Abstract
Summary
Thalassemia bone disease is well described, but the prevalence of nephrolithiasis has not been characterized. The association between nephrolithiasis, reduced bone density, and increased fractures has been demonstrated through this retrospective study of 166 participants with transfusion-dependent thalassemia. The findings support the need for increased vigilance of kidney and bone disease in this cohort.
Introduction
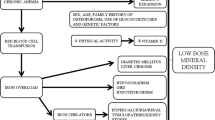
Previous studies have revealed that thalassemia is associated with reduced bone mineral density (BMD) and fractures. Many causes are implicated including hypogonadism, growth hormone deficiency, marrow expansion, and iron overload. Nephrolithiasis is associated with reduced BMD and increased fractures in the general population. However, the prevalence of nephrolithiasis and its association with bone density and fractures have not been characterized in thalassemia.
Methods
We have addressed this question by performing a retrospective cohort study of 166 participants with transfusion-dependent thalassemia who had undergone dual-energy X-ray absorptiometry between 2009 and 2011. Logistic regression modeling was used to adjust for potential confounders.
Results
We found a high prevalence of kidney stones (18.1 %) which was greater in males compared to females (28.7 vs 9.7 %, respectively). Renal stones were associated with reduced femoral neck Z-score and fractures in men after adjusting for potential confounders. These results indicate that nephrolithiasis is highly prevalent in patients with transfusion-dependent thalassemia and is significantly associated with reduced BMD and increased fractures.
Conclusions
The findings from this study strongly support the need for ongoing surveillance of BMD, fractures, and nephrolithiasis in the management of transfusion-dependent thalassemia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Thalassemia is a disorder of hemoglobin synthesis due to mutations in the globin chains (α or β). In its more severe form, it requires treatment with chronic transfusion and concomitant iron chelation therapy. Iron overload develops due to the body’s limited capacity to excrete iron, and therefore, the clinical course is similar to hemochromatosis with cardiac, liver, endocrine, and bone complications. The latter includes evidence of nephrolithiasis and decreased bone mineral density (BMD), although neither has been systematically examined.
The bone disease which accompanies nephrolithiasis is a significant but poorly addressed issue. There are large epidemiological studies which have demonstrated the association between reduced bone density and fractures with nephrolithiasis in the general community [1, 2]. While the exact pathophysiological mechanism of bone loss and fragility is not known, the hypercalciuria associated with calcium nephrolithiasis is a potential mechanism. There has been anecdotal evidence of an increased risk of kidney stones and reports of hypercalciuria [3–5] and reduced phosphate tubular reabsorption [5] in patients with thalassemia. However, despite the high prevalence of reduced BMD and fractures, the link between nephrolithiasis and urinary calcium and phosphate defects on bone disease has not been characterized in patients with thalassemia.
Transfusion-dependent thalassemia has been associated with reduced bone density and increased risk of fractures in the pediatric and particularly young adult population [6–8]. The etiology of bone disease is multifactorial and may include ineffective erythropoiesis with marrow expansion, iron toxicity, hypogonadism, and growth hormone deficiency, leading to failure to achieve peak bone mass. The measurement of BMD using dual-energy X-ray absorptiometry (DXA) is a well-established tool for assessing fracture risk in the adult population. However, the discriminative ability of DXA parameters in predicting fractures in thalassemia is limited by studies with comparatively small sample sizes and variable treatment regimens. Furthermore, the accuracy of DXA may be compromised due to differences in bone loss at cortical and trabecular sites in patients undergoing transfusion [9–11].
We conducted a retrospective cohort study of all patients with transfusion-dependent thalassemia at Monash Medical Centre (MMC) who had undergone DXA study between 2009 and 2011. A detailed medical review was performed to ascertain the prevalence of kidney stones, fractures, relevant endocrine disease, medications, and biochemistry. We investigated the relationship between DXA parameters and fractures. The associations between kidney stones, DXA parameters, and fractures were established after adjusting for relevant confounding factors. We have confirmed that nephrolithiasis in transfusion-dependent thalassemia is highly prevalent and independently associated with reduced BMD and increased fractures.
