Abstract
Purpose
Calcium carbonate was previously shown to interfere with l-thyroxine absorption. To estimate the magnitude of tablet l-thyroxine malabsorption caused by calcium carbonate, with resulting increase in serum thyrotropin (TSH), we performed a cohort study in a referral care center.
Methods
Fifty postmenopausal hypothyroid l-thyroxine-treated women (age 71.7 ± 5.1 years) who added calcium supplementation (600–1000 mg/day) were considered. They were taking l-thyroxine 45–60 min before breakfast (setting 1). After 4.4 ± 2.0 years from initiation of l-thyroxine therapy, they took calcium supplemaentation within 2 h after l-thyroxine taking (setting 2) for 2.3 ± 1.1 years. Hence, we recommended postponing calcium intake 6–8 h after l-thyroxine (setting 3). We evaluated TSH levels, the prevalence of women with elevated TSH (>4.12 mU/L), total cholesterolemia, fasting glycemia, blood pressure, and the prevalence of hypercholesterolemia, hyperglycemia, and hypertension.
Results
TSH levels were 3.33 ± 1.93 mU/L versus 1.93 ± 0.51 or 2.16 ± 0.54 comparing setting 2 with setting 1 or 3 (P < 0.001, both). In setting 2, 18% women had elevated TSH versus none in setting 1 or 3 (P < 0.01). Total cholesterolemia, fasting glycemia, systolic, and diastolic blood pressure were also significantly higher in setting 2 compared to settings 1 and 3. For every 1.0 mU/L increase within the TSH range of 0.85–6.9 mU/L, total cholesterolemia, glycemia, systolic, and diastolic blood pressure increased by 12.1, 3.12 mg/dL, 2.31, and 2.0 mmHg, respectively.
Conclusions
Monitoring of hypothyroid patients who ingest medications that decrease l-thyroxine absorption should not be restricted to solely measuring serum TSH.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hypothyroidism and osteoporosis are frequent disorders, both having a prevalence that increases as age advances and a predilection for women [1,2,3,4]. Accordingly, postmenopausal women are the largest group of persons who take L-T4 for the therapy of hypothyroidism and calcium carbonate for the prevention or therapy of osteoporosis [5]. The recommended replacement hormone for the therapy of both primary and central hypothyroidism is levothyroxine (L-T4) [2, 3], and such therapy in primary hypothyroidism is monitored by periodic measurements of serum thyrotropin (TSH) [2, 3]. Target levels of TSH are recommended to be ≤4.12 mU/L [2, 3]. Though the upper normal limit of TSH in the age band 61–80 years ranges from 4.62 to 5.23 mU/L (depending on month of the year, with no gender differences at any of the 12 months) [6], the vast majority of endocrinologists aim to TSH levels ≤4.0 mU/L even in hypothyroid patients in their 9th decade of life [7]. Failure of TSH to reach target levels, viz. undertreated hypothyroidism, is frequently caused by the oral ingestion of medications that impair the intestinal absorption of L-T4 [2, 3, 8,9,10].
The increased need of the L-T4 daily dose caused by calcium supplementation is due to non-specific hormone binding [5], a physical sequestration that diminishes the free L-T4 available for the intestinal absorption. Of calcium carbonate, calcium citrate, and calcium acetate, clear evidence of such sequestration exists for carbonate [5, 11,12,13]. Such interference of calcium and other drugs refers to the classic tablet formulation of L-T4. Recent reports indicate that novel formulations (soft gel capsule, oral solution) resist to the interference caused by drugs, food, beverages, and gastrointestinal disorders [14,15,16,17,18]. However, these novel formulations are currently available only in some countries.
There is abundant evidence for metabolic and cardiovascular complications associated with increased TSH (overt and subclinical hypothyroidism) and even with TSH in the upper part of the normal range [19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35]. Regardless of the presence of metabolic syndrome or type 2 diabetes mellitus, these complications include the direct, unfavorable correlation of TSH with fasting blood glucose [20,21,22,23,24], insulin resistance [21, 22, 25, 26], total and LDL-cholesterol [18,19,20, 23, 24, 26, 27], systolic and/or diastolic blood pressure (DBP) [20, 25, 26, 29,30,31,32,33,34,35,36]. One or more of those relationships were statistically significant in women [23, 26, 27, 30, 31]. Thus, it is not surprising that subclinical hypothyroidism and TSH levels in the upper part of the normal range are associated with mortality from cardiovascular disease, cerebrovascular disease, or even all-cause mortality [36,37,38,39,40,41,42].
