Abstract
Background
Low mineral mass and reduced bone strength with increased fracture risk are the main causes of morbidity in Thalassemia Major (TM). The pathogenesis is multifactorial and includes ineffective erythropoiesis with medullary expansion, multiple endocrine dysfunctions, direct iron bone deposition, deferoxamine-induced bone dysplasia, and reduced physical activity associated with disease complications. Dual-energy X-ray absorptiometry (DXA) is the “gold standard” for bone mineral density (BMD) assessment and for bone strength and quality evaluation. This method identifies patients at greater risk of fragility fractures, guiding treatment and monitoring response to therapy. In TM, DXA shows limitations concerning BMD calculation accuracy and fracture risk prediction. One of the main challenges in the assessment of bone health in patients with TM is the accurate interpretation of densitometric results.
Purpose
This review investigates the major pitfalls in DXA implementation and interpretation in TM.
Methods
Available literature has been assessed.
Conclusions
DXA shows limitations in assessing bone mineral “status” in TM, especially in the paediatric population, due to the peculiar characteristics of bone architecture and deformities associated with the disease. A radiological technique adjustment in this population is mandatory.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Thalassemia-associated osteoporosis (TAO) is a complex pathology due both to multiple hormonal deficiencies and multiorgan involvement. The high prevalence of bone disease in transfusion-dependent Thalassemia Major (TM) is observed in both young and old patients, as life expectancy continues to improve, with a lifetime fracture rate up to 71% [1] even in properly treated TM patients of both genders [2, 3]. Diagnostic methods and management of osteoporosis in TM patients are still uncertain since multiple factors are involved in the pathogenesis of this disease. Several studies have been conducted describing the prevalence of decreased bone density and fragility fractures in thalassemia, but the direct comparison of individual studies is difficult because the employed criteria are often different [4,5,6,7,8] (Table 1). Dual-energy X-ray absorptiometry (DXA) is the most used method in assessing bone mass in this population but requires adjustments. Indeed, there are several open issues concerning bone mass evaluation in these patients due to their young age, the presence of bone deformities, and the reduced bone size due to the impaired ability to reach peak bone mass.
The role of DXA in assessing fracture risk as well as the most suitable assessment anatomical site (spine vs. femoral neck) is far less clear in this population as compared to postmenopausal patients. Fracture incidence in this population has not been precisely defined yet, owing to the limited number of studies, their small sample size, and short follow-up. Furthermore, the high prevalence of spinal deformity in this population impairs the detection of vertebral fractures. Therefore, alternative approaches have been explored but are not employed in clinical practice. This review investigates the major pitfalls in DXA implementation and interpretation in TM, taking into account the open issues concerning the evaluation of skeletal fragility in this specific population.
Methods
We performed a literature search using MEDLINE (PubMed database) in April 2019 to detect articles in English on bone mineral assessment in TM. To conduct literature research, we used the PICO research tool focussed on the TM (Population), DXA (Intervention), quantitative computed tomography (QCT) or trabecular bone score (TBS) (Comparison), and bone mineral density (BMD) and fractures (Outcomes). We used the search terms by using Keywords chosen according to Medical Subject Heading (MeSH) terms “thalassemia major” AND “dual energy x-ray absorptiometry” OR “trabecular bone score” OR “quantitative computer tomography” AND “bone mineral density” OR “fracture” OR “vertebral deformities” OR “risk fractures”. The predefined inclusion criteria for study selection were as follows: systematic review of cross-sectional studies, prospective cohort study, case–control study, or cross-sectional study. Editorials, Letters, and abstracts from conference proceedings were excluded. The full articles of selected studies were examined, and additional searches of their reference lists performed to identify other potentially eligible articles.
Pathophysiology of TM-associated osteoporosis
Thalassemia represents a group of hereditary disorders of haemoglobin (Hb) synthesis, resulting in various degrees of anaemia: “TM” is a form of severe anaemia and dependence on chronic transfusion therapy as well as chelation therapy to prevent iron overload [9].
In addition to low BMD traditional risk factors (gender, age, osteoporosis family history, glucocorticoid treatment, and hypogonadism) [10,11,12], TM also associates with bone marrow expansion, iron overload, and nephrolithiasis, which reduce BMD and correlate with greater fracture incidence [13]. Vertebral body is an important site of marrow expansion due to ineffective haemopoiesis and significant cortical thinning, leading to trabecular bone disruption providing an additional pathophysiological explanation for vertebral fractures [14, 15], also confirming that marrow expansion can result in reduced BMD. Moreover, a longitudinal study showed a clear positive correlation between Hb levels and BMD [13]. Iron overload can lead to loss of bone mass through direct toxicity, pituitary and/or gonadal dysfunction, and chronic liver disease [16]. RANK/RANKL/OPG system changes, favouring osteoclast activity, are typical of TM, due to chronic anaemia, iron toxicity, and endocrine complications. In TM patients, BMD, already low in childhood [17], further decreases during and after puberty, especially in patients with primary amenorrhea or delayed puberty [18]. TM adolescents who failed to progress normally through puberty also fail to preserve satisfactory bone mineralization and peak bone mass attainment [19]. Hypogonadism contribution to low BMD in severe TM is important in both genders [20] (34% of females and 38% of males) [4]. Genetic factors know to be implicated in TAO are polymorphisms at the Sp1 site of the collagen type Ia1 (COLIA 1) gene, vitamin D receptor, and calcitonin receptor genes [21] [Fig. 1]. Even though iron chelation represents an invaluable tool in the management of thalassemia, several data confirm the skeletal deleterious effects of deferoxamine (DFO) [22].
