Abstract
Purpose
Maternal nicotine exposure negatively impacts offspring’s health and metabolism, leading to obesity and insulin resistance. Here we investigated the pancreatic islet function, glycemic homeostasis, and insulin signaling in adult rat offspring that were nicotine-exposed during breastfeeding.
Methods
For this, lactating Wistar rat dams were divided into two groups: Nicotine (implanted with osmotic minipumps containing 6 mg/Kg, NIC) and Control (saline, CON). Solutions were released from postnatal (PN) day 2–16. At PN110 and PN170, 10 offspring per litter/sex/group were submitted to the oral glucose tolerance test (OGTT). PN180 offspring were killed and glycemia, insulinemia, adiponectinemia, pancreas morphology as well as pancreatic islet protein expression (related to insulin secretion) and skeletal muscle (related to insulin action) were evaluated. Males and females were compared to their respective controls.
Results
Adult NIC offspring of both sexes showed glucose intolerance in the OGTT. Despite normoglycemia, NIC males showed hyperinsulinemia while females, although normoinsulinemic, had hyperglycemia. Both sexes showed increased IRI, reduced adiponectin/visceral fat mass ratio and higher ectopic deposition of lipids in the pancreatic tissue adipocytes. In pancreatic islets, NIC males showed lower PDX-1 expression while females had higher PDX-1 and GLUT2 expressions plus lower α2 adrenergic receptor. In the muscle, NIC offspring of both sexes showed reduction of GLUT4 expression; NIC males also had lower insulin receptor and pAKT expressions.
Conclusions
Thus, glycemic homeostasis and peripheral insulin signaling in adult offspring of both sexes are affected by nicotine exposure through the milk, increasing the risk for type 2 diabetes development.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Type 2 diabetes mellitus is considered a pandemic, mainly caused by insulin resistance (IR), which is closely associated with obesity [1]. However, in recent years, experimental and epidemiological evidence have shown that the growing incidence of type 2 diabetes and the obesity pandemic may also be due to insults during critical early life periods [2]. In the 1980s, Barker raised the “thrifty phenotype hypothesis” to explain the relationship between the fetal environment and the diseases found in adulthood [3]. This concept is now known as “Developmental Origins of Health and Disease” (DOHaD), which postulates that specific environmental insults during a critical period of early life can induce developmental plasticity by programming the individual for disease development [4].
The period of breastfeeding is considered a window of susceptibility to metabolic programming, because organs involved in the maintenance of glycemic homeostasis, such as the brain, liver and pancreatic islets continue to differentiate during this period. These organs have been shown to be vulnerable during this period since programming insults can have negative, long-lasting and sometimes late-emerging effects on glycemic homeostasis [5].
Smoking exposure during critical periods of development is related to metabolic programming. It is well known that cigarette smoke contains several compounds that can be harmful to the developing individual. Studies show that smoking during pregnancy is dangerous for both mother and fetus [6]. Although it has been shown that many pregnant women quit smoking, some studies show that most women who quit smoking during gestation resume it during breastfeeding, erroneously believing that smoking during this period is no longer harmful to the baby. Unfortunately, babies are not only exposed to second-hand smoke but also to those components of tobacco smoke that will be present in the mother’s milk, nicotine included [7].
Nicotine is a psychoactive substance and is the main agent leading to tobacco dependence [8]. Nicotine is considered an endocrine disruptor, interfering with the action of hormones [9]. To mimic the late-emerging effects of maternal smoking during early life, our research group has developed a programming model to investigate the effects of early, involuntary exposure to nicotine via breast milk on the offspring. In this experimental model, lactating rats are exposed to nicotine through implantation of osmotic minipumps that release a controlled amount of nicotine (equivalent to a heavy smoker) into the bloodstream [10]. In this model, lactating rats show a large amount of cotinine, the main metabolite of nicotine, in serum and milk, confirming the efficacy of the model [11]. Male animals programmed by exposure to nicotine during lactation are overweight and have increased visceral fat accumulation, hyperleptinemia, central leptin resistance, and hepatic steatosis at adulthood [12, 13]. However, the female offspring show no alteration of body mass (BM), adiposity, and leptinemia [14, 15]. Regarding glycemic homeostasis, male offspring are normoglycemic and hyperinsulinemic, suggesting IR. They also have lower plasma adiponectin/adipose tissue ratio [13]. It is worth mentioning that the aforementioned results were not studied in females.
The present study aimed to investigate the mechanisms involved in the IR that has been previously observed in the male offspring of this programming model [12], and to investigate, for the first time, the glycemic homeostasis of the female offspring. Our hypothesis is that animals early-exposed to nicotine will develop pancreatic dysfunction and alterations in proteins involved with peripheral insulin signaling in the muscle, which is responsible for 70–80% uptake of postprandial glucose.
Materials and methods
The Institutional Ethical Committee for the Use of Laboratory Animals of the Biology Institute of the State University of Rio de Janeiro approved all experimental procedures (CEUA/038/2018). Experimental procedures were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Experimental model of maternal exposure to nicotine
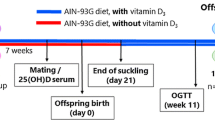
Three-month-old virgin female Wistar rats were caged with male rats at a 3:1 ratio for 2 weeks. After the detection of pregnancy, dams were allocated in individual cages with free access to water and standard chow for rodents. At birth, litters were adjusted to six pups, three males and three females, to maximize lactation performance. According to the manufacturer’s recommendation, in order to ensure proper operation of the osmotic minipumps (continuous and homogeneous release of solutions), they were filled with the solution of interest and immersed in saline solution for 24 h prior to implantation. Thus, implantation of osmotic minipumps was performed at postnatal (PN) day 2. Lactating dams were randomly assigned to one of two groups: Nicotine group (NIC): 10 dams were lightly anaesthetized with thiopental (30 mg/Kg; Thiopentax, Itapira, SP, Brazil), a 3 × 6 cm area on the back was shaved and an incision was performed to allow for the subcutaneous insertion of the osmotic minipumps (Alzet, 2ML2, California, USA). Minipumps were prepared with nicotine free-base diluted in a saline solution (NaCl 0.9%) to deliver a dose rate of 6 mg/Kg of nicotine per day during 2 weeks [10]. This protocol produces plasma nicotine concentrations similar to those found in typical smokers [16]. Control group (CON): 10 dams were implanted, using the procedures indicated above, with osmotic minipumps containing only NaCl 0.9%.