Methods
We reviewed the medical records of patients with transfusion-dependent thalassemia of all genotypes at MMC. Those who had undergone DXA study during 2009–2011 were included in the analysis of which there were 166 participants. Patients with β-thalassemia were classified as major if they had undergone more than eight transfusions with the others considered to have the minor form. The remaining thalassemia genotypes were determined from documentation in the medical records.
BMD was assessed at the lumbar spine (L2–L4) and femoral neck using a GE Lunar Prodigy (software version 12; Madison, Wisconsin), and all scans were performed in the one center at MMC. BMD was expressed as gram per centimeter square, and Z-scores were calculated relative to gender- and age-specific norms.
Nephrolithiasis and fractures were confirmed based on radiological reports or documentation in the medical record. Fractures were broadly divided into vertebral and non-vertebral fractures. All vertebral fractures were confirmed on spinal X-ray by a radiologist and available for review. Non-vertebral fractures were confirmed through a combination of documentation in the medical records or X-ray reports. Patient age and anthropometric measurements of weight and height were determined at the time of DXA with body mass index (BMI) calculated as kilogram per meter square.
Biochemical variables were determined at the time or within 6 months of the DXA study and included serum creatinine, 25(OH) vitamin D, calcium and phosphate. A low vitamin D level was defined as less than 75 nmol/L. The results of 24-h urine calcium excretion were documented where available. Hypercalciuria was defined as a 24-h urine calcium excretion greater than 7.5 mmol/day.
A past history of hypogonadism was established from the medical record. This was defined in males as the use of androgen replacement therapy or low testosterone levels (<8 nmol/L) documented on two separate occasions. In females, hypogonadism was defined as menarche occurring after the age of 16, use of hormone replacement therapy for induction of pubertal development, menopause before the age of 40, or documentation of low estradiol levels (<73 pmol/L) on two separate occasions at least 6 months apart. Hypoparathyroidism was defined as a low corrected serum calcium level (<2.10 mmol/L) in the setting of low or normal serum PTH (normal range 1.5–7.5 pmol/L). The daily dose of supplemental elemental calcium (in milligram per day), ergocalciferol (in international unit per day), and iron chelation therapy was determined at the time of DXA study from review of medication charts. A previous history of bisphosphonate use was documented.
We used logistic regression modeling to adjust for potential confounders. The association between areal BMD and fractures was determined after adjusting initially for age and, then subsequently, for age and nephrolithiasis. The independent association between nephrolithiasis and DXA Z-scores was determined after adjusting for BMI, calcium, and vitamin D supplementation. The relationship between kidney stones and fractures was calculated after adjusting for BMI, previous bisphosphonates use, 25(OH) vitamin D levels, hypogonadism, and femoral and lumbar spine Z-score separately. As the Z-score was matched to age and gender controls, these two variables were not included in the regression models.
We used the Mann–Whitney test to examine the relationship between gender, nephrolithiasis, and fractures to biochemical variables, medications, and hypogonadism for continuous variables and Fisher exact test for categorical variables. All normally distributed data were expressed as mean with standard deviation, and nonparametric data were expressed as median with minimum and maximum ranges. A p value of <0.05 was considered to be statistically significant, and all tests were two sided. Analyses were conducted using SPSS 15 (version 15.0; SPSS, Inc., Chicago, IL).
Results
We reviewed the medical records of 166 patients with transfusion-dependent thalassemia at MMC who had undergone DXA study during 2009–2011. There were 73 (44 %) male and 93 (56 %) female participants aged between 4 and 66 years with a median age of 35 years. There were 153 (92 %) participants with β-thalassemia major and 11 (6.6 %) with HbH disease; 2 (1.4 %) participants had other thalassemia genotypes. The prevalence of hypogonadism was 50 % and was more common in females (53.7 %) than males (45.2 %) (Table 1).
There were 30 (18.1 %) patients with documented nephrolithiasis with the majority occurring in men (28.8 %) compared to women (9.7 %). These patients had a higher BMI (p = 0.01), were older (p = 0.02), and more likely to be male (p = 0.001). They also had higher serum creatinine (p = 0.001) and 25(OH) vitamin D (p = 0.01) levels, although the median value was still within the normal reference range (Table 2). The serum ferritin (p = 0.02) and phosphate levels (p = 0.02) were lower in the renal stone group. Hypoparathyroidism was documented in 10.2 % of patients but was not significantly associated with nephrolithiasis. Patients with hypoparathyroidism had lower serum calcium levels than those without (2.25 vs 2.34 mmol/L, p = 0.094; data not shown). A 24-h urine calcium excretion was available in 13 patients with a history of kidney stones, and 5 patients had documented hypercalciuria.
All DXA parameters were significantly lower in participants with a history of fracture, and male patients had lower BMD values than females (Table 3). All DXA parameters were inversely associated with fractures and were statistically significant. The prevalence of fractures was 33 (19.9 %) with the majority being vertebral fractures (94 %) localized to the thoracic spine. There was a history of previous bisphosphonate use in 21 (12.7 %) participants, and men sustained more fractures than women (27.4 vs 14 %, p = 0.03). Older participants were more likely to have had fractures (p = 0.04), but BMI was similar between the fracture and non-fracture group (p = 0.60) (Table 3).
After adjusting for BMI, calcium, and vitamin D supplementation, the risk of nephrolithiasis per unit reduction in femoral neck Z-score was 1.63 [95 % confidence interval (CI) 1.1–2.42, p = 0.02] (Table 4). There was no significant association between fractures and kidney stones in the overall analysis. However, in a subgroup analysis, nephrolithiasis was highly associated with increased fracture prevalence in men after accounting for BMI, previous bisphosphonates use, hypogonadism, and 25(OH) vitamin D levels and separately adjusting for lumbar spine and femoral neck Z-score [odds ratio (OR) = 5.59, 95 % CI 1.16–27.03; OR = 5.21, 95 % CI 1.06–25.64, respectively] (Table 5). The OR of fracture for each standard deviation reduction in femoral neck and lumbar spine areal BMD was 2.13 and 2.31, respectively, after adjusting for age. However, there was not a significant change in OR of fracture for areal BMD after both age and kidney stones were taken into account (Table 6).
Discussion
Bone disease represents a significant disease burden, and fracture prevalence had been reported to be 12.1 % in young patients with thalassemia [6]. This compares to a 5-year fracture incidence of 4.9 % in people aged 35 to 44 in the general population [12]. We confirmed the prevalence of nephrolithiasis in transfusion-dependent thalassemia to be 18.1 % and nephrolithiasis to be significantly associated with reduced femoral neck Z-score and increased fracture risk in males. The relationship between nephrolithiasis with BMD and fractures was maintained despite adjusting for confounding factors. While each standard deviation reduction in areal BMD was associated with an approximate doubling of fracture risk, this risk was not significantly attenuated when controlled for kidney stones.
There are some limitations to the use of DXA, a two-dimensional measure of bone density, in pubertal patients with thalassemia. The age of peak bone mass is delayed, and the effect of changing bone size, shape, and strength in the growing adolescent are important considerations. The degree of bone loss at cortical and trabecular sites is also different in thalassemia. We demonstrated lower Z-scores at the lumbar spine compared to the femoral neck which is consistent with other studies [7]. A possible explanation may be the significant marrow expansion occurring in the spine, leading to reduced bone mass [13]. Peripheral quantitative computer tomography (pQCT) allows cortical and trabecular sites to be studied separately. There have been small studies comparing the use of DXA and pQCT which have demonstrated reduced pQCT trabecular and cortical parameters in thalassemia compared to controls [9, 10].
We found increasing age to be a significant risk factor for fractures. Although hypogonadism was common in this study, most participants were adequately treated with sex hormone replacement, and hypogonadism was not found to be highly associated with fractures. The significant relationship between previous bisphosphonate use and calcium supplementation to fractures likely reflects a treatment effect bias. While increased erythropoiesis from suboptimal treatment leads to reduced bone density [13], we did not find any relationship between the dose of deferasirox (iron chelator) or serum ferritin with fractures.
Nephrolithiasis has a prevalence of 4.9–6.3 % in men and 2.8–4.1 % in women [14] in the general community and is more common with increasing age and weight [14–16]. The burden of nephrolithiasis in our thalassemia cohort was far greater with a prevalence of 28.8 % in males and 9.7 % in females. The relationship between calcium and vitamin D intake to nephrolithiasis had been examined in many large population-based studies including the Women’s Health Initiative, which showed a 17 % increased risk of calculi in women taking calcium and vitamin D [17]. Many studies have not shown an increased risk of calculi formation with dietary calcium [18, 19] but suggested an increased risk with supplemental calcium when taken without food [20, 21]. In the absence of food, calcium is unable to bind to dietary oxalate, leading to increased calcium absorption and urinary excretion [20]. As most patients will intentionally reduce their intake of calcium and vitamin D after a kidney stone, this may erroneously suggest a relationship between low calcium and vitamin D intake to renal calculi.
Kidney stones are associated with reduced BMD and increased fractures in the general population [1, 2]. The exact etiology of this phenomenon is unclear, but much research has focused on the role of idiopathic hypercalciuria which is thought to cause stones through calcium oxalate and calcium phosphate supersaturation [22]. Although we were unable to obtain data on renal stone composition or urine calcium excretion in all our patients, the increased prevalence of hypercalciuria in thalassemia suggests that this may be a possible pathogenic mechanism for bone loss. Hypercalciuria may be associated with bone loss through increased bone resorption in an attempt to maintain normal serum calcium. Renal phosphate wasting has been reported to predispose to calcium stone formation [23, 24]. These defects in renal tubular handling of calcium and phosphate are not fully understood, but a Fanconi-like syndrome has been described in patients with thalassemia [4, 5, 25]. We have previously reported a case of deferasirox-induced renal phosphate wasting leading to hypophosphatemic osteomalacia in a patient with β-thalassemia major [26].
Iron overload is a universal complication of transfusion therapy and is highly correlated with increasing disease morbidity and mortality in patients with thalassemia [27]. We found that reduced ferritin levels were associated with kidney stones. As serum ferritin is a good surrogate marker of the adequacy of iron chelation treatment, iron chelators may have a pathogenic role in kidney stone formation. The majority of our patients were on deferasirox, which is known to cause a mild reversible increase in creatinine [28] and phosphate wasting as part of a Fanconi-like syndrome [29, 30]. We have shown reduced serum ferritin and phosphate and increased creatinine to be highly associated with nephrolithiasis. To our knowledge, renal tubulopathies have not been described with other iron-chelating agents such as desferrioxamine. While it is not possible to establish a causal link to deferasirox in this case, further studies are warranted.
Kidney stones were more common in males, and this is similar to that in the general community. While we believe that transfusion-related complications are important factors in stone formation, there are likely to be other dietary, environmental, or gender-specific factors which contribute to the sexual dimorphism. The relationship between nephrolithiasis to BMD and fractures appears to be stronger in men than in women in the general population [1]. In our subgroup analysis, we found renal stones to be associated with fractures in males but not in females. There could be several reasons for this observation. Males had lower Z-scores and are transfused to a higher hemoglobin level compared to females. The greater prevalence of fractures in young males may also reflect participation in higher risk-taking activities. Although this study is relatively large for transfusion-dependent thalassemia, it is small compared to the majority of postmenopausal osteoporosis studies. This may explain why a statistically significant relationship between fractures and nephrolithiasis was not seen in the overall analysis.
There are several caveats with this study. A definitive causal link between renal stones, low BMD, and increased fractures cannot be formally established due to the retrospective nature of this study, nor can the pathogenesis of renal stones, as data on stone composition and 24-h urine calcium measurements were not available for all patients. The findings from this study cannot be extrapolated to patients with less severe thalassemia where complications related to chronic transfusion are less likely.
We have shown for the first time a significant association between nephrolithiasis, reduced BMD, and increased fractures in patients with transfusion-dependent thalassemia after adjusting for confounding factors. This strongly argues that enquiring about a history of nephrolithiasis along performing urine calcium and DXA studies is required in all patients with transfusion-dependent thalassemia. Further studies are needed to determine the pathogenesis and composition of kidney stones and the influence of gender on the prevalence of nephrolithiasis.
References
Lauderdale DS, Thisted RA, Wen M, Favus MJ (2001) Bone mineral density and fracture among prevalent kidney stone cases in the third national health and nutrition examination survey. J Bone Miner Res 16:1893–1898
Melton Iii LJ, Crowson CS, Khosla S, Wilson DM, O’Fallon WM (1998) Fracture risk among patients with urolithiasis: a population-based cohort study. Kidney Int 53:459–464
Vogiatzi MG, MacKlin EA, Trachtenberg FL et al (2009) Differences in the prevalence of growth, endocrine and vitamin D abnormalities among the various thalassaemia syndromes in North America. Br J Haematol 146:546–556
Quinn CT, Johnson VL, Kim HY et al (2011) Renal dysfunction in patients with thalassaemia. Br J Haematol 153:111–117
Ponticelli C, Musallam KM, Cianciulli P, Cappellini MD (2010) Renal complications in transfusion-dependent beta thalassaemia. Blood Rev 24:239–244
Vogiatzi MG, Macklin EA, Fung EB, Vichinsky E, Olivieri N, Kwiatkowski J, Cohen A, Neufeld E, Giardina PJ (2006) Prevalence of fractures among the thalassemia syndromes in North America. Bone 38:571–575
Vogiatzi MG, Macklin EA, Fung EB et al (2009) Bone disease in thalassemia: a frequent and still unresolved problem. J Bone Miner Res 24:543–557
Jensen CE, Tuck SM, Agnew JE, Koneru S, Morris RW, Yardumian A, Prescott E, Hoffbrand AV, Wonke B (1998) High prevalence of low bone mass in thalassaemia major. Br J Haematol 103:911–915
Fung EB, Vichinsky EP, Kwiatkowski JL, Huang J, Bachrach LK, Sawyer AJ, Zemel BS (2011) Characterization of low bone mass in young patients with thalassemia by DXA, pQCT and markers of bone turnover. Bone 48:1305–1312
Ladis V, Raptou P, Rigatou E, Chouliaras G, Galanos A, Korres D, Kattamis C (2008) Study of bone density by pQCT analysis in healthy adults and patients with B-thalassemia major and intermedia. Pediatr Endocrinol Rev 6:127–131
Ladis VA, Gandaifis N, Papadopoulos EC, Gavras GM, Papassotiriou I, Korres DS, Kattamis CA (2004) Bone density study at the distal radius, using pQCT analysis, in Greek thalassemic patients. Pediatr Endocrinol Rev 2:307–309
Knowelden J, Buhr AJ, Dunbar O (1964) Incidence of fractures in persons over 35 years of age. A report to the M.R.C. working party on fractures in the elderly. Br J Prev Soc Med 18:130–141
Mahachoklertwattana P, Pootrakul P, Chuansumrit A, Choubtum L, Sriphrapradang A, Sirisriro R, Rajatanavin R (2006) Association between bone mineral density and erythropoiesis in Thai children and adolescents with thalassemia syndromes. J Bone Miner Metab 24:146–152
Stamatelou KK, Francis ME, Jones CA, Nyberg LM Jr, Curhan GC (2003) Time trends in reported prevalence of kidney stones in the United States: 1976–1994. Kidney Int 63:1817–1823
Taylor EN, Stampfer MJ, Curhan GC (2005) Obesity, weight gain, and the risk of kidney stones. JAMA 293:455–462
Daudon M, Lacour B, Jungers P (2006) Influence of body size on urinary stone composition in men and women. Urol Res 34:193–199
Jackson RD, LaCroix AZ, Gass M et al (2006) Calcium plus vitamin D supplementation and the risk of fractures. N Engl J Med 354:669–683
Curhan GC, Willett WC, Knight EL, Stampfer MJ (2004) Dietary factors and the risk of incident kidney stones in younger women: nurses’ health study II. Arch Intern Med 164:885–891
Heaney RP (2008) Calcium supplementation and incident kidney stone risk: a systematic review. J Am Coll Nutr 27:519–527
Curhan GC, Willett WC, Speizer FE, Spiegelman D, Stampfer MJ (1997) Comparison of dietary calcium with supplemental calcium and other nutrients as factors affecting the risk for kidney stones in women. Ann Intern Med 126:497–504
Favus MJ (2011) The risk of kidney stone formation: the form of calcium matters. Am J Clin Nutr 94:5–6
Pak CY (1969) Physicochemical basis for formation of renal stones of calcium phosphate origin: calculation of the degree of saturation of urine with respect to brushite. J Clin Invest 48:1914–1922
Prié D, Ravery V, Boccon-Gibod L, Friedlander G (2001) Frequency of renal phosphate leak among patients with calcium nephrolithiasis. Kidney Int 60:272–276
Prié D, Huart V, Bakouh N et al (2002) Nephrolithiasis and osteoporosis associated with hypophosphatemia caused by mutations in the type 2a sodium-phosphate cotransporter. N Engl J Med 347:983–991
Yacobovich J, Stark P, Barzilai-Birenbaum S, Krause I, Pazgal I, Yaniv I, Tamary H (2010) Acquired proximal renal tubular dysfunction in β-thalassemia patients treated with deferasirox. J Pediatr Hematol Oncol 32:564–567
Milat F, Wong P, Fuller PJ, Johnstone L, Kerr PG, Doery JCG, Strauss BJ, Bowden DK (2012) A case of hypophosphatemic osteomalacia secondary to deferasirox therapy. J Bone Miner Res 27:219–222
Borgna-Pignatti C, Rugolotto S, De Stefano P et al (2004) Survival and complications in patients with thalassemia major treated with transfusion and deferoxamine. Haematologica 89:1187–1193
Cappellini MD, Cohen A, Piga A et al (2006) A phase 3 study of deferasirox (ICL670), a once-daily oral iron chelator, in patients with β-thalassemia. Blood 107:3455–3462
Rheault MN, Bechtel H, Neglia JP, Kashtan CE (2011) Reversible Fanconi syndrome in a pediatric patient on deferasirox. Pediatr Blood Cancer 56:674–676
Wei HY, Yang CP, Cheng CH, Lo FS (2011) Fanconi syndrome in a patient with β-thalassemia major after using deferasirox for 27 months. Transfusion 51:949–954
Acknowledgments
P. W. is supported by an Australian Postgraduate Scholarship and Royal Australian College of Physicians and Osteoporosis Australia scholarship. This work was supported through an unrestricted educational grant from Servier, Merck Sharp, and Dohme and Sanofi-Aventis to F. M. and P. W., and a National Health and Medical Research Council (Australia) Senior Principal Research Fellowship to P. J. F. (grant number 1002559). Prince Henry’s Institute is supported by the Victorian Government’s Operational Infrastructure Support program.
Conflicts of interest
The authors declare that they have no disclosures.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wong, P., Fuller, P.J., Gillespie, M.T. et al. Thalassemia bone disease: the association between nephrolithiasis, bone mineral density and fractures. Osteoporos Int 24, 1965–1971 (2013). https://doi.org/10.1007/s00198-012-2260-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-012-2260-y