Relevant for our present study is a recent observational study on 611 hospitalized elderly patients, in whom TSH and free thyroxine (FT4) levels were measured between at least 30 days after discharge and until death or end of follow-up [43]. The main outcome measure was all-cause mortality up to 66 months after discharge based on TSH and FT4 categories. The authors concluded that (i) in treated hypothyroid adult patients, median TSH levels of 5–10 IU/L are associated with increased mortality and (ii) treatment should aim at achieving euthyroidism to improve survival [43].
In brief, in a population of L-T4-treated postmenopausal hypothyroid women with stable target levels of TSH (≤4.12 mU/L) under no interfering drugs, and who subsequently added calcium supplementation, we aimed to evaluate (i) the magnitude of the interference on tablet L-T4 malabsorption caused by calcium supplementation (namely, average serum TSH and proportion of women with elevated TSH levels [>4.12 mU/L]) and (ii) the magnitude of worsening of relevant factors (namely, average total cholesterolemia, fasting glycemia, blood pressure, and proportion of hypercholesterolemia, hyperglycemia, and hypertension) while TSH was above target levels.
Materials and methods
Patients
To maximize our search for hypothyroid patients who were simultaneously taking calcium salts, we took advantage of the outpatient ambulatory facility for Osteoporosis at our University hospital. This facility has a computerized database that records all past and concurrent diseases and medications, particularly endocrino-metabolic diseases and associated both medications and laboratory data. Physical examination, including measurement of blood pressure, is also recorded.
Of the approximate 1400 women in the database, 203 (14.5%) were taking L-T4 tablet for primary hypothyroidism, had TSH and at least two clinical chemistry data (total cholesterolemia and fasting glycemia) recorded at all the three time points of our study and always measured in the same laboratory. Of these 203 women, 50 (24.6%) added oral calcium salts to the initial L-T4 replacement therapy, with (n = 44) or without (n = 6) oral vitamin D supplementation but no other medication/supplement known to impair the intestinal absorption of L-T4. Mean age of these 50 patients at the time data were collected for the present study was 71.7 ± 5.1 years (median 71). The fundamental causes of hypothyroidism were Hashimoto’s thyroiditis or surgical thyroidectomy. Calcium was always calcium carbonate, and it was taken at the daily dose of 600 or 1000 mg/day elemental calcium. When vitamin D supplementation had been given, it was taken at 800 IU/day. Calcium carbonate with or without vitamin D supplementation had been prescribed by the family physicians almost always. Tablet L-T4 was either one of the two brand medication marketed in Italy (Eutirox, Merck, Darmstadt, Germany or Tirosint, IBSA s.r.l., Lodi).
Tablet L-T4 was always taken with plain water 45-60 min prior to breakfast, and calcium carbonate 60–120 min after ingestion of the L-T4 tablet in all the patients. The daily dose of L-T4 was 1.43 ± 0.24 µg/kg bw (median = 1.47). At the time the database was probed to collect data reported here, L-T4 therapy had been taken for 4.4 ± 2.0 years prior to adding the said supplementation with calcium carbonate ± vitamin D. In turn, the supplementation with calcium carbonate ± vitamin D had been taken for 2.3 ± 1.1 years, during which tablet L-T4 continued to be taken.
Knowing that an interval of 1–2 h between ingestion of L-T4 and the subsequent ingestion of calcium salts is insufficient to avoid the sequestration of L-T4 by calcium, we instructed the 50 women to postpone ingestion of calcium carbonate after lunch, corresponding to 6–8 h after ingestion of L-T4 in the morning. Patients were seen again after 3 months, and pertinent data recorded, including information on hypercholesterolemia, hypertension, and hyperglycemia, as defined by authoritative panel of experts [44, 45].
Informed consent was obtained from all individual participants included in the study.
Statistics
Data are reported as mean ± SD. Differences between means were analyzed by the two-tailed Student’s t-test, with log10 transformation for serum levels of TSH due to their non-Gaussian distribution. Differences between proportions were analyzed by the two-tailed Fisher’s exact test or chi square (χ2) test, as appropriate. P values of <0.05 or below were considered statistically significant, while P values comprised between 0.10 and 0.05 as borderline significant.