After weaning (PN21), NIC and CON pups received free access to water and standard rodent chow. The offspring’s BM was monitored every 4 days until PN180, when animals were euthanized: After a 12 h-fasting period, animals were anesthetized with thiopental (Thiopentax, 30 mg/Kg) and blood was collected by cardiac puncture in tubes with heparin. Blood samples were centrifuged (1500 g for 20 min at 4 °C) to obtain plasma, which was kept at −20 °C. Afterwards, the retroperitoneal, gonadal and mesenteric white adipose tissues were collected, weighted and the sum of these tissues was used as the visceral fat mass (VFM) variable. The pancreas and soleus skeletal muscle were quickly removed to be used in the specific techniques described below.
Glycemic homeostasis
The oral glucose tolerance test (OGTT) was performed at PN110 and PN170. A blood sample was collected, after a 12 h-fasting period, for the determination of basal glycemia (time 0) using a glucometer (ONETOUCH ULTRA®, Johnson & Johnson, São Paulo, Brazil). Glucose solution (50%) was injected in sterile saline via an oral probe at 2 g/Kg BM and glycemia was measured after 15, 30, 60 and 120 min. Fasting glycaemia was analyzed at PN180 in blood samples obtained from the tail vein using reagent strips that were read in a glucometer. Plasma insulin and adiponectin concentrations were measured by Elisa Kit (Millipore Corporation, MA, USA) according to the manufacturer’s instructions. Plasma adiponectin was normalized by total VFM [17]. Insulin sensitivity was assessed using the insulin resistance index (IRI), which was calculated as follow: fasting glucose (mg/mL) × fasting insulin (mUI/mL).
Pancreas morphology
Due to the lack of standardized nomenclature in the literature, the term pancreatic steatosis was used in the current study to describe all types of accumulation of fat in the pancreas, as suggested by Smits & Van Geenen [18].
The pancreases of seven animals per litter/sex/group were removed, weighed and quickly fixed in 4% paraformaldehyde for 24 h. Then, the entire tissue was incorporated into Paraplast® Plus (Sigma-Aldrich Chemicals, St Louis, MO, USA) and prepared for light microscopy. Consecutive 5 μm serial sections were obtained and the slides were stained with hematoxylin-eosin. Ten images/animal/group were captured randomly (magnification ×20, Leica Microsystems CTR6000, Wetzlar, Germany) and quantification of pancreatic steatosis was performed by stereology. Pancreatic fat (interlobular, intralobular and perilobular) was quantified by volume density (Vv) using a point test system superimposed on the tissue image (36 points), calculated as: Vv = PP/PT, where PP represents the number of points that reach the fat cells and PT is the total number of test points.
Western blotting
The pancreatic islets were isolated using the collagenase method [19]. The islets were collected using a stereomicroscope and approximately 300 islets were stored in a −80 °C freezer until the Western blot analysis was carried out.
For protein expression analysis, samples of isolated islets of six animals per litter/sex/group were resuspended and lysed by sonication (two times, 10 s pulses, Sonic Dismembrator Model 100, Thermo Fisher Scientific) in T-PER™ Tissue Protein Extraction Reagent (78510, Sigma St. Louis, MO, USA) buffer. The cocktail of protease inhibitors (complete EDTA-free, Roche Applied Science, Mannheim, Germany) was added and samples were centrifuged at 15,294 g for 5 min at 4 °C (Eppendorf 5417R Refrigerated Centrifuge). The skeletal muscle of 6–7 animals per litter/sex/group were homogenized in RIPA buffer, (20 mM TRIS HCl, 10 mM NaF, 1% NP40, 150 mM NaCl, 5 mM EDTA, 0.1% SDS) containing a protease inhibitor cocktail and centrifuged twice at 15,294 g for 15 min at 4 °C. The total protein content was determined using a BCA Protein Assay Kit (Thermo Scientific, IL, USA) and cell lysates were treated with Laemmli sample buffer (50 mM Tris-HCl, pH 6.8, 1% SDS, 5% 2-mercaptoethanol, 10% glycerol, 0.001% bromophenol blue).
Total protein extracts were separated by gel electrophoresis (SDS-PAGE, 8.5–12%) at 150 V/60 A. The proteins were then transferred from the gel to a nitrocellulose membrane (Hybond ECL; Amersham Pharmacia Biotech, NJ, USA) by the Trans-Blot® Turbo™ Transfer System (Bio-Rad®, Hercules, CA, USA) at 2.5 A/15 V/45 min. Membranes were blocked with 5% BSA in Tween-TBS buffer (containing 20 mM Tris-HCl, pH 7.5; 500 mM NaCl and 0.1% Tween-20) for 1 h with continuous shaking. Then, membranes were incubated with different primary antibodies (described in Table 1) overnight at 4 °C. Then, membranes were washed 3 times with Tween-TBS, followed by 1 h incubation with the appropriate secondary antibody at room temperature. After this period, membranes were incubated with streptavidin-horseradish peroxidase conjugated HRP (RPN1231V, GE Healthcare Life Sciences, USA) when necessary. After another series of washes, targeted proteins were detected by enhanced chemiluminescence (Clarity™ and Clarity Max™ Western ECL Blotting Substrates, cat 170-5061, Bio-Rad, California, USA). Images were scanned and bands were quantified by densitometry, using Image J 1.34 s software (Wayne Rasband, National Institute of Health, MA, USA). The glyceraldehyde-3-phosphate dehydrogenase protein content (GAPDH, 5174, Cell Signaling, Massachusetts, USA; diluted 1:1000 in TTBS) and B-actin (Santa Cruz Biotechnology®, CA, USA; diluted 1:1000 in TTBS) were used for the normalization of the data.
Statistical analysis
The results are expressed as mean ± SEM. Data were analyzed using the statistical program GraphPad Prism 6.0 (GraphPad Software, Inc., La Jolla, CA, USA). Unpaired Student’s t test were used separately when analyzing the programming effects in males and females. Differences were considered significant when P < 0.05.
Results
Somatic parameters of the offspring
At PN180, NIC males showed increased BM and VFM [15% (P < 0.01) and 33% (P < 0.001), respectively] when compared to CON group (Table 2), while NIC females did not have changes in these parameters (Table 2). The absolute and relative mean pancreases masses were similar between groups (Table 2).
Oral glucose tolerance test (OGTT) and glucose homeostasis
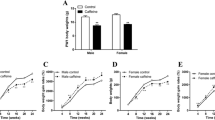
At PN110, after 30 min of glucose administration, we observed an increase in glucose in all groups, which was followed by a gradual reduction, without significant differences between them (Fig. 1a, c). The area under curve (AUC) of the OGTT was unchanged in both NIC males and NIC females (Fig. 1b, d).
Oral glucose tolerance test (OGTT) at PN110 and PN170. OGTT (a and e) and area under curve (AUC) of OGTT (b and f) of male rat offspring. OGTT (c and g) and AUC of OGTT (d and h) of female rat offspring. Groups: CON: Control, NIC: Nicotine. Values represent mean ± SEM of different litters per sex per group (n = 10). Student’s t tests were used for the comparisons. *P < 0.05, **P < 0.01
At PN170, NIC males showed increased blood glucose at 60 and 120 min after glucose administration when compared to CON males (P < 0.05 and P < 0.01, respectively, Fig. 1e). The AUC of OGTT is compatible with the aforementioned data, showing an 18% increase in NIC males when compared to the CON group (P < 0.05, Fig. 1f). NIC females had higher glucose levels at 30, 60 and 120 min after glucose administration (P < 0.05, P < 0.01 and P < 0.05, respectively, Fig. 1g). Regarding the AUC, NIC females showed increased glycemia during OGTT compared to the CON ones (11%, P < 0.01, Fig. 1h).
At PN180, NIC males whose mothers were exposed to nicotine during lactation showed no difference in fasting glycemia (Fig. 2a), but had higher insulinemia (51% vs CON, P < 0.05, Fig. 2b) and IRI (66% vs CON, P < 0.01, Fig. 2c). In addition, these animals showed no differences in plasma adiponectin (Fig. 2d), although they showed a lower adiponectin/VFM when compared to CON males (−39%, P < 0.01, Fig. 2e).
Glycemia (a), Insulinemia (b), IRI (c), Serum adiponectin (d) and Serum adiponectin/visceral fat mass (e) of male rat offspring at PN180. Glycemia (f), Insulinemia (g), IRI (h), Serum adiponectin (i) and Serum adiponectin /visceral fat mass (j) of female rat offspring at PN180. Groups: CON: Control, NIC: Nicotine. Values represent mean ± SEM of different litters per sex per group (n = 10). Student’s t tests were used for the comparisons. *P < 0.05, **P < 0.01
Regarding NIC females at PN180, we observed hyperglycemia (12%, P < 0.05, Fig. 2f), normoinsulinemia (Fig. 2g), higher IRI (80%, P < 0.01, Fig. 2h), hypoadiponectinemia (−24%, P < 0.05, Fig. 2i) and reduced adiponectin/VFM when compared to CON females (−53%, P < 0.01, Fig. 2j).
Pancreas parameters in adult offspring
Concerning the histological analysis of the pancreas (Fig. 3), NIC males showed increased fat accumulation in the pancreatic tissue compared to CON males (183%, P < 0.01, Fig. 3b). NIC females also showed increased pancreatic steatosis when compared to CON females (438%, P < 0.001, Fig. 3d).
Pancreas parameters adult offspring. Representative photomicrographs of pancreatic tissue (a and c) of adult rat offspring show lipid accumulation with H&E staining (scale bar: 50 µm - magnification ×10). Groups: CON: Control, NIC: Nicotine. NIC groups have a large adipocyte infiltration (asterisk). The arrows indicate lipid droplets that are found in the exocrine pancreas of CON animals. Percentage of steatosis in male (b) and female (d) offspring. We used different litters per sex per group (n = 7). Protein expression involved in insulin secretion (PDX1, GLUT2, GCK, AdRα2, AdRβ2, mAChM3, mAChM4) and in the exocytosis of insulin granules (Sintaxin 1A, SNAP25, Munc18a, Synaptotagmin VII) by beta cells in male (e) and female (f) offspring with their respective representative bands. GAPDH or B-actin contents were used as internal controls for protein normalization. Values represent mean ± SEM of different litters per sex per group (n = 6). Student’s t tests were used for the comparisons. *P < 0.05, **P < 0.01, *** P < 0.001
The expression of proteins related to the insulin secretion are depicted in Fig. 3. NIC males showed a reduction only of PDX1 expression when compared to CON males (−20%, P < 0.05, Fig. 3e). The other proteins were not altered (Fig. 3e). NIC females had an increase of GLUT2 and PDX1 expressions (33%, P < 0.05 in both cases, Fig. 3f) and a reduction of α2 adrenergic receptor in islets (−25%, P < 0.05, Fig. 3f) in relation to CON females. The expressions of the other proteins were unaltered (Fig. 3f).
Insulin signaling pathway in the skeletal muscle of adult offspring
The main proteins involved with insulin signaling in skeletal muscle are shown in Fig. 4. NIC males showed reduced expression of IR-beta (−32%, P < 0.05, Fig. 4a), GLUT4 (−24%, P < 0.05, Fig. 4a) and less degree of phosphorylation of AKT (pAKT) (−19%, P < 0.05, Fig. 4a) and in relation to the CON males, although there was no change in the expression of total AKT (Fig. 4a). The evaluation of total pAKT/AKT ratio from NIC animals was not changed (CON male: 98.9 ± 11.7; NIC male: 81.4 ± 11.6; CON female: 87.5 ± 8.7; NIC female: 79.7 ± 8.1). In addition, NIC males did not show changes in the expression of SNARE proteins (Syntaxin 4, SNAP23 and Munc18c), a complex involved in GLUT4 transport (Fig. 4a). The skeletal muscle of NIC females only showed a reduction of GLUT4 expression when compared to CON females (−37%, P < 0.05, Fig. 4b). The other proteins were not affected in this tissue (Fig. 4b).
Insulin signaling pathway in the skeletal muscle of adult offspring. IR-beta, AKT, pAKT, GLUT4, Syntaxin 4, SNAP23 and Munc18c protein expression at PN180 in male (a) and female (b) rat offspring with their respective representative bands. Groups: CON: Control, NIC: Nicotine. GAPDH content we used as internal control for protein normalization. Values represent mean ± SEM of different litters per sex per group (n = 6–7). Student’s t tests were used for the comparisons. *P < 0.05
Discussion
In the present study we demonstrated that changes in the glycemic homeostasis previously found [12] in male rats programmed by early exposure to nicotine during lactation occur only after PN110, since OGTT, which is considered an indicative test for IR, was unchanged at that age. At PN170, animals of both sexes had glucose intolerance and, at PN180, they showed pancreatic steatosis and IR, characterized by reduced GLUT4 in the skeletal muscle, increased IRI and reduced adiponectin/VFM ratio. Specifically, males also had hyperinsulinemia, reduced expression of PDX1 in the pancreatic islets and decreased expression of the insulin receptor and less degree of phosphorylation of AKT in muscle, while females had hyperglycemia, hypoadiponectinemia, increased expression of GLUT2 and PDX1 and reduced α2-adrenergic receptor in the islets.
IR associated with progressive beta cell dysfunction is characteristic of the development of type 2 diabetes [18]. Unlike hepatic steatosis, pancreatic steatosis is histologically characterized by infiltration, increased number of adipocytes in this tissue [20, 21] and increased intracellular lipids [22]. Here, males and females exposed to nicotine during breastfeeding showed increased lipids accumulation in fat cells in the pancreas. Although controversial, the effect of ectopic fat associated with IR and pancreatic beta cell dysfunction has been investigated in clinical and experimental studies [21, 23]. Pancreatic steatosis may potentiate the metabolic syndrome, resulting in hyperglycemia, reduction of insulin secretion [24], loss of mass and function of pancreatic beta cells [25]. The pancreatic infiltrated fat cells also could be associated with the increased oxidative stress and proinflammatory cytokines production resulting in localized inflammation and fibrosis, impairing the architecture [26, 27] and causing inflammation in the pancreatic islets [28], apoptosis of pancreatic beta cells [26, 27] and pancreatic dysfunction [21,22,23].
In humans, a positive association between increased lipid infiltration in the pancreas and IR has been reported, suggesting that pancreatic fat enhances this condition [22,23,24,25,26,27,28,29,30,31,32]. In a cohort of 8097 individuals, Wang et al. [30] demonstrated that there was increased risk of developing diabetes in patients with pancreatic fat accumulation. Also, it has been reported that individuals newly diagnosed with type 2 diabetes have significantly higher amounts of pancreatic fat when compared to healthy patients [31]. After analysis of pancreatic fat content in men with and without type 2 diabetes, Tushuizen et al. [32] demonstrated that the average pancreatic fat content in diabetic patients was 20% higher than in nondiabetic patients. In addition, the pancreatic fat observed in these patients was associated with the pancreatic beta cell dysfunction [31, 32]. It appears that may occur a combined destructive effect between increased free fatty acids (FAs) and pancreatic beta cell function, associated with increased lipotoxicity and increased local chemokine production, resulting in long-term beta cell injury and/or death [18, 21, 23]. However, the role of pancreatic steatosis on beta cell function is not fully understood and remains contradictory in the literature. Not all human studies have found a relationship between pancreatic adipocyte infiltration and pancreatic beta cell function in individuals with impaired glucose metabolism [33,34,35]. These contradictory results can be attributed to differences in applied methodology, number of individuals evaluated, age, and ethnicity of the population studied.
In addition to local inflammation possibly caused by adipocyte infiltration, pancreatic beta cells can be affected by a lipotoxic mechanism, since it seems that the islet cells have no adipocyte infiltration and only the exocrine pancreas show the ectopic fat. It is well described in the literature that the adipocyte-stored triglycerides are composed of different FAs, which have positive and negative effects on insulin secretion and on the survival of beta cells [36]. For example, monounsaturated FAs, are generally associated with protective effects, participating in the regulation of normoglycemia, increased insulin sensitivity, and prevention of apoptosis [37]. However, saturated FAs are widely associated with lipotoxicity, associated with reduced beta cell proliferation, insulin gene expression and induction of cell death [38, 39]. Chronic exposure to FAs results in disturbances in the regulation of lipid metabolism, which contribute to decreased function and beta cells, through lipotoxicity and, consequently, inducing T2DM [37, 40]. Here, we hypothesize that accumulated pancreatic lipids may act as a source of FAs or other lipid-derived metabolites, which can gradually affect the beta cell function and insulin secretion through lipotoxic pathways, which seems to be more pronounced in NIC females. In fact, the percentage of adipocytes infiltrating the female pancreas (+438%) is more pronounced than the percentage of male steatosis (+183%). Therefore, NIC female is more affected; its beta cell does not produce enough insulin resulting in hyperglycemia, while NIC male is able to release more insulin, perhaps due to less fat in the pancreas, maintaining normoglycemia. This hypothesis was also based on available literature. Some authors suggest a relationship between pancreatic steatosis and the severity of pancreatitis [18, 41, 42]. Although we propose that the endocrine dysfunction observed in animals exposed to NIC could in part be related to mild pancreatitis, lipotoxicity or increased local chemokine production and the infiltration of immune cells through a paracrine effect mediated by adipocytes that are infiltrated in the pancreas, further studies are needed to clarify this idea. This is a limitation of our study, since we only evaluated the percentage of adipocytes that were infiltrated in the pancreatic tissue.
It is worth mentioning that there are few and controversial studies in the literature on exocrine dysfunction caused by pancreatic steatosis. Three mechanisms have been described that can cause exocrine pancreatic disorder in patients with pancreatic steatosis: acinar cell lipotoxicity, negative paracrine effect mediated by infiltrated adipocytes, or direct destruction of acinar cells [21, 43]. Therefore, further research in animal models is needed to assess the influence of pancreatic steatosis on the exocrine pancreas, especially using immunostaining techniques.
Epidemiological data demonstrate the negative impacts of smoking during pregnancy and the increased risk of developing type 2 diabetes at adulthood [44]. A clinical study showed that newborns of mothers who reported smoking in the middle and late gestation had hyperglycemia, reduced fetal IGF-I concentration with changes in fetal pancreatic beta cell function [45]. In animals, maternal cigarette smoke exposure during pregnancy and/or lactation caused glucose intolerance in the offspring and reduced insulin sensitivity [46], besides leading to reduction in size and number of pancreatic islets [47]. In addition to overweight and increased visceral fat, male offspring whose mothers were exposed to nicotine concentrations equivalent to women who smoke moderately during pregnancy and lactation had glucose intolerance and IR, effects that may be mediated by reduced pancreatic beta cell mass in early life [47, 48] and possibly associated with greater susceptibility for the development of metabolic syndrome in the adult offspring.
In our programming model of nicotine exposure exclusively during lactation, males had normoglycemia, but had glucose intolerance and increased IRI. Normoglycemia is possibly being maintained by the hyperinsulinemia. In humans, most diabetic patients initially have glucose intolerance, which is considered the intermediate phase in type 2 diabetes progression [49]. To compensate for IR, pancreatic beta cells adapt to different situations to improve function and maintain glycemic homeostasis. In this phase, structural adaptations occur in the beta cells that lead to hyperfunction, i.e., increase insulin secretion in response to hyperglycemia, ensuring glycemic homeostasis, even if only for a temporary period [50]. A limitation in the present study was that the area and mass of the pancreatic islets were not evaluated, parameters that were assessed by other authors in models of pregnancy + lactation exposure [47, 48].
Unlike males, NIC females had hyperglycemia, which may be due to beta cell failure to produce sufficient amounts of insulin to maintain normoglycemia. Increased metabolic insulin demand may lead to reduced pancreatic beta cell function [50]. Chronic hyperglycemia can lead to pancreatic beta cell depletion, probably by glucotoxicity of the beta cell [51], a phase that characterizes a critical metabolic state that precedes beta cell dysfunction leading to cell death [50]. This difference observed on the glycemic profile between adult men and women in these two human studies [50, 51] may be related to the amount of adipocytes infiltrated in pancreatic tissue, in which women are most affected, because they showed higher percentage of adipocytes infiltrated. However, we know that pancreatic steatosis is only one of the factors that contribute to the development of T2DM. To support this idea, a limitation of our work was not to have assessed insulin secretion in vitro, therefore, further studies are needed to support our hypothesis.
Proper functioning of pancreatic islets depends on a number of regulators, among them PDX-1, which is considered one of the most important transcription factors involved in the regulation, development and maintenance of pancreatic beta cells, and that controls the expression of the GLUT2, GCK, and insulin [52]. A change in metabolic state reduces PDX1 transcription, mediating a cascade of modifications that culminate in the silencing of this gene [53]. Here, we demonstrated that early life nicotine exposure reduces PDX1 expression in male pancreatic islets, although it did not alter GLUT2 and GCK expressions. Downregulation of PDX1 expression in beta cells may underlie the pathogenesis of beta cell failure and type 2 diabetes [54]. NIC females showed increased expressions of PDX1 and GLUT2, mainly due to the hyperglycemia. However, due a primary impairment in insulin production, the compensatory increase in GLUT2 was unable to increase insulin secretion.
Pancreatic islets are innervated by branches of the autonomic nervous system and sympathetic innervation is responsible for inhibiting glucose-induced insulin release [55]. In adult females, nicotine exposure via breast milk also seems to change the autonomic nervous system that controls insulin secretion, since these animals show a reduction in α2 adrenergic receptor expression. This reduction could be a compensatory effect to the lower insulin production.
Adiponectin is a fat tissue hormone that increases insulin sensitivity, as well as has antioxidant and anti-inflammatory actions [56]. The concentration of adiponectin in the umbilical cord of newborns of smoking women and in children exposed to cigarette smoke was significantly lower when compared to children of healthy mothers [57]. The present study lends support to the idea that a correlation exists between glucose intolerance, IR and hypoadiponectinemia, since NIC offspring of both sexes had a reduction in adiponectin/VFM and NIC females had a reduction in plasma adiponectin.
Especially in the skeletal muscle, IR is considered the primary defect in the progression of this disease. Glucose uptake in the muscle is substantially higher than in the adipose tissue or liver [58]; the former tissue is the predominant site of postprandial insulin-mediated glucose uptake through GLUT4 stimulation and translocation to the plasma membrane. Thus, after a meal, ~80% of the glucose uptake occurs in the muscle [58, 59]. Insulin signaling in muscle depends on it binding to its receptor, which activates a cascade of protein phosphorylation, including insulin receptor substrate (IRS), phosphatidylinositol 3-kinase, and a serine/threonine protein kinase (AKT). AKT stimulates GLUT4 translocation, therefore, participating in insulin-dependent glucose transport in the muscle and adipose tissue [60]. Thus, the deregulation of critical proteins in the insulin signaling pathway is related to the pathogenesis of IR and type 2 diabetes [61]. Specifically, muscle GLUT4 defects contribute to glucose intolerance and IR [58, 59]. In our model, NIC rats of both sexes showed a reduction in GLUT4. In addition, NIC males also had decreased muscle insulin receptor and pAKT. Alteration in the expression of these proteins may be contributing to IR in animals programmed by nicotine exposure during lactation.
Most studies investigating the negative effects of nicotine only consider pregnancy or pregnancy plus breastfeeding, i.e., there are few studies that evaluate the effects of exposure to tobacco smoke exclusively during the early PN period. As already mentioned, many women resume smoking during lactation, and here we highlight the deleterious effects of early nicotine exposure, causing pancreatic steatosis, beta-cell dysfunction and IR in the offspring of both sexes. Maternal smoking during breastfeeding is still a serious public health problem, as this attitude contributes to the type 2 diabetes pandemic.
Conclusion
We evidence that smoking during breastfeeding predisposes the progeny of both sexes to the development of type 2 diabetes at adulthood, since we demonstrated that early, involuntary nicotine-only exposure via breast milk led to the development of pancreatic steatosis, glucose intolerance and IR. In our programming model, it seems that the female offspring are more prone to develop severe diabetes earlier in life. However, further studies are needed to understand how pancreatic steatosis can impair beta cell function by lipotoxicity, cytotoxicity or both and contributes to type 2 diabetes or whether its presence is only a marker of pancreatic beta cell dysfunction. In addition, we need to understand how the females are more prone to pancreatic beta cell failure.
References
E. Wilmot, I. Idris, Early onset type 2 diabetes: risk factors, clinical impact and management. Ther. Adv. Chronic Dis. (2014). https://doi.org/10.1177/2040622314548679
A. Vaag, C. Brons, L. Gillberg, NS. Hansen, L. Hjort, GP. Arora, N. Thomas, C. Brohon, R. Ribel-Madsen, LG. Grunnet, Genetic, nongenetic and epigenetic risk determinants in developmental programming of type 2 diabetes. Acta Obstet. Gynecol. Scand. (2014). https://doi.org/10.1111/aogs.12494
DJ. Barker, Intrauterine programming of adult disease. Mol. Med. Today. (1995). https://doi.org/10.1136/bmj.311.6998.171
M. Mandy, M. Nyirenda, Developmental Origins of Health and Disease: the relevance to developing nations. Int. Health. (2018). https://doi.org/10.1093/inthealth/ihy006
L. Ellsworth, E. Harman, V. Padmanabhan, B. Gregg, Lactational programming of glucose homeostasis: a window of opportunity. Reproduction. (2018). https://doi.org/10.1530/REP-17-0780
M. Napierala, J. Mazela, TA. Merritt, E. Florek, Tobacco smoking and breastfeeding: effect on the lactation process, breast milk composition and infant development: a critical review. Environ. Res. (2016). https://doi.org/10.1016/j.envres.2016.08.002
W. Hannover, K. Röske, JR. Thyrian, J. Grempler, HJ. Rumpf, U. Hapke, U. John, Interventions for smoking cessation in pregnancy and postpartum. Modalities, efficacy, introduction to motivational interviewing and social-cognitive models for behaviour changes. Z Geburtshilfe Neonatol. (2008). https://doi.org/10.1055/s-2008-1076836
B. Le Foll, SR. Goldberg, Effects of nicotine in experimental animals and humans: an update on addictive properties. Handb. Exp. Pharmacol. (2009). https://doi.org/10.1007/978-3-540-69248-5_12
MM. Tabb, B. Blumberg, New modes of action for endocrine disrupting chemicals. Mol. Endocrinol. (2006). https://doi.org/10.1210/me.2004-0513
E. Oliveira, EG. Moura, AP. Santos-Silva, AT. Fagundes, AS. Rios, Y. Abreu-Villaça, JF. Nogueira Neto, MC. Passos, PC. Lisboa, Short- and long-term effects of maternal nicotine exposure during lactation on body adiposity, lipid profile, and thyroid function of rat offspring. J. Endocrinol. (2009). https://doi.org/10.1677/JOE-09-0020
E. Oliveira, CR. Pinheiro, AP. Santos-Silva, IH. Trevenzoli, Y. Abreu-Villaça, JF. Nogueira Neto, AM. Reis, MC. Passos, EG. Moura, PC. Lisboa, Nicotine exposure affects mother’s and pup’s nutritional, biochemical, and hormonal profiles during lactation in rats. J. Endocrinol. (2010a). https://doi.org/10.1677/JOE-09-0430
E. Oliveira, EG. Moura, AP. Santos-Silva, CR. Pinheiro, NS. Lima, JF. Nogueira-Neto, AL. Nunes-Freitas, Y. Abreu-Villaça, MC. Passos, PC. Lisboa, Neonatal nicotine exposure causes insulin and leptin resistance and inhibits hypothalamic leptin signaling in adult rat offspring. J. Endocrinol. (2010b). https://doi.org/10.1677/JOE-10-0104
EP. Conceição, N. Peixoto-Silva, CR. Pinheiro, E. Oliveira, EG. Moura, PC. Lisboa, Maternal nicotine exposure leads to higher liver oxidative stress and steatosis in adult rat offspring. Food Chem. Toxicol. (2015). https://doi.org/10.1016/j.fct.2015.01.025
CR. Pinheiro, E. Oliveira, IH. Trevenzoli, AC. Manhães, AP. Santos-Silva, V. Younes-Rapozo, S. Claudio-Neto, AC. Santana, CC. Nascimento-Saba, EG. Moura, PC. Lisboa, Developmental plasticity in adrenal function and leptin production primed by nicotine exposure during lactation: gender differences in rats. Horm. Metab. Res. (2011). https://doi.org/10.1055/s-0031-1285909
TC. Peixoto, EG. Moura, PN. Soares, IM. Bertasso, CB. Pietrobon, FAH. Caramez, RA. Miranda, E. Oliveira, AC. Manhães, PC. Lisboa, Nicotine exposure during breastfeeding reduces sympathetic activity in brown adipose tissue and increases in white adipose tissue in adult rats: sex-related differences. Food Chem. Toxicol. (2020). https://doi.org/10.1016/j.fct.2020.111328
E. Lichtenstein, The smoking problem: a behavioral perspective. J. Consult. Clin. Psychol. (1982). https://doi.org/10.1037//0022006x.50.6.804
SY. Park, YR. Cho, HJ. Kim Unraveling the temporal pattern of diet-induced insulin resistance in individual organs and cardiac dysfunction in C57BL/6 mice. Diabetes. (2005). https://doi.org/10.2337/diabetes.54.12.3530
MM. Smits, EJ. Van Geenen, The clinical significance of pancreatic steatosis. Nat. Rev. Gastroenterol. Hepatol. (2011). https://doi.org/10.1038/nrgastro.2011.4
CB. Pietrobon, IM. Bertasso, RA. Ribeiro, ACP. Alegre-Maller, C. Lubaczeuski, AC. Boschero, ACF. Araújo, SL. Balbo, ML. Bonfleur, Maternal Roux-en-Y gastric bypass impairs insulin action and endocrine pancreatic function in male F1 offspring. Eur. J. Nutr. (2019). https://doi.org/10.1007/s00394-019-01968-9
RF. Ogilvie, The islands of Langerhans in 19 cases of obesity. J. Pathol. Bacteriol. (1933). https://doi.org/10.1002/path.1700370314
R. Catanzaro, B. Cuffari, A. Italia, F. Marotta, Exploring the metabolic syndrome: nonalcoholic fatty pancreas disease. World J. Gastroenterol. (2016). https://doi.org/10.3748/wjg.v22.i34.7660
NS. Sakai, SA. Taylor, MD. Chouhan, Obesity, metabolic disease and the pancreas-Quantitative imaging of pancreatic fat. Br. J. Radiol. (2018). https://doi.org/10.1259/bjr.20180267
JS. Lee, SH. Kim, DW. Jun, JH. Han, EC. Jang, JY. Park, BK. Son, SH. Kim, YJ. Jo, YS. Park, YS. Kim, Clinical implications of fatty pancreas: correlations between fatty pancreas and metabolic syndrome. World J. Gastroenterol. (2009). https://doi.org/10.3748/wjg.15.1869
M. Heni, J. Machann, H. Staiger, NF. Schwenzer, A. Peter, F. Schick, CD. Claussen, N. Stefan, HU. Häring, A. Fritsche, Pancreatic fat is negatively associated with insulin secretion in individuals with impaired fasting glucose and/or impaired glucose tolerance: a nuclear magnetic resonance study. Diabetes Metab. Res. Rev. (2010). https://doi.org/10.1002/dmrr.1073
I. Kharroubi, L. Ladrière, AK. Cardozo, Z. Dogusan, M. Cnop, DL. Eizirik, Free fatty acids and cytokines induce pancreatic beta-cell apoptosis by different mechanisms: role of nuclear factor-kappaB and endoplasmic reticulum stress. Endocrinology. (2004). https://doi.org/10.1210/en.2004-0478
X. Zhang, Y. Cui, L. Fang, F. Li, Chronic high-fat diets induce oxide injuries and fibrogenesis of pancreatic cells in rats. Pancreas. (2008). https://doi.org/10.1097/MPA.0b013e3181744b50
ZZ. Zhao, LL. Xin, JH. Xia, SL. Yang, YX. Chen, K. Li, Long-term High-fat High-sucrose Diet Promotes Enlarged Islets and β-Cell Damage by Oxidative Stress in Bama Minipigs. Pancreas. (2015). https://doi.org/10.1097/MPA.0000000000000349
T. Horii, Y. Fujita, C. Ishibashi, K. Fukui, H. Eguchi, J. Kozawa, I. Shimomura, Islet inflammation is associated with pancreatic fatty infiltration and hyperglycemia in type 2 diabetes. BMJ Open Diabetes Res. Care. (2020). https://doi.org/10.1136/bmjdrc-2020-001508
NS. Patel, MR. Peterson, DA. Brenner, E. Heba, C. Sirlin, R. Loomba, Association between novel MRI-estimated pancreatic fat and liver histology-determined steatosis and fibrosis in non-alcoholic fatty liver disease. Aliment Pharmacol. Ther. (2013). https://doi.org/10.1111/apt.12237
CY. Wang, HY. Ou, MF. Chen, TC. Chang, CJ. Chang, Enigmatic ectopic fat: prevalence of nonalcoholic fatty pancreas disease and its associated factors in a Chinese population. J. Am. Heart Assoc. (2014). https://doi.org/10.1161/JAHA.113.000297
J. Chai, P. Liu, E. Jin, T. Su, J. Zhang, K. Shi, XU. Hong, J. Yin, H. Yu: MRI chemical shift imaging of the fat content of the pancreas and liver of patients with type 2 diabetes mellitus. Exp. Ther. Med. (2016). https://doi.org/10.3892/etm.2015.2925
ME. Tushuizen, MC. Bunck, PJ. Pouwels, S. Bontemps, JH. Van Waesberghe, RK. Schindhelm, A. Mari, RJ. Heine, M. Diamant, Pancreatic fat content and beta-cell function in men with and without type 2 diabetes. Diabetes Care. (2007). https://doi.org/10.2337/dc07-0326
NJV. Zijl, GH. Goossens, CCM. Moors, DH. van Raalte, MHA. Muskiet, PJW. Pouwels, EE. Blaak, E. Diamant, Ectopic fat storage in the pancreas, liver, and abdominal fat depots: impact on β-cell function in individuals with impaired glucose metabolism. J. Clin. Endocrinol. Metab. (2011). https://doi.org/10.1210/jc.2010-1722
VWS. Wong, GLH. Wong, DKW. Yeung, JM. Abrigo, APS. Kong, RSM. Chan, AML. Chim, J. Shen, CS. Ho, J. Woo, WCW. Chu, HLYC. Chan, Fatty pancreas, insulin resistance, and β-cell function: a population study using fat-water magnetic resonance imaging. Am. J. Gastroenterol. (2014). https://doi.org/10.1038/ajg.2014.1
R. Murakami, Y. Saisho, Y. Watanabe, J. Inaish, T. Tsuchiya, K. Kou, S. Sato, M. Kitago, Y. Kitagawa, T. Yamada, H. Itoh: Pancreas Fat and b Cell Mass in Humans with and Without Diabetes: an Analysis in the Japanese Population. J. Clin. Endocrinol. Metab. (2017). https://doi.org/10.1210/jc.2017-00828
P. Newsholme, D. Keane, HJ. Welters, NG. Morgan, Life and death decisions of the pancreatic beta-cell: the role of fatty acids. Clin. Sci. (Lond). (2007). https://doi.org/10.1042/CS20060115
P. Acosta-Montaño, V. García-González, Effects of Dietary Fatty Acids in Pancreatic Beta Cell Metabolism, Implications in Homeostasis. Nutrients. (2018). https://doi.org/10.3390/nu10040393
DK. Hagman, LB. Hays, SD. Parazzoli, V. Poitout, Palmitate inhibits insulin gene expression by altering PDX-1 nuclear localization and reducing MafA expression in isolated rat islets of Langerhans. J. Biol. Chem. (2005). https://doi.org/10.1074/jbc.M506000200
X. Palomer, J. Pizarro-Delgado, E. Barroso, M. Vázquez-Carrera, Palmitic and Oleic Acid: The Yin and Yang of Fatty Acids in Type 2 Diabetes Mellitus. Trends Endocrinol. Metab. (2018). https://doi.org/10.1016/j.tem.2017.11.009
M. Lytrivi, AL. Castell, V. Poitout, M. Cnop, Recent Insights Into Mechanisms of β-Cell Lipo- and Glucolipotoxicity in Type 2 Diabetes. J. Mol. Biol. (2020). https://doi.org/10.1016/j.jmb.2019.09.016
T. Ronti, G. Lupattelli, E. Mannarino, The endocrine function of adipose tissue: an update. Clin. Endocrinol. (2006). https://doi.org/10.1111/j.1365-2265.2006.02474.x
HY. Ou, CY. Wang, YC. Yang, MF. Chen, CJ. Chang, The association between nonalcoholic fatty pancreas disease and diabetes. PLoS ONE. (2013). https://doi.org/10.1371/journal.pone.0062561.
P. Dite, M. Blaho, M. Bojkova, P. Jabandziev, L. Kunovsky, Nonalcoholic Fatty Pancreas Disease: clinical Consequences. Dig. Dis. (2020). https://doi.org/10.1159/000505366.
MA. La Merrill, PM. Cirillo, NY. Krigbaum, BA. Cohn, The impact of prenatal parental tobacco smoking on risk of diabetes mellitus in middle-aged women. J. Dev. Orig. Health Dis. (2015). https://doi.org/10.1017/S2040174415000045
F. Fang, ZC. Luo, A. Dejemli, E. Delvin, J. Zhang, Maternal Smoking and Metabolic Health Biomarkers in Newborns. Plos ONE. (2015). https://doi.org/10.1371/journal.pone.0143660
H. Chen, M. Iglesias, V. Caruso, MJ. Morris, Maternal Cigarette Smoke Exposure Contributes to Glucose Intolerance and Decreased Brain Insulin Action in Mice Offspring Independent of Maternal Diet. Plos ONE. (2011). https://doi.org/10.1371/journal.pone.0027260
E. Somm, VM. Schwitzgebel, DM. Vauthay, EJ. Camm, CY. Chen, JP. Giacobino, SV. Sizonenko, ML. Aubert, PS. Hüppi, Prenatal nicotine exposure alters early pancreatic islet and adipose tissue development with consequences on the control of body weight and glucose metabolism later in life. Endocrinology. (2008). https://doi.org/10.1210/en.2008-0361
JE. Bruin, LD. Kellenberger, HC. Gerstein, KM. Morrison, AC. Holloway, Fetal and neonatal nicotine exposure and postnatal glucose homeostasis: identifying critical windows of exposure. J. Endocrinol. (2007). https://doi.org/10.1677/JOE070050
KS. Polonsky, J. Sturis, GI. Bell, Seminars in Medicine of the Beth Israel Hospital, Boston. Non-insulin-dependent diabetes mellitus - a genetically programmed failure of the beta cell to compensate for insulin resistance. N. Engl. J. Med. (1996). https://doi.org/10.1056/NEJM199603213341207
JH. Cho, JW. Kim, JA. Shin, J. Shin, KH. Yoon, β-cell mass in people with type 2 diabetes. J. Diabetes Investig. (2011). https://doi.org/10.1111/j.20401124.2010.00072.x
S. Del Prato, Role of glucotoxicity and lipotoxicity in the pathophysiology of Type 2 diabetes mellitus and emerging treatment strategies. Diabet. Med. (2009). https://doi.org/10.1111/j.1464-5491.2009.02847.x
F. Pedica, S. Beccari, S. Pedron, L. Montagna, P. Piccoli, C. Doglioni, M. Chilosi, PDX-1 (pancreatic/duodenal homeobox-1 protein 1). Pathologica. (2014). https://doi.org/10.1097/00006676-200403000-00001
JH. Park, DA. Stoffers, RD. Nicholls, RA. Simmons, Development of type 2 diabetes following intrauterine growth retardation in rats is associated with progressive epigenetic silencing of Pdx1. J. Clin. Investig. (2008). https://doi.org/10.1172/JCI33655
C. Liang, F. Hao, X. Yao, Y. Qiu, L. Liu, S. Wang, C. Yu, Z. Song, Y. Bao, J. Yi, Y. Huang, Y. Wu, L. Zheng, Y. Sun, G. Wang, X. Yang, S. Yang, L. Sun, Y. Li, Hypericin maintians PDX1 expression via the Erk pathway and protects islet β-cells against glucotoxicity and lipotoxicity. Int. J. Biol. Sci. (2019). https://doi.org/10.7150/ijbs.33817
KV. Prates, JC. de Oliveira, A. Malta, CCI. Matiusso, RA. Miranda, TA. Ribeiro, FA. Francisco, CCS. Franco, VM. Moreira, VS. Alves, R. Torrezan, PCF. Mathias, LF. Barella, Sympathetic innervation is essential for metabolic homeostasis and pancreatic beta cell function in adult rats. Mol. Cell Endocrinol. (2018). https://doi.org/10.1016/j.mce.2017.09.031
T. Kadowaki, T. Yamauchi, N., Kubota, K., Hara, K., Ueki, K., Tobe, Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J. Clin. Investig. (2006). https://doi.org/10.1172/JCI29126.
M. Chełchowska, J. Ambroszkiewicz, J. Mazur, L. Lewandowski, TM. Maciejewski, M. Ołtarzewski, J. Gajewski, Effect of tobacco smoking on the maternal and fetal adipokine axis in relation to newborn birth weight and length. Przegl Lek. 71(11),567–571 (2014).
D. Thiebaud, E. Jacot, RA. DeFronzo, E. Maeder, E. Jequier, JP. Felber, The effect of graded doses of insulin on total glucose uptake, glucose oxidation, and glucose storage in man. Diabetes. (1982). https://doi.org/10.2337/diacare.31.11.957
MC. Petersen, GI. Shulman, Mechanisms of Insulin Action and Insulin Resistance. Physiol. Rev. (2018). https://doi.org/10.1152/physrev.00063.2017
DR. Alessi, M. Deak, A. Casamayor, FB. Caudwell, N. Morrice, DG. Norman, P. Gaffney, CB. Reese, CN. MacDougall, D. Harbison, A. Ashworth, M. Bownes, 3-Phosphoinositide-dependent protein kinase-1 (PDK1): structural and functional homology with the Drosophila DSTPK61 kinase. Curr. Bio. (1997). https://doi.org/10.1016/s0960-9822(06)00336-8
CM. Taniguchi, B. Emanuelli, CR. Kahn, Critical nodes in signalling pathways: insights into insulin action. Nat. Rev. Mol. Cell Biol. (2006). https://doi.org/10.1038/nrm1837
Acknowledgements
We thank Mr. Ulisses Risso Siqueira for animal care as well as Mrs. Fabiana Gallaulckydio and Mr. Leandro Bezerra for technical assistance.
Funding
This work was supported by Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ); the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq); and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES).
Author information
Authors and Affiliations
Contributions
C.B.P., P.C.L. and E.G.M. designed the study. C.B.P. and P.C.L. wrote the paper. A.C.M. and E.O. were responsible for nicotine exposure. T.C.P. and P.N. were responsible for animal programming. C.B.P. and I.M.B. were responsible for biochemical and molecular procedures. K.R. and J.J.C. were responsible for pancreas histology. C.B.P., P.C.L., A.C.M. and E.G.M. revised the paper. All authors contributed to and approved the final version of the paper.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The Institutional Ethical Committee for the use of laboratory animals of the Biology Institute, State University of Rio de Janeiro approved all experimental procedures (CEUA/038/2018). This article does not contain any studies with human participants performed by any of the authors.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Pietrobon, C.B., Lisboa, P.C., Bertasso, I.M. et al. Pancreatic steatosis in adult rats induced by nicotine exposure during breastfeeding. Endocrine 72, 104–115 (2021). https://doi.org/10.1007/s12020-020-02579-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-020-02579-9