Role of dual energy X-ray absorption
In TM, the best modality to assess bone health and estimate fracture risk needs to be determined. As in the general population, DXA is commonly considered the gold standard not only to assess bone health but also for its relatively low cost and low radiation dose (2–5 mrem) [23, 24]. Lumbar spine and femoral neck are the two most common sites of BMD measurement by DXA. In daily practice, BMD is more often described as T- or Z-score expressed as units of standard deviation (SD) from appropriate reference data. The last official positions of The International Society of Clinical Densitometry (ISCD) define that the diagnosis of osteoporosis in children and adolescents should not be made on the basis of densitometric criteria alone but of the finding of ≥1 vertebral fractures, in the absence of local disease or high energy trauma, emphasizing the importance of vertebral fractures and resizing the importance of BMD measurement. In the absence of crush fractures, osteoporosis diagnosis is indicated by the presence of both a clinically significant fracture history and BMD Z-score of ≤−2.0. Therefore, a Z-score of bone mineral content (BMC)/BMD >−2.0 does not exclude the probability of skeletal fragility and increased risk of fractures [25]. On the other hand, in postmenopausal women and in men aged ≥50 years, according to the WHO international reference standards, osteoporosis may be diagnosed when T-score of the lumbar spine (LS), total hip, or femoral neck is ≤−2.5 [26]. Among children, an osteoporosis-intervention study revealed 88% of scan errors; 62% of these errors involved a misdiagnosis of osteoporosis due to inappropriate T-score use [19, 20, 27, 28]. In TM patients, lower BMD T- and Z-scores [3, 29] and fractures are strongly correlated, especially in hypogonadal patients despite hormone replacement treatment [3, 25, 30, 31]. For each 1-SD decrease in spine or femoral BMD Z-score, the mean fracture rate increases by 37% and 47%, respectively [3]. The fracture risk scoring system based on worsening BMD by DXA is less clear in paediatric metabolic bone disease as compared to osteoporosis in postmenopausal women [32]. In a retrospective study, the average BMD Z- and T-scores were −0.85 SD among patients with a history of fractures [31]. Femoral neck has been suggested as the best site for monitoring BMD longitudinal changes in TM patients [33], since femoral neck shows a lower degree of bone deformity and red marrow activity along with no soft tissue interference. This hypothesis is further strengthened by the evidence that BMD at the LS is quite stable or may even increase over time, providing false longitudinal evaluations. Indeed, the spine is more severely affected than other skeletal sites, with lower BMD Z-scores at LS as compared to the hip [4], which, on the contrary, displays a lower fracture incidence [4, 29].
BMD and short stature
Short stature, delayed puberty, and/or delayed bone age are common clinical conditions in TM. Multiple factors such as growth hormone deficiency and hypogonadism, together with the important impact of anaemia and the need for chronic transfusions, contribute to reduced skeletal size and failure to achieve peak bone mass in TM [4]. Short stature is present in 49% of cases with height Z-score <−2 [34] and some studies report short stature as a risk factor for reduced BMD [35]. The bone status is assessed by DXA as the quantity of mineral within a given bone area calculated by assessing the areal BMD (aBMD), expressed as g/cm2, where aBMD is the measurement of the projected bone geometry (length and width, cm2) and BMC is the bone mineral content (expressed in grams, g). Size-related artefacts in BMD assessed by this method are well consolidated: DXA measurements (aBMD) depend on bone size, since the measurements derive from a two-dimensional projection of the image of a three-dimensional structure, not taking into account the bone depth [36].
During childhood and adolescence, body size and maturation are major determinants of BMC and BMD assessed by DXA. With bone growth and expansion, BMC increases exponentially. As for height and weight, the age-related increase in BMC and BMD is non-linear, and this variability increases with age. Consequently, in paediatric patients, BMD and BMC are expressed as Z-scores (SD scores) [37] allowing appropriate comparison with age and gender peers. The BMD reference ranges in growing children provided by DXA manufacturers are due to bone growth with age and not to a real increase in the absolute bone density [38]. The importance of this issue is crucial when performing a scan on a child with a chronic disease or genetic condition such as TM patients who are short as compared to age-related peers: in these patients obtained low BMD can simply reflect their short stature [39].
aBMD implementation is controversial in paediatric subjects, therefore BMC would be preferred [40, 41]. Although aBMD by DXA takes into account bone and body size, differently from BMC, bone size adjustment is incomplete. Therefore, the two-dimensional images provided by DXA, not including bone depth, do not correspond to the actual volumetric density [42]. Consequently, in small patients, DXA underestimates the BMD compared to those with normal size for the chronological age [43, 44]. As a consequence, shorter patients display a lower aBMD as compared to normal subjects with identical “true” volumetric BMD [45, 46]. In conclusion, low BMD or BMC Z-score in the context of short stature or delayed maturation can be difficult to interpret, raising the question of whether the detected lower bone density is due to smaller age-related bone size [Figs 2 and 3].
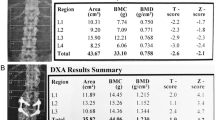
Dual-energy X-ray absorptiometry (DXA) positioning and size artifacts in Thalassemia Major (TM: a DXA analysis in a 54-year-old TM male patient with spinal scoliotic angulation (red dashed line) opposed to the straight (vertical) required positioning, affecting the projectional presentation of bone structures on the coronal plane; b DXA size artifact in a 44-year-old TM patient with platyspondyly and short stature (150 cm) with reduced bone area in the L1–L2–L3 vertebrae (red square) compared to L4 and resulting in higher bone mineral density despite a lower bone mineral content
BMD and degenerative spine changes
In TM, spine is a site of significant bone deformity secondary to bone marrow expansion and DFO-induced bone dysplasia [22, 29, 47]. The presence of several degenerative changes in the spine such as osteophytes and osteochondrosis can influence BMD as measured by the DXA, decreasing the sensitivity of this analysis if the structural pathology elements are included in the scan of the LS [48]. Moreover, spinal abnormalities interfere with DXA analysis that is based on reference scores from normal subjects, not affected by these deformities. In these patients, the detection of intranuclear gas and calcification within discs, platyspondyly, and endplate irregularity are extremely common [49]. The mechanism of the development of disc changes in TM patients is unclear, but a metabolic basis with the weakening of the annulus fibrosus also seems to be implicated owing to the chelating agent DFO, which can adversely affect the integrity and resistance of its fibres [49]. DFO also appears to be involved in endplate deformation causing platyspondyly and bone defects of the upper and lower edges of the ventral vertebrae, either due to direct toxicity or to chelation of other minerals or trace elements, thus interfering with endochondral ossification [49,50,51,52,53,54]. The harmful effect of iron overload due to tissue damage from the generation of free radicals could be an alternative causative factor [55]. Studies performed mainly on animal models have shown that the generation of free radicals is recognized as a cause of joint arthropathy and may exacerbate disc degeneration [56]. Some studies show that degeneration of the vertebral endplate occurs, with associated osteophytic alterations, may couple with osteocondrotic changes in intervertebral disc [57, 58]. An increased incidence of scoliosis in TM has been described since 20 years ago [59,60,61]. Scoliosis is the most important source of error in BMD analyses performed by DXA, due to incorrect positioning of the patient: any bone deformity and abnormalities affecting the projectional presentation of bone structures (such as scoliosis) on the coronal plane can potentially influence the output of BMD measurements [62]. This is evident for scoliosis or any type of axial/lateral rotation or lordosis/kyphosis of the lumbar tract in case of assessment of the LS BMD.
Mild vertebral deformities are common and usually with no apparent hyperkyphosis in asymptomatic or scarcely symptomatic patients. Platyspondyly (flattened and anteriorly wedged vertebral bodies) is a typical skeletal change conceivably related to iron chelation therapy [63] and is significantly associated with vertebral deformity. Homozygous TM patients, chelated with DFO to correct iron overload secondary to transfusion treatment, show a new constellation of spinal changes. In a retrospective study on transfused patients receiving DFO [52], 16 out of 22 subjects showed morphological deformities, including decreased spinal height, increased thoracic kyphosis, vertebral flattening, anterior elongation, and disk calcification. These changes were probably consequent to an impaired spinal growth-plate development. Studies investigating DFO effects according to different treatment dose support the conclusion that spinal changes are DFO induced. Spinal changes observed in DFO-treated patients differ both morphologically and pathogenetically from earlier reports of vertebral deformities occurring as a consequence of compensatory marrow hyperplasia in poorly transfused patients [52]. Iron overload is a consolidated issue in TM patients and is actively monitored in hepatic, cardiac, and pancreatic districts. In severe cases, liver iron overload, due to its high radiographical density, may lead to potential inaccurate aBMD assessment given the frequent overlap of the L2–L4 tract to the liver. However, there is no study in the literature on the pseudo effect of the soft tissue on BMD evaluation in this population [64, 65] [Figs 4 and 5].
Vertebral deformities in Thalassemia Major (TM): a 28-year-old male TM patient with iron chelation therapy with upper thoracic spine flattening (platyspondyly); b 34-year-old female TM patient with prominent vertical trabeculations and demineralization due to significant marrow expansion (reformatted computed tomographic scan)
Acquisition and analysis of DXA studies
DXA results may be misinterpreted if not evaluated by skilled professionals. BMD measurement is often reported as standard deviation score from the mean of age-, ethnic-, and sex-matched healthy individuals (Z-score). This score, however, does not take into account body and bone size as a confounding factor. There are no established guidelines to incorporate body size into the clinical interpretation of DXA-based bone outcomes. In addition, standards for bone health in childhood have not been validated yet, therefore size-related adjustments of bone outcomes are difficult to assess. Several adjustment approaches are available, but their eligibility in evaluating bone quality in TM has not been established and represents a significant challenge. Various approaches with size adjustment techniques have been developed to solve this problem. The most popular methods are the following:
(1) Bone mineral apparent density (BMAD) has been used as a size-adjusted measure of DXA BMD although not specifically designed to address growth-related size adjustments [66]. In this method, assuming that a vertebral body has a cuboid or a cylindric shape, BMC value obtained from DXA-derived data can be used to estimate volumetric bone density (g/cm3) by calculating bone volume of the lumbar vertebrae. Regulatory reference data for children BMAD are available in the UK Alphabetical study [67]. This method has been shown to be related to fracture risk in children [68].
(2) The second approach is the calculation of BMC for height or height by age: reporting BMC for height is a simple size adjustment tool that does not require any information on bone size. It has been shown to be useful for comparing populations with short stature. However, this was not predictive of fracture risk, but it was shown to be correlated with the bone resistance assessed by peripheral QCT (pQCT) [41]. The ISCD recommends adjusting total body less head (TBLH) BMC and aBMD results in children with short stature or growth delay using the BMAD or height Z-score for spine; when assessing TBLH, adjusting for height Z-score is indicated [69]. The ISCD has developed a two-step approach in which the height Z-score for age (HAZ) is calculated using the growth charts of the Centers for disease prevention and control [70] and spine aBMD-for-age Z-score is then adjusted for HAZ (aBMDHAZ) [44]. The regulatory models are based on >10,000 BMD performed in children [71]. Several studies showed that spine BMAD and aBMDHAZ partially reduced the confounding effect of stature on childhood bone density Z-scores [44, 72]. Molgaard et al. were among the first to propose a scheme including growth status in the clinical interpretation of DXA measurements. They propose a three-stage approach to understand whether a child’s low aBMD and BMAD is due to short bones (height for age), narrow bones (bone area for height), or light bones as possible sources of bone deficits, reporting an appropriate or reduced BMC for the bone area. This approach may be applied to both LS and total body results [73]. This technique takes advantage of unique sex- and age-specific measurement distributions of height and BMC but does not combine them into a single measure. Clinical utility of this approach was never validated. DXA data can also be adapted to the pubertal state because puberty affects body size, skeletal maturation, and bone strength. In clinical practice, it may be important to consider advanced or delayed puberty when interpreting BMD results. Since few studies have included the Tanner stage or the gynaecological age as the main factor in their regulatory data sets [74, 75], the actual correction for pubertal stage is generally reserved for research studies. Another simple correction, which does not take into account body size, considers bone age instead of chronological age: the effect of delayed or advanced skeletal maturation on BMD measurements is evidenced by a specific paediatric software that plots the results on the age- and sex-specific curves [76]. In clinical practice, a commonly used technique is to replace bone age or “height age” (the age at which a child’s height is the median height-for-age on the growth chart) with chronological age as a means of adjusting for short stature. It should be noted that the use of the “height age” approach might compare children who are short-for-age with younger children of similar height and at an earlier stage of sexual maturation, with a consequent risk of underestimating bone density [44]. A similar problem may occur using height-specific Z-scores because these do not take age into account. Since it is shown that lean muscle mass is essential for bone formation and that there are strong associations between body composition indices and bone mass measurements, the correction for lean body mass (LBM) [77, 78] highly correlates with BMC/BMD and is an indicator of the mechanical forces that bones are exposed to. After adjustment for age, additional adjustments are made considering whether lean muscle mass is appropriate for age and whether BMD is appropriate for lean muscle mass. This basic assumption allowed to determine two conditions that need to be satisfied in a “normal skeletal–muscle unit” in which bone has to be appropriate for muscle and muscle has to be appropriate for height [76]. In order to evaluate the risk of fracture in children, several studies have recommended to consider the ratio between total body BMC and LBM (BMC/LBM) as an index of relative bone strength [79, 80]. The amount of soft tissue changes drastically in healthy individuals during growth. Discrepancies are more striking when comparing healthy children with children with chronic illnesses and children underweight or overweight. Because soft tissue mass influences BMD and BMC measurements, DXA scans must be performed on devices with specific paediatric software with validated algorithms in healthy, obese, and chronically ill children [81]. In fact, the use of an “adult” algorithm will significantly overestimate the BMD in a child with respect to the results obtained using the “low-density paediatric” algorithm because lower-density “bone pixels” will be excluded [82].
Considering that osteophytes, sclerosis, and scoliosis can falsify BMD measurements even in children, a correct DXA scan that takes into account the structural changes within the spine is of fundamental importance. Furthermore, fractured vertebra display an increased BMD because of the fracture itself, underlining the need of excluding all the artefacts in data analysis. A recent study shows that iron overload leads to an underdiagnosed low bone mass by spine DXA in at-risk patient populations. Indeed, clinicians should closely follow-up patients with a falsely normal vertebral BMD and combine these data with data taken from the hip [83].
Fractures in Thalassemia
After the last position statement of the ISCD, greater importance has been attributed to vertebral fractures as a manifestation of osteoporosis in young populations affected by chronic diseases. The importance of combining fracture assessment with BMD evaluation relies in their asymptomatic presence despite normal bone density [84]. Accurate evaluation and optimal management of TM bone disease is hampered by the lack of large longitudinal studies examining fracture incidence. Indeed, spinal X-rays are not routinely performed in this population. In recent studies, fracture rates range from 12.1% to 38.8% based on the study population and data collection method [2, 31, 34, 85]. Vertebral fractures are the most frequent osteoporotic fractures, but they are often underdiagnosed and undertreated [86, 87]. The vertebral morphometry of the thoracic and LS, an available and easy tool, is the most useful exam to evaluate vertebral fractures. However, diagnosis and evaluation of their severity is difficult due to their characteristics and diversity. To overcome inconsistencies related to the subjective evaluation of vertebral radiographs that depends very much on radiologist’s experience and low evaluation reproducibility, semi-quantitative and quantitative approaches have been developed. In the semi-quantitative approach (SQ), also known as Genant method [88], thoracic and lumbar vertebrae from T4 to L4 are graded on visual inspection and without direct vertebral measurement as normal (grade 0), mildly deformed (grade 1: reduction of 20–25% in height and 10–20% in projected vertebral area), moderately deformed (grade 2: reduction of 26–40% in height and 21–40% in projected vertebral area), and severely deformed (grade 3: reduction of >40% in height and projected vertebral area) compared with other heights of the same and/or adjacent vertebrae. On the other hand, in the quantitative morphometry, fracture type and degree are assigned to each vertebra in the images of the lateral spine according to measurements made by using six points positioned on each of the four corners of the vertebral body and two additional points positioned in the centre of the upper and lower endplates to obtain the anterior height, centre (average or medium vertebral height), and posterior height [89]. In a disease such as TM in which vertebral deformities are frequent, spinal deformity may be difficult to distinguish from fractures, since vertebral deformity does not always represent a vertebral fracture, but a vertebral fracture always causes vertebral deformity [90]. Applying the common threshold values of reduction in vertebral heights by 20–25%, a considerable number of slight deformities detected by the visual reading is lost by the quantitative technique. Furthermore, a significant number of false positives are found with quantitative methods. However, based on our experience, only a visual evaluation of an experienced radiologist can detect mild and subtle deformities, as well as appreciate technical, anatomical, and pathological issues that lead to fracture detection. Visual interpretation, when performed by an expert eye, also discerns true deformities from normal or anomalous vertebrae. In fact, osteoarthritis is characterized by osteophytes and elongated vertebral body shape and generally does not lead to a reduction in height or to the development of a wedged or shortened vertebral body shape. In addition to changes in size, vertebral deformities are generally detected visually by the presence of endplate deformities, the lack of endplate parallelism, and general altered morphology compared with the nearest vertebrae. Some of these visual aspects are not captured by the six-digitization points used in quantitative assessments; this can lead to failure to detect certain spine deformities. For example, only a trained observer can identify the subtle hallmarks differentiating a fractured endplate and a wedge-shaped appearance caused by vertebral body remodelling in degenerative disc disease [84]. Furthermore, low BMD may suggest the presence of a fracture rather than a spinal deformity: patients with prevalent vertebral fractures have a significantly lower BMD measured by DXA, QCT, and trabecular BMD measurement compared to patients without fractures [91]. In TM, low bone mass is commonly reported, along with limb fractures, but vertebral fractures are likely underestimated [3, 11, 33, 92]. Some studies report that limb fractures are the most common complications, with the upper extremities as the predominant fracture sites [3]. Unrecognized vertebral deformities are prevalent in 2.6–13% of TM patients [3, 92], and in our experience, the column is the most frequent site of fracture, since 25% of TM patients display spine deformities while femoral fractures affect 1.7% of TM patients (unpublished data). Moreover, as a consequence of bone marrow expansion, mild vertebral deformities may be misdiagnosed in radiological reports. Mild vertebral fracture diagnosis remains an open question due to the Genant SQ method that could lead to overdiagnosis. The Genant SQ method is not specifically validated in TM, as in many other diseases known to affect bone metabolism, but is the more frequently employed method for evaluating vertebral fracture prevalence. This method evaluates not only height reductions but also vertebral shape alterations and configuration, in comparison with the adjacent vertebrae and the expected normal appearance. These features add a strong qualitative aspect to the morphometry interpretation that, in expert hands, can help to differentiate a deformity from a fracture [93]. The high prevalence of asymptomatic vertebral fractures in adolescents and young adults with thalassemia syndromes suggests the use of lateral thoracolumbar spine radiography in the surveillance of these patients [Fig. 6].
Alternative methods of BMD evaluation
Few studies investigated alternative methods for BMD assessment in TM in order to improve correlations with fracture risk, avoiding the bias related to height and age. In different forms of secondary osteoporosis, fractures are associated with damage to bone microarchitecture in addition to a quantitative alteration [94]; therefore, the evaluation of microarchitecture seems to be useful to obtain adequate information on bone strength. BMD sensitivity in evaluating fracture risk is low: indeed more fragile fractures occur in osteopenic patients (T-score between −2.5 and −1.0) compared to osteoporotic subjects as assessed by DXA (T-score −2.5) [95]. DXA is not informative concerning bone quality [96], since aBMD measurement does not provide information about bone microarchitecture.
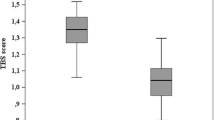
TBS is a grey-level textural measurement related to bone texture extrapolated from two-dimensional LS DXA images. TBS, based on experimental variograms of the projected DXA image, seems to correlate with bone fragility, regardless of BMD [97]. An elevated TBS score correlated with better skeletal texture is a reflection of better micro-architecture; a low TBS value correlates with poor skeletal texture, a reflection of degraded micro-architecture.
The World Health Organization FRAX calculator allows the use of TBS as an independent clinical risk factor for computing the absolute 10-year fracture risk [98]. TBS assessment could complete the quantitative assessment of aBMD measured by the DXA, which, despite being considered the gold standard for TAO, has the above-mentioned limits and is often not strictly correlated with fracture risk. Indeed, fracture prevalence appears to be lower than expected on the basis of low BMD values obtained from DXA [99]. TBS could be useful to sub-categorize patients with low BMD and low TBS who have a higher fracture incidence as compared to patients with low BMD but high TBS [99,100,101]. Baldini et al. showed that BMD and TBS are significantly lower in the TM patients, supporting the hypothesis that in TAO both qualitative and quantitative factors are involved [99] [Fig. 7]. A further DXA limitation is represented by the issue that it does not distinguish cortical bone from trabecular bone.
Trabecular bone score (TBS) assessment: a 46-year-old female Thalassemia Major patient with multiple fractures showing a TBS Z-score = −2.1 SD and a dual-energy X-ray absorptiometry Z-score = −3.7 SD. Bone texture is worse in L4 as compared to the adjacent vertebrae, despite higher areal bone mineral density values, indicating microarchitectural deterioration
High-resolution QCT was used for studying trabecular bone microstructure in TM with special evidence on trabecular architecture and cortical properties of lumbar vertebrae [96].
QCT is considered a reliable tool for bone strength examination providing a volumetric measure not affected by body size with the distinction between cortical and trabecular BMD. pQCT is an alternative bone densitometry technique able to assess volumetric BMD (vBMD) at peripheral sites as well as to estimate bone geometric properties that are directly proportional to bone strength. Radius or tibia scan, in addition to vBMD quantification, provides information on muscle and bone geometry, such as muscle and cortical bone cross-section as well as periosteal and endosteal circumference [102]. An additional advantage of pQCT is the assessment of peripheral sites devoid of iron overload. Several studies have reported better bone mass values derived from QCT compared to DXA in LS of TM patients [103, 104]. The discrepancy between the results of the two techniques has been attributed to the differential involvement of cortical and trabecular bone detected only by QCT [105]. Other authors have proposed that iron deposition may cause an overestimation of X-ray attenuation values at vertebral sites in patients inadequately chelated, when assessed with single energy techniques, such as QCT. This phenomenon is less important when vertebral bone density is assessed with DXA, which uses two X-ray energies [106, 107]. In our opinion, QCT may be superior to DXA due to its three-dimensionality, which overcomes size artefacts. However, its limited availability, higher radiation exposure, and interference in the X-ray attenuation values at vertebral sites (because of local iron deposition in not adequately chelated patients) are major limits [99] [Fig. 8].
Quantitative computed tomography (CT) in a 46-year-old female Thalassemia Major patient. Regions of interest for vertebral (black arrow), muscle (white arrow), and fat tissue (blue arrow) compartments with their respective CT Hounsfield Units value are displayed on the left. The European reference database with the superimposed red, yellow, and green colour-coding chosen to calculate the absolute density values expressed in mg/cm3; T-score and Z-score depicted on the table. The bone mineral density values relate to high fracture risk
Conclusions
The absence of a gold standard to determine bone mineral “status” in children imposes the definition of a radiological technique adjustment in this population. Although many studies report bone mass deficiency in TM patients [3, 14, 108,109,110,111], few have examined potential confounding factors or evaluated other measures to assess bone strength. Comparisons to healthy children are fundamental to identify potential biases in various techniques taking into account short stature in children with chronic diseases, who may have additional factors affecting their bone growth. A correct evaluation of bone mineral content and density in this population is mandatory because inaccurate interpretation of DXA can lead to inappropriate pharmacological treatments and physical activity limitations. Moreover, clinical factors influencing bone fragility in children have still to be fully determined. Bone densitometry by DXA is part of a comprehensive skeletal health screening, including review of nutrition, physical activity, pubertal stage, disease severity, patient and family fracture history, medication exposure, and analysis of clinical data. All these factors could play an important role in the pathogenesis of osteoporosis, microarchitectural impairment, and fragility fractures. The issue of undiagnosed spine fractures due to bone marrow expansion and overdiagnosis of minor fractures indicate the need to establish a standardized method for evaluating vertebral deformities in this population.
References
E. Voskaridou et al. A national registry of haemoglobinopathies in Greece: deducted demographics, trends in mortality and affected births. Ann. Hematol. 91, 1451–1458 (2012)
M. Baldini et al. Endocrine and bone disease in appropriately treated adult patients with beta-thalassemia major. Ann. Hematol. 89, 1207–1213 (2010)
M. Di Stefano et al. Bone mass and metabolism in thalassemic children and adolescents treated with different iron-chelating drugs. J. Bone Miner. Metab. 22, 53–57 (2004)
M.G. Vogiatzi et al. Bone disease in Thalassemia: a frequent and still unresolved problem. J. Bone Miner. Res. 24, 543–557 (2009)
E.B. Fung et al. Characterization of low bone mass in young patients with thalassemia by DXA, pQCT and markers of bone turnover. Bone 48, 1305–1312 (2011)
Y.G. Chen et al. Risk of fracture in transfusion-naïve thalassemia population: a nationwide population-based retrospective cohort study. Bone 106, 121–125 (2018)
A.A. Shamshirsaz et al. Bone mineral density in Iranian adolescents and young adults with β-thalassemia major. Pediatr. Hematol. Oncol. 24, 469–479 (2007)
W. Sutipornpalangkul et al. Prevalence of fractures among Thais with Thalassaemia syndromes. Singap. Med. J. 51(10), 817–821 (2010)
D.J. Weatherall, Fortnightly review: the thalassaemias. BMJ (1997). https://doi.org/10.1136/bmj.314.7095.1675
R. Haidar et al. Bone disease and skeletal complications in patients with β thalassemia major. Bone 48, 425–432 (2011)
M. Angastiniotis, A. Eleftheriou, Thalassaemic bone disease: an overview. Pediatr. Endocrinol. Rev. 6, 73–80 (2008)
J.A. Kanis et al. FRAXTM and the assessment of fracture probability in men and women from the UK. Osteoporos. Int. 19, 385–397 (2008)
P. Wong et al. Thalassemia bone disease: the association between nephrolithiasis, bone mineral density and fractures. Osteoporos. Int. 24, 1965–1971 (2013)
P. Mahachoklertwattana et al. Association between bone mineral density and erythropoiesis in Thai children and adolescents with thalassemia syndromes. J. Bone Miner. Metab. (2006). https://doi.org/10.1007/s00774-005-0661-0
P. Pootrakul, Relation between erythropoiesis and bone metabolism in thalassemia. J. Pediatr. Orthop. (1981). https://doi.org/10.1097/01241398-198112000-00054
T. Diamond et al. Osteoporosis in hemochromatosis: iron excess, gonadal deficiency, or others factors? Ann. Intern. Med. (1989). https://doi.org/10.7326/0003-4819-110-6-430
A. Filosa et al. Longitudinal monitoring of bone mineral density in thalassemic patients. Genetic structure and osteoporosis. Acta Paediatr. Int. J. Paediatr. (1997). https://doi.org/10.1111/j.1651-2227.1997.tb09019.x
R. Lala et al. Bone density and metabolism in thalassaemia. J. Pediatr. Endocrinol. Metab. 11(Suppl 3), 785–790 (1998)
B.K. Bielinski et al. Impact of disordered puberty on bone density in β-thalassaemia major. Br. J. Haematol. 120, 353–358 (2003)
M. L. G. Anapllotou et al. The contribution of hypogonadism to the development of osteoporosis in thalassaemia major: new therapeutic approaches. Clin. Endocrinol. (Oxf.) (2008). https://doi.org/10.1111/j.1365-2265.1995.tb01876.x
M. D. Cappellini et al. (eds), Guidelines for the Management of Transfusion Dependent Thalassemia (TDT) (Thalassemia International Federation, Nicosia, 2014)
Y.L. Chan et al. Patterns of bone diseases in transfusion-dependent homozygous thalassaemia major: predominance of osteoporosis and desferrioxamine-induced bone dysplasia. Pediatr. Radiol. 32, 492–497 (2002)
E.M. Lewiecki, Update on bone density testing. Curr. Osteoporos. Rep. (2005). https://doi.org/10.1007/s11914-996-0016-3
H.E. Meema, H. Meindok, Advantages of peripheral radiogrametry over dual-photon absorptiometry of the spine in the assessment of prevalence of osteoporotic vertebral fractures in women. J. Bone Miner. Res. 7(8), 897–903 (1992)
C.M. Gordon et al. 2013 pediatric position development conference: executive summary and reflections. J. Clin. Densitom. 17, 219–224 (2014)
J.A. Shepherd et al. Executive Summary of the 2015 ISCD Position Development Conference on Advanced Measures From DXA and QCT: fracture prediction beyond BMD. J. Clin. Densitom. (2015). https://doi.org/10.1016/j.jocd.2015.06.013
E. Voskaridou, E. Terpos, New insights into the pathophysiology and management of osteoporosis in patients with beta thalassaemia. Br. J. Haematol. (2004). https://doi.org/10.1111/j.1365-2141.2004.05143.x
R.I. Gafni, J. Baron, Overdiagnosis of osteoporosis in children due to misinterpretation of dual-energy X-ray absorptiometry (DEXA). J. Pediatr. (2004). https://doi.org/10.1016/j.jpeds.2003.08.054
E.B. Fung et al. Fracture prevalence and relationship to endocrinopathy in iron overloaded patients with sickle cell disease and thalassemia. Bone 43, 162–168 (2008)
J. A. Kanis, Osteoporosis III: diagnosis of osteoporosis and assessment of fracture risk. Lancet (2002). https://doi.org/10.1016/S0140-6736(02)08761-5
M.G. Vogiatzi et al. Prevalence of fractures among the Thalassemia syndromes in North America. Bone 38, 571–575 (2006)
N. Bishop et al. Fracture prediction and the definition of osteoporosis in children and adolescents: The ISCD 2013 Pediatric Official Positions. J. Clin. Densitom. 17, 275–280 (2014)
P. Wong et al. Thalassemia bone disease: a 19-year longitudinal analysis. J. Bone Miner. Res. 29, 2468–2473 (2014)
F. Najafipour et al. A cross-sectional study of metabolic and endocrine complications in beta-thalassemia major. Ann. Saudi Med. 28(5), 361–366 (2008)
I. Aslan et al. Bone mineral density in thalassemia major patients from Antalya, Turkey. Int. J. Endocrinol. 2012, 573298 (2012)
A. Prentice et al. Uncritical use of bone mineral density in absorptiometry may lead to size- related artifacts in the identification of bone mineral determinants. Am. J. Clin. Nutr. (1994). https://doi.org/10.1093/ajcn/60.6.837
C.M. Gordon et al. Dual energy X-ray absorptiometry interpretation and reporting in children and adolescents: The 2007 ISCD Pediatric Official Positions. J. Clin. Densitom. 11, 43–58 (2008)
K.A. Ward et al. UK reference data for the Hologic QDR Discovery dual-energy x ray absorptiometry scanner in healthy children and young adults aged 6-17 years. Arch. Dis. Child. (2007). https://doi.org/10.1136/adc.2006.097642
N. Shaw, N. Crabtree, Bone density in children: what are we measuring? Arch. Dis. Child. (2019). https://doi.org/10.1136/archdischild-2019-316940
R.P. Heaney, Bone mineral content, not bone mineral density, is the correct bone measure for growth studies. Am. J. Clin. Nutr. 78, 350–351 (2003)
M.B. Leonard et al. Interpretation of whole body dual energy X-ray absorptiometry measures in children: comparison with peripheral quantitative computed tomography. Bone 34, 1044–1052 (2004)
L.A. Binkovitz, M.J. Henwood, Pediatric DXA: technique and interpretation. Pediatr. Radiol. 37, 21–31 (2007)
M.S. Fewtrell et al. Dual X-ray absorptiometry (DXA) of the lumbar spine in a clinical paediatric setting: does the method of size-adjustment matter? Bone 37, 413–419 (2005)
B.S. Zemel et al. Height adjustment in assessing dual energy x-ray absorptiometry measurements of bone mass and density in children. J. Clin. Endocrinol. Metab. 95, 1265–1273 (2010)
L.K. Bachrach et al. Clinical report—bone densitometry in children and adolescents. Pediatrics 127, 189–194 (2011)
L.K. Bachrach, Osteoporosis in children: still a diagnostic challenge. J. Clin. Endocrinol. Metab. 92, 2030–2032 (2007)
P.W. Brill et al. Deferoxamine-induced bone dysplasia in patients with thalassemia major. Am. J. Roentgenol. 156, 561–565 (1991)
T. Rand et al. Impact of spinal degenerative changes on the evaluation of bone mineral density with dual energy x-ray absorptiometry (DXA). Calcif. Tissue Int. 60, 430–433 (1997)
S. Desigan et al. Degenerative disc disease as a cause of back pain in the thalassaemic population: a case-control study using MRI and plain radiographs. Skelet. Radiol. 35, 95–102 (2006)
S. De Virgillis et al. Deferoxamine-induced growth retardation in patients with thalassemia major. J. Pediatr. 113, 661–669 (1988)
Y.L. Chan et al. Desferrioxamine-induced long bone changes in thalassaemic patients — radiographic features, prevalence and relations with growth. Clin. Radiol. 55, 610–614 (2000)
M.J. Hartkamp et al. Spinal deformities in deferoxamine-treated homozygous beta-thalassemia major patients. Pediatr. Radiol. 23, 525–528 (1993)
A. Naselli et al. Long-term follow-up of skeletal dysplasia in thalassaemia major. J. Pediatr. Endocrinol. Metab. 11(Suppl 3), 817–825 (1998)
T.L. Levin et al. Deferoxamine-induced platyspondyly in hypertransfused thalassemic patients. Pediatr. Radiol. 25(Suppl 1), S122–S124 (1995)
L.N. Grinberg et al. Hydroxyl radical generation in β-thalassemic red blood cells. Free Radic. Biol. Med. 18, 611–615 (1995)
T. Yamaza et al. Oxidative stress-induced DNA damage in the synovial cells of the temporomandibular joint in the rat. J. Dent. Res. (2004). https://doi.org/10.1177/154405910408300807
D.J. Peacock et al. Lateral bone density measurements in osteoarthritis of the lumbar spine. Ann. Rheum. Dis. (1996). https://doi.org/10.1136/ard.55.3.196
G. Jones et al. A longitudinal study of the effect of spinal degenerative disease on bone density in the elderly. J. Rheumatol. 22(5), 932–936 (1995)
P. Korovessis et al. Incidence of scoliosis in β-thalassemia and follow-up evaluation. Spine (Phila. Pa. 1976). 21, 1798–1801 (1996)
P.G. Korovessis et al. Prevalence of scoliosis in beta-thalassemia. J. Spinal Disord. 9, 170–173 (1996)
D.A. Papanastasiou et al. Correlative analysis of the sagittal profile of the spine in patients with β-thalassemia and in healthy persons. J. Spinal Disord. (2000). https://doi.org/10.1097/00002517-200004000-00004
J.C. Cheng et al. The effect of vertebral rotation of the lumbar spine on dual energy X-ray absorptiometry measurements: observational study. Hong Kong Med. J. 7(3), 241–245 (2001)
P.A. Tyler et al. The radiological appearances of thalassaemia. Clin. Radiol. 61, 40–52 (2006)
M. Yildiz, D. Canatan, Soft tissue density variations in thalassemia major: a possible pitfall in lumbar bone mineral density measurements by dual-energy X-ray absorptiometry. Pediatr. Hematol. Oncol. 22, 723–726 (2005)
E.E. Drakonaki et al. Bone marrow changes in beta-thalassemia major: quantitative MR imaging findings and correlation with iron stores. Eur. Radiol. 17, 2079–2087 (2007)
L.K. Bachrach et al. Bone mineral acquisition in healthy Asian, Hispanic, black, and Caucasian youth: a longitudinal study. J. Clin. Endocrinol. Metab. (1999). https://doi.org/10.1210/jc.84.12.4702
N.J. Crabtree et al. Amalgamated reference data for size-adjusted bone densitometry measurements in 3598 children and young adults—the ALPHABET Study. J. Bone Miner. Res. 32, 172–180 (2017)
N.J. Crabtree et al. Diagnostic evaluation of bone densitometric size adjustment techniques in children with and without low trauma fractures. Osteoporos. Int. 24, 2015–2024 (2013)
N.J. Crabtree et al. Dual-energy x-ray absorptiometry interpretation and reporting in children and adolescents: The Revised 2013 ISCD Pediatric Official Positions. J. Clin. Densitom. 17, 225–242 (2014)
R.J. Kuczmarski et al. CDC growth charts: United States. Adv. Data 1–27 (2000)
D.F. Short et al. Anthropometric models of bone mineral content and areal bone mineral density based on the bone mineral density in childhood study. Osteoporos. Int. (2015). https://doi.org/10.1007/s00198-014-2916-x
J.M. Kindler et al. Lumbar spine bone mineral apparent density in children: results from the bone mineral density in childhood study. J. Clin. Endocrinol. Metab. (2019). https://doi.org/10.1210/jc.2018-01693
C. Mølgaard et al. Whole body bone mineral content in healthy children and adolescents. Arch. Dis. Child. (1997). https://doi.org/10.1136/adc.76.1.9
J.P. Bonjour et al. Critical years and stages of puberty for spinal and femoral bone mass accumulation during adolescence. J. Clin. Endocrinol. Metab. 73, 555–563 (1991)
A. Arabi et al. Bone mineral density by age, gender, pubertal stages, and socioeconomic status in healthy Lebanese children and adolescents. Bone (2004). https://doi.org/10.1016/j.bone.2004.06.015
N. Di Iorgi et al. Update on bone density measurements and their interpretation in children and adolescents. Best. Pract. Res. Clin. Endocrinol. Metab. 32, 477–498 (2018)
N.J. Crabtree et al. The relationship between lean body mass and bone mineral content in paediatric health and disease. Bone 35, 965–972 (2004)
W. Högler et al. Importance of lean mass in the interpretation of total body densitometry in children and adolescents. J. Pediatr. (2003). https://doi.org/10.1016/S0022-3476(03)00187-2
M.A. Petit et al. Examining the developing bone: what do we measure and how do we do it? J. Musculoskelet. Neuronal Interact. 5, 213–224 (2005)
N.J. Crabtree, K. Kent, in Bone Health Assessment in Pediatrics, ed. by E. Fung, L. Bachrach, A. Sawyer (Springer, Cham, 2016), pp. 89–114
G.M. Blake et al. Comparison of effective dose to children and adults from dual X-ray absorptiometry examinations. Bone 38, 935–942 (2006)
J. Damilakis et al. Radiation exposure in X-ray-based imaging techniques used in osteoporosis. Eur. Radiol. 20, 2707–2714 (2010)
H.M. Allard et al. Vertebral bone density measurements by DXA are influenced by hepatic iron overload in patients with hemoglobinopathies. J. Clin. Densitom. (2018). https://doi.org/10.1016/j.jocd.2018.07.001
C. Rodd et al. Incident vertebral fractures 12 months following glucocorticoid initiation in children with rheumatic disorders. Pediatr. Rheumatol. (2012)
L. Ruggiero, V. De Sanctis, Multicentre study on prevalence of fractures in transfusion-dependent thalassaemic patients. J. Pediatr. Endocrinol. Metab. 11(Suppl 3), 773–778 (1998)
P.D. Delmas et al. Underdiagnosis of vertebral fractures is a worldwide problem: the IMPACT study. J. Bone Miner. Res. (2005). https://doi.org/10.1359/JBMR.041214
N. Kim et al. Underreporting of vertebral fractures on routine chest. Radiogr. Am. J. Roentgenol. 182, 297–300 (2004)
H.K. Genant et al. Vertebral fracture assessment using a semiquantitative technique. J. Bone Miner. Res. 8, 1137–1148 (2009)
D.M. Black et al. A new approach to defining normal vertebral dimensions. J. Bone Miner. Res. 6, 883–892 (2009)
M. Grigoryan et al. Recognizing and reporting osteoporotic vertebral fractures. Eur. Spine J. 12, S104–S112 (2003)
W. Yu et al. Spinal bone mineral assessment in postmenopausal women: a comparison between dual X-ray absorptiometry and quantitative computed tomography. Osteoporos. Int. 5, 433–439 (1995)
P. Engkakul et al. Unrecognized vertebral fractures in adolescents and young adults with thalassemia syndromes. J. Pediatr. Hematol. Oncol. 35, 212–217 (2013)
A. Guermazi et al. Identification of vertebral fractures in osteoporosis. Semin. Musculoskelet. Radiol. 06, 241–252 (2002)
F.M. Ulivieri et al. Utility of the trabecular bone score (TBS) in secondary osteoporosis. Endocrine 47, 435–448 (2014)
O. Johnell et al. Predictive value of BMD for hip and other fractures. J. Bone Miner. Res. 20, 1185–1194 (2005)
M. Mylona et al. Comparison of DXA, QCT and trabecular structure in beta-thalassaemia. Eur. J. Haematol. 74, 430–437 (2005)
B.C. Silva et al. Trabecular bone score: a noninvasive analytical method based upon the DXA image. J. Bone Miner. Res. 29, 518–530 (2014)
B.C. Silva et al. Fracture risk prediction by non-BMD DXA measures: The 2015 ISCD Official Positions. Part 2: Trabecular bone score. J. Clin. Densitom. (2015). https://doi.org/10.1016/j.jocd.2015.06.008
M. Baldini et al. Spine bone texture assessed by trabecular bone score (TBS) to evaluate bone health in thalassemia major. Calcif. Tissue Int. 95, 540–546 (2014)
L. Pothuaud et al. Evaluation of the potential use of trabecular bone score to complement bone mineral density in the diagnosis of osteoporosis: a preliminary spine BMD–matched, case-control study. J. Clin. Densitom. 12, 170–176 (2009)
L. Pothuaud et al. Correlations between grey-level variations in 2D projection images (TBS) and 3D microarchitecture: applications in the study of human trabecular bone microarchitecture. Bone 42, 775–787 (2008)
J.M. Burnham et al. Bone density, structure, and strength in juvenile idiopathic arthritis: Importance of disease severity and muscle deficits. Arthritis Rheum. 58, 2518–2527 (2008)
J. Kalef-Ezra et al. Body composition in homozygous β-thalassemia. Ann. NY Acad. Sci. 904, 621–624 (2006)
S. Akpek et al. Evaluation of osteoporosis in thalassemia by quantitative computed tomography: is it reliable? Pediatr. Hematol. Oncol. 18, 111–116 (2001)
L. Danesi et al. Evaluation of spine and hip bone density by DXA and QCT in thalassemic patients. J. Pediatr. Endocrinol. Metab. 11(Suppl 3), 961–962 (1998)
T.L. Levin et al. MRI marrow observations in thalassemia: the effects of the primary disease, transfusional therapy, and chelation. Pediatr. Radiol. (1995). https://doi.org/10.1007/BF02011827
M. Tunacı et al. Imaging features of thalassemia. Eur. Radiol. 9, 1804–1809 (1999)
Jensen et al. High prevalence of low bone mass in thalassaemia major. Br. J. Haematol. 103, 911–915 (1998)
E.P. Vichinsky, The morbidity of bone disease in thalassemia. Ann. NY Acad. Sci. 850, 344–348 (1998)
B. Wonke, et al. Genetic and acquired predisposing factors and treatment of osteoporosis in thalassaemia major. J. Pediatr. Endocrinol. Metab. 11, 795–801 (1998)
U. Dundar, et al. Bone metabolism and mineral density in patients with beta-thalassemia major. Saudi Med. J. 28, 1425–1429 (2007)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Informed consent
Informed consent was obtained from all individual participants whose radiological images were included in the study.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pellegrino, F., Zatelli, M.C., Bondanelli, M. et al. Dual-energy X-ray absorptiometry pitfalls in Thalassemia Major. Endocrine 65, 469–482 (2019). https://doi.org/10.1007/s12020-019-02003-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-019-02003-x