Results
The frequency of L-T4 undertreatment caused by calcium carbonate taken in the morning within two hours after the L-T4 tablet was 18%, in that serum TSH levels >4.12 mU/L were recorded in 9/50 women. These 9 women will be referred to as group A, and the remaining 41 as group B. The 9/50 proportion is significantly greater than the 0/50 proportion (P < 0.01) observed when the same women had been under L-T4 alone. Group A women did not differ from group B women in terms of age (72.6 ± 6.6 vs. 71.6 ± 4.8 years, P = 0.62), body weight (70.4 ± 4.7 vs. 67.3 ± 9.3 kg, P = 0.34), daily dose of L-T4 (105.6 ± 24.3 vs. 96.1 ± 21.5 µg/kg bw/d, P = 0.25) or supplementation with calcium carbonate alone (1/9 [11%] vs 5/41 [12%]).
Calcium carbonate taken within two hours after tablet L-T4 ingestion
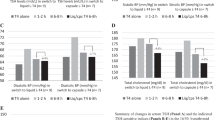
Data handled as continuos and categorical variables, for all 50 women and for group A and group B separately, are presented in Figs. 1 and 2, respectively. In the 50 women, all variables increased significantly when L-T4 was accompanied by treatment with calcium carbonate compared to baseline (ingestion of L-T4 alone). The increase of TSH was 73%, while the increases of total cholesterolemia, fasting glycemia, systolic blood pressure (SBP), and DBP were 7.5%, 7.8%, 4.8%, and 7.0%, respectively (Fig. 1, top panel).
Top panel: Mean ± SD levels of the indices evaluated in the 50 women as a whole under the three specified conditions (baseline [before adding calcium carbonate to the tablet l-thyroxine replacement therapy], calcium supplementation with calcium taken in the morning (AM) 1–2 h after l-thyroxine or calcium supplementation with calcium taken in the afternoon (PM) 6–8 h after l-thyroxine). Bottom panel: Mean ± SD levels of the indices evaluated in the two groups of women (group A and group B) under the three conditions specified in Top panel. Comparisons are within group and between groups. *P between 0.10 and 0.05; **P < 0.05; ***P < 0.01; ****P < 0.001. The symbols for at least a borderline significant difference (P value between 01.0 and 0.05) appearing to the right of a given mean ± SD value for an index of the A group refer to the intergroup comparison
Top panel: Frequency of elevated TSH, total cholesterolemia, fasting glycemia, systolic blood pressure, and diastolic blood pressure in the 50 women under the three conditions specified in Fig. 1. Bottom panel: Frequency of elevated TSH, total cholesterolemia, fasting glycemia, systolic blood pressure, and diastolic blood pressure in the two groups of women (group A and group B) under the three conditions specified in Fig. 1. *P between 0.10 and 0.05; **P < 0.05; ***P < 0.01; and ****P < 0.001. The symbols for at least a borderline significant difference (P comprised between 01.0 and 0.05) that appear to the right of a given mean ± SD value for an index of the A group refer to the intergroup comparison
When women took L-T4 alone, serum TSH was not statistically greater in group A compared to group B and so were total cholesterol, fasting glucose, and blood pressure (Fig. 1, bottom panel). However, when group A women added calcium supplementation, TSH increased by 124%, trenspassing the 4.12 mU/L threshold in all nine women of group A by definition. The increase in serum TSH was of lower magnitude, yet highly significant (+57%), in group B. In either group, each index increased compared with the period of ingestion of L-T4 alone (from 6.1% [SBP] to 17.3% [total cholesterolemia] in group A, and from 4.5% [SBP] to 7.1% [fasting glycemia] in group B) (Fig. 1, bottom panel).
In the inter-group comparison, mimiking serum TSH, each index measured after ingesting calcium carbonate was consistently greater in group A compared with group B, but only total cholesterolemia was significantly so (Fig. 1, bottom panel).
Handling indices categorically (Fig. 2, top panel) and comparing ingestion of calcium within 2 h after taking L-T4 vs. L-T4 alone in the 50 women as a whole, the greatest increase in frequency (four-fold) was observed for total cholesterolemia. Considering groups separately, the prevalence of hypertension, hyperglycemia, or hypercholesterolemia at baseline (therapy with L-T4 alone) in group A was statistically similar to its counterpart in group B. In either group, frequency of each index increased and was the highest when L-T4 was accompanied by taking calcium within the next 2 h (Fig. 2, bottom panel).
Using a four-tier stratification (no complications, any one, any two, or all three complications), compared to baseline (taking L-T4 alone) the ingestion of calcium carbonate within 2 h after having taken the L-T4 tablet was associated with a significantly worse pattern of complications in the 50 patients (P = 0.012) (Fig.3), with the frequency of no complication falling from 50% to 25% and frequency of any two complications increasing from 0% to 22%. When the two groups were analyzed separately (Fig. 4), the pattern of complications was worse at a borderline significant level in either group (P = 0.07 in group A and P = 0.076 in group B). The statistically significant inter-group difference in this 4-category stratification (Df = 3, χ2 = 8.8, P = 0.03) was accounted for by the 0% vs. 32% frequencies (P = 0.089) in the no complication category at one extreme, and the 33% vs. 5% frequencies (P = 0.031) in the all-three-complications category at the other extreme (Fig. 4). In brief, ingestion of calcium carbonate within 2 h after having taken the L-T4 tablet resulted in a 1.5-fold increased rate of having at least one complication (50–74%), a difference that was accounted for by group A (55–100% vs. 49–68%) (Fig. 4).
Frequency of complications (hypercholesterolemia, hyperglycemia, hypertension) in the 50 women under the three conditions specified in Fig. 1. In the left part of the figure, women are presented on a four-tier stratification (no complication, any one, any two, or all three complication), while in the right part of the figure, women are presented on a two-tier stratification (no complications and at least one complication). For the definition of complications, see text (section Patients and Methods)
Frequency of complications (hypercholesterolemia, hyperglycemia, hypertension) in the two groups of women (group A and group B) under the three conditions specified in Fig. 1. For stratification based on complications, see Fig. 5. For the definition of complications, see text (section Patients and Methods)
Calcium carbonate ingestion postponed (6–8 h after tablet L-T4 ingestion)
Data are presented in Figs. 1–4. Postponing calcium carbonate ingestion, TSH and other indices decreased. However, cholesterolemia, glycemia, and SBP remained consistently greater compared to the corresponding levels when L-T4 alone was taken. Indeed, Δ-cholesterolemia was +5.6 mg/dL in group A and +0.6 mg/dL in group B; Δ-glycemia was +1.5 mg/dL in group A and +2.1 mg/dL in group B, the latter being statistically significant; Δ-SBP +2.2 and +0.9 mmHg, respectively (Fig. 1, bottom panel).
In the comparison between group A and group B, total cholesterolemia was greater (P < 0.05) and glycemia was borderline significantly greater (P = 0.096) (Fig. 1, bottom panel). The pattern of complications was similar to the baseline counterpart, including the frequency of having at least one complication (54% vs. 50% at baseline in all women, 55% vs. 55% in group A, and 54% vs. 49% in group B) (Figs. 3 and 4).
Correlation and linear regression analysis
Table 1 summarizes the linear correlation between TSH (independent variable) and each of the other indices (dependent variables) in the 50 women as a whole and in the two groups separately.
In the regression analysis (not shown), for every 1.0 mU/L increase within the TSH range of 0.85–6.9 mU/L measured in the 50 women, total cholesterolemia, glycemia, SBP, and DBP increase by 12.1 mg/dL (95% CI 9.1–14.4), 3.12 mg/dL (−0.42 to 6.66), 2.31 mmHg (0.82–3.80), and 2.0 mmHg (0.94–3.06), respectively. All slopes deviate significantly from zero (P < 0.0001 [TSH], P = 0.0029 [SBP], P = 0.0003 [DBP]), except glycemia that deviates borderline significantly (P = 0.086).
Discussion
One major finding of the study reported here is that the impaired absorption of tablet L-T4 caused by insufficiently postponed ingestion of calcium continues to be poorly appreciated by practitioners and some endocrinologists. In our study, approximately one-fifth of postmenopausal women had serum levels >4.12 mU/L upon ingesting calcium carbonate one to two hours after ingestion of tablet L-T4. In contrast, as we also report here, if calcium carbonate is postponed by 6–8 h, all women had TSH below that threshold.
In the 3-month duration study by Singh et al. [5], 20 hypothyroid patients (age range, 27–78 years; women = 9/20) were taking a stable long-term regimen of L-T4 and had TSH in the normal range. These 20 patients were instructed to take 1200 mg/day of elemental calcium as calcium carbonate, ingested with their L-T4, for 3 months. No further details on timing were given, except stating that both calcium carbonate and L-T4 were ingested “in the morning on an empty stomach”. Serum TSH increased significantly from 1.60 ± 0.22 to 2.88 ± 0.41 at month 2, and 2.71 ± 0.43 mU/L at month 3. This average increase by 80% and 69%, respectively, agrees nicely with our 73% in our sample. Serum TSH returned to baseline after stopped taking calcium carbonate. However, this was not the case of our patients, in whom calcium carbonate was postponed (not withdrawn) and TSH remained significantly greater than baseline.
Interestingly, a binding study revealed that at a gastric pH 2, but not at pH 7.4, a significant fraction of thyroxine was adsorbed to calcium carbonate in a dose-dependent manner, pointing out for the role of gastric pH, which is known to be possibly impaired in hypothyroid patients or under specific treatment regimen in current clinical practice [46].
A second major finding is that the elevation in serum TSH (on the average, +67% in the all 50 women) is accompanied by a change of blood pressure, total cholesterolemia, and fasting glycemia in the unfavorable direction (increase). The increase of these indices was more pronounced in the group of women with TSH which passed the 4.12 mU/L threshold. On the average, the 1.93 mU/L difference in concentration of TSH (4.91 vs. 2.98 mU/L) was accompanied by a 27 mg/dL difference in total cholesterolemia, 8.0 mg/dL difference in glycemia, and 3.4 and 3.0 mmHg difference in SBP and DBP. These data and their amelioration upon postponing calcium carbonate ingestion are consistent with the direct, positive relation between TSH and each of these indices [19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35] and are also consistent with the recent study by Inoue et al. [42]. This prospective cohort study was conducted using a nationally representative sample of 12,584 US adults aged ≥20 years with TSH levels within the reference range from the National Health and Nutrition Examination Survey (NHANES) III. At baseline, the frequency of diabetes, hypertension, and hypercholesterolemia, increased significantly across TSH tertiles (diabetes = 6.3%, 7.7%, and 9.1%; hypertension = 20.9%, 24.8%, and 29.8%; hypercholesterolemia = 13.2%, 15.2%, and 19.4%).
In the present study, upon managing the issue of the impaired intestinal absorption of L-T4 by postponing ingestion of calcium carbonate to 6–8 h after having taken the L-T4 tablet, serum TSH fell, with all the above indices accompanying this fall. However, serum TSH levels and indices were not exactly superimposable to the corresponding levels measured when the 50 women took L-T4 alone. Indeed, serum TSH was 12% significantly greater, fasting glycemia was 3.2% significantly greater, total cholesterolemia was 0.9% insignificantly greater, and SBP and DBP both 0.6% insignificantly greater. Thus, at least posmenopausal women of age similar to our women (65–84-year old) are particularly sensitive to minimal changes of TSH, even within a range of ∼1.0–4.0 mU/L. In the regression analysis, for every 1.0 mU/L increase of serum TSH over the 0.85–6.9 mU/L range, total cholesterolemia, glycemia, SBP and DBP increase on the average by 12.1, 3.1 mg/dL, 2.3 and 2.0 mmHg, respectively.
The importance of “keeping in order” serum TSH levels in L-T4-treated hypothyroid patients is also underscored by the direct association of serum TSH (even within the normal reference range) and mortality [36,37,38,39,40,41,42,43]. Two recent studies are worthy of note. In the prospective part of one aforementioned study [42], the median follow-up period was 19.1 years, with 3395 all-cause deaths. A significantly higher risk of all-cause mortality (adjusted hazard ratio [HR] 1.27; CI 1.10–1.47), and cardiovascular mortality (HR 1.30 [CI 1.02–1.67]), and cancer mortality (HR 1.43 [CI 1.01–2.01]) was observed in the high normal TSH group (1.90–4.60 mU/L) than in the medium normal TSH group (1.16–1.89 mU/L). The second study [42], was on L-T4-treated patients aged 60–80 years (72% females) followed-up for 18–66 months (1.5–5.5 years) after discharge from the hospital, and it was summarized in the Introduction. Mortality rate for TSH in the band 5.0–10.0 mUL was double than in the band 2.5–5.0 and 0.5–2.5 mU/L. During a period of time of 2.3 ± 1.1 years, when TSH peaked at 3.33 ± 0.95 mU/L, no death occurred in our 50 outpatients.
In conclusion, the interference of calcium salts on the intestinal absorption of L-T4 remains poorly appreciated by patients and physicians, particularly the general practitioners. This is also favored by the somewhat vague information provided by leaftlets, with statetements, such as “calcium salts may impair the intestinal absorption of thyroid hormones” or “calcium salts should be taken at interval of at least two hours from the thyroid hormone ingestion”. Leaflets of some calcium carbonate preparations do not even report interaction with L-T4, while providing details for other interactions, such as underscoring the interval of 4–6 h with respect to the ingestion of tetracyclines. Our data show that calcium carbonate has to be taken 6 or more hours after the ingestion of the L-T4 tablet. An insufficiently long interval not only increases serum TSH levels but also cholesterolemia, glycemia, and blood pressure values. It is possible that such complications may apply to the L-T4 malabsoprtion caused by other medicines. Should our data be confirmed, monitoring of hypothyroid patients who ingest medications interfering with the intestinal absorption of L-T4 should be more careful and not restricted to the sole measurement of serum TSH.
References
A. Garmendia Madiaraga, S. Santos Palacios, F. Guillén-Grima, J.C. Galofré, The incidence and prevalence of thyroid dysfunction in Europe: a meta-analysis. J. Clin. Endocrinol. Metab. 99, 923–931 (2014)
J.R. Garber, R.H. Cobin, H. Gharib et al.. Clinical practice guidelines for hypothyroidism for hypothyroidism in adults: cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Thyroid 22, 1200–1235 (2012)
J. Jonklaas, A.C. Bianco, A.J. Bauer, K.D. Burman, A.R. Cappola, F.S. Celi, D.S. Cooper, B.W. Kim, R.P. Peeters, M.S. Rosenthal, A.M. Sawka, American Thyroid Association Task Force on Thyroid Hormone Replacement. Guidelines for the treatment of hypothyroidism: prepared by the American thyroid association task force on thyroid hormone replacement. Thyroid 24, 1670–1751 (2014)
A. Catalano, N. Morabito, G. Basile, S. Fusco, G. Castagna, F. Reitano, R.C. Albanese, A. Lasco, Fracture risk assessment in postmenopausal women referred to an Italian center for osteoporosis: a single day experience in Messina. Clin. Cases Miner. Bone Metab. 10(3), 191–194 (2013)
N. Singh, P.N. Singh, J.M. Hershman, Effect of calcium carbonate on the absorption of levothyroxine. JAMA 283, 2822–2825 (2000)
J. Ehrenkranz, P.R. Bach, G.L. Snow, A. Schneider, J.L. Lee, S. Ilstrup, S.T. Bennett, S. Benvenga, Circadian and circannual rhythms in thyroid hormones: determining the TSH and free T4 reference intervals based upon time of day, age, and sex. Thyroid 25, 954–961 (2015)
H.B. Burch, K.D. Burman, D.S. Cooper, J.V. Hennessey, A 2013 survey of clinical practice patterns in the management of primary hypothyroidism. J. Clin. Endocrinol. Metab. 99, 2077–2085 (2014)
L. Liwanpo, J.M. Hershman, Conditions and drugs interfering with thyroxine absorption. Best Pract. Res. Clin. Endocrinol. Metab. 23, 781–792 (2009)
P. Colucci, C. Seng Yue, M. Ducharme, S. Benvenga, A review of the pharmacokinetics of levothyroxine for the treatment of hypothyroidism. Eur. Endocrinol. 9, 40–47 (2013)
S. Benvenga, When thyroid hormone replacement is ineffective? Curr. Opin. Endocrinol. Diabetes Obes. 20, 467–477 (2013)
C.R. Schneyer, Calcium carbonate and reduction of levothyroxine efficacy [letter]. JAMA 279, 750 (1998)
I. Zamfirescu, H.E. Carlson, Absorption of levothyroxine when coadministered with various calcium formulations. Thyroid 21, 483–486 (2011)
C.J. Diskin, T.J. Stokes, L.M. Dansby, L. Radcliff, T.B. Carter, Effect of phosphate binders upon TSH and L-thyroxine dose in patients on thyroid replacement. Int. Urol. Nephrol. 39, 599–602 (2007)
S. Benvenga, F. Di Bari, R. Vita, Undertreated hypothyroidism due to calcium oriron supplementation corrected by oral liquid levothyroxine. Endocrine 56, 138–145 (2017)
R. Vita, G. Saraceno, F. Trimarchi, S. Benvenga, Switching levothyroxine from the tablet to the oral solution formulation corrects the impaired absorption oflevothyroxine induced by proton-pump inhibitors. J. Clin. Endocrinol. Metab. 99, 4481–4486 (2014)
R. Vita, F. Di Bari, S. Benvenga, Oral liquid levothyroxine solves the problem of tablet levothyroxine malabsorption due to concomitant intake of multiple drugs. Expert Opin. Drug Deliv. 14, 467–472 (2017)
C. Virili, P. Trimboli, F. Romanelli, M. Centanni, Liquid and softgel levothyroxine use in clinical practice: state of the art. Endocrine 54, 3–14 (2016)
A.M. Formenti, G. Mazziotti, R. Giubbini, A. Giustina, Treatment of hypothyroidism: all that glitters is gold? Endocrine 52, 411–413 (2016)
I.L. Klein, S. Danzi, The cardiovascular system in hypothyroidism. In: Werner and Ingbar’s The thyroid: a fundamental and clinical text, 10th ed, ed. by L.E. Bravermann, D.S. Cooper (Wolters Kluver, Lippincott, Willimas & Wilkins, Philadelphia, 2013), pp. 575–582.
Jahangir Alam, MostNur-E.-TajMokarrama Mukti, Mominul Hoque, Intaz Ali, Mostafa Kamal, SunilC. Mallik, Subhagata Choudhury, Cross sectional evaluation of thyroid hormone levels in non-diabetic and diabetic patients in Bangladeshi population. Asian J. Biol. Sci. 6, 228–233 (2013)
A. Javed, P.B. Balagopal, A. Vella, P.R. Fischer, F. Piccinini, C. Dalla Man, C. Cobelli, P.D. Giesler, J.M. Laugen, S. Kumar, Association between thyrotropin levels and insulin sensitivity in euthyroid obese adolescents. Thyroid 25, 478–484 (2015)
I.M. Benseñor, A.C. Goulart, C. Molina Mdel, É.J. de Miranda, I.S. Santos, P.A. Lotufo, Thyrotropin levels, insulin resistance, and metabolic syndrome: a cross-sectional analysis in the Brazilian Longitudinal Study of Adult Health (ELSA-Brasil). Metab. Syndr. Relat. Disord. 13, 362–369 (2015)
B. Xu, H. Yang, Z. Wang, T. Yang, H. Guo, P. Cheng, W. He, M. Sun, H. Chen, Y. Duan, Elevated thyroid stimulating hormone levels are associated with metabolic syndrome in a Chinese community-based population of euthyroid people aged 40 years and older. J. Biomed. Res 30, 476–482 (2016)
T.N. Le, F.S. Celi, E.P. Wickham 3rd, Thyrotropin levels are associated with cardiometabolic risk factors in euthyroid adolescents. Thyroid 26, 1441–1449 (2016)
H.T. Park, G.J. Cho, K.H. Ahn, J.H. Shin, S.C. Hong, T. Kim, J.Y. Hur, Y.T. Kim, K.W. Lee, S.H. Kim, Thyroid stimulating hormone is associated with metabolic syndrome in euthyroid postmenopausal women. Maturitas 62, 301–305 (2009)
A. Giandalia, G.T. Russo, E.L. Romeo, A. Alibrandi, P. Villari, A.A. Mirto, G. Armentano, S. Benvenga, D. Cucinotta, Influence of high-normal serum TSH levels on major cardiovascular risk factors and Visceral Adiposity Index in euthyroid type 2 diabetic subjects. Endocrine 47, 152–160 (2014)
Y. Zhang, P. Lu, L. Zhang, X. Xiao, Association between lipids profile and thyroid parameters in euthyroid diabetic subjects: a cross-sectional study. BMC Endocr. Disord. 15, 12 (2015)
V. Velkoska Nakova, B. Krstevska, M. Bosevski, Ch. Dimitrovski, V. Serafimoski, Dyslipidaemia and hypertension in patients with subclinical hypothyroidism. Prilozi 30, 93–102 (2009).
L. Petrosyan, Relationship between high normal TSH levels and metabolic syndrome components in type 2 diabetic subjects with euthyroidism. J. Clin. Transl. Endocrinol. 2, 110–113 (2015)
W.X. Jian, J. Jin, L. Qin, W.J. Fang, X.R. Chen, H.B. Chen, Q. Su, H.L. Xing, Relationship between thyroid-stimulating hormone and blood pressure in the middle-aged and elderly population. Singap. Med. J. 54, 401–405 (2013)
D. Liu, F. Jiang, Z. Shan, B. Wang, J. Wang, Y. Lai, Y. Chen, M. Li, H. Liu, C. Li, H. Xue, N. Li, J. Yu, L. Shi, X. Bai, X. Hou, L. Zhu, L. Lu, S. Wang, Q. Xing, W. Teng, A cross-sectional survey of relationship between serum TSH level and blood pressure. J. Hum. Hypertens. 24, 134–138 (2010)
R. Luboshitzky, A. Aviv, P. Herer, L. Lavie, Risk factors for cardiovascular disease in women with subclinical hypothyroidism. Thyroid 12, 421–425 (2002)
Y. Duan, W. Peng, X. Wang, W. Tang, X. Liu, S. Xu, X. Mao, S. Feng, Y. Feng, Y. Qin, K. Xu, C. Liu, C. Liu, Community-based study of the association of subclinical thyroid dysfunction with blood pressure. Endocrine 35, 136–142 (2009)
T. Ittermann, D. Tiller, C. Meisinger, C. Agger, M. Nauck, R. Rettig, A. Hofman, T. Jørgensen, A. Linneberg, J.C. Witteman, O.H. Franco, K.H. Greiser, K. Werdan, A. Döring, A. Kluttig, B.H. Stricker, H. Völzke, High serum thyrotropin levels are associated with current but not with incident hypertension. Thyroid 23, 955–963 (2013)
V.L. Langén, T.J. Niiranen, P. Puukka, J. Sundvall, A.M. Jula, V.L. Langén, T.J. Niiranen, P. Puukka, J. Sundvall, A.M. Jula, Association between thyroid-stimulating hormone and blood pressure in adults: an 11-year longitudinal study. Clin. Endocrinol. 84, 741–747 (2016)
A.E. Hak, H.A. Pols, T.J. Visser, H.A. Drexhage, A. Hofman, J.C. Witteman, Subclinical hypothyroidism is an independent risk factor for atherosclerosis and myocardial infarction in elderly women: the Rotterdam Study. Ann. Intern. Med. 132, 270–278 (2000)
M. Imaizumi, M. Akahoshi, S. Ichimaru, E. Nakashima, A. Hida, M. Soda, T. Usa, K. Ashizawa, N. Yokoyama, R. Maeda, S. Nagataki, K. Eguchi, Risk for ischemic heart disease and all-cause mortality in subclinical hypothyroidism. J. Clin. Endorinol. Metab. 89, 3365–3370 (2004)
N. Rodondi, W.P. den Elzen, D.C. Bauer, A.R. Cappola, S. Razvi, J.P. Walsh, B.O. Asvold, G. Iervasi, M. Imaizumi, T.H. Collet, A. Bremner, P. Maisonneuve, J.A. Sgarbi, K.T. Khaw, M.P. Vanderpump, A.B. Newman, J. Cornuz, J.A. Franklyn, R.G. Westendorp, E. Vittinghoff, J. Gussekloo, Thyroid Studies Collaboration. Subclinical hypothyroidism and the risk of coronary heart disease and mortality. JAMA 304, 1365–1374 (2010)
A. Hassan, A. Altamirano-Ufion, B. Zulfiqar, P. Boddu, