lymphocytes infiltrating a human breast medullary carcinoma. Immunol Lett. 1999;65:143–51.
Conra K, Bachmann M. Autoantibodies. In: Shoenfeld Y, Gerschwin ME, Meroni PL, editors. Autoantibodies, summary 2/e. 2nd ed. Amsterdam: Elsevier; 2007. p. 423–35.
Vollmers HP, Brandlein S. Nature’s best weapons to fight cancer. Revival of human monoclonal IgM antibodies. Hum Antibodies. 2002;11:131–42.
Kotlan B, Toth J, McKnight M, Glassy MC. Characteristics of tumor gangliosides revealed by B cells infiltrating human breast carcinomas. Hum Antibodies. 2006;15(1, 2):9–13.
Sherer Y, Levy Y, Shoenfeld Y. IVIG in autoimmunity and cancer efficacy versus safety. Expert Opin Drug Saf. 2002;1:153–8.
Schwartz-Albiez R, Monteiro RC, Rodriguez M, Binder CJ, Shoenfeld Y. Natural antibodies, intravenous immunoglobulin and their role in autoimmunity, cancer and inflammation. Clin Exp Immunol. 2009;158:43–50.
Kotlan B, Stroncek DF, Marincola FM. Intravenous immunoglobulin-based immunotherapy: an arsenal of possibilities for patients and science. Immunotherapy. 2009;1(6):995–1015. doi:10.2217/imt.09.67.
Baharav E, Merimski O, Shoenfeld Y, Zigelman R, Gilbrud B, Yecheskel G, et al. Tyrosinase as an autoantigen in patients with vitiligo. Clin Exp Immunol. 1996;105(1):84–8.
Stein R, Witz IP, Ovadia J, Goldenberg DM, Yron I. CD5+ B cells and naturally occurring autoantibodies in cancer patients. Clin Exp Immunol. 1991;85(3):418–23.
Shoenfeld Y, Fishman P. Gamma-globulin inhibits tumor spreaf in mice. Int. Immunol. 1999;11:1247–52.
Merimsky O, Meller I, Moshe I, Bar-Yehuda S, Shoenfeld Y, Fishman P. A possible role for IVIg in the treatment of soft tissue sarcoma: a clinical case and an experimental model. Int J Oncol. 2002;20(4):839–43.
Fishman P, Shoenfeld Y. Intravenous immunoglobulin (IVIG) as an inhibitor of tumor growth: from autoimmunity to cancer, chap VII. In: Heidt PJ, Rusch VD, van der Waaij D, editors. Old Herborn University seminar monograph. Germany: Herborn Litrature, Herborn Dill; 2000. p. 93–107.
Shoenfeld Y, Levy Y, Fishman P. Shrinkage of melanoma metastases following high dose intravenous immunoglobulin treatment. IMAJ. 2001;3:698–9.
Shoenfeld Y, Krause I. IVIG for autoimmune, fibrosis and malignant conditions: our experience with 200 patients. J Clin Immunol. 2004;24:107–14.
Kotlan B, Glassy MC. Antibody phage display. Overview of a powerful technology that has quickly translated to the clinic. In: Aitken R, editor. Methods in molecular biology, vol. 562. Humana Press Inc.; 2009. p. 1–16.
Ladanyi A, Timar J, Bocsi J, Tovari J, Lapis K. Sex-dependent liver metastasis of human melanoma lines in SCID mice. Melanoma Res. 1995;5:83–6.
Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162(1):156–9.
Shoenfeld Y, Blank M, Branch DR, Vassilev T, Käsermann F, Bayry J, et al. IVIG pluripotency and the concept of Fc-sialylation: challenges to the scientist. Nat Rev Immunol. 2014;14(5):349. doi:10.1038/nri3401-c1.
Acknowledgments
We acknowledge the Harry J. Lloyd Charitable Trust Melanoma Research Award (2010) given to B. Kotlan, previous Fulbright No. 1206103 and OTKA T048933 Grants to B. Kotlan as well as her being supported by the Hungarian Cancer Foundation, Budapest, Hungary and a second Fulbright No. 1214104 Grant 2014.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kotlan, B., Liszkay, G., Blank, M. et al. The novel panel assay to define tumor-associated antigen-binding antibodies in patients with metastatic melanomas may have diagnostic value. Immunol Res 61, 11–23 (2015). https://doi.org/10.1007/s12026-014-8600-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12026-014-8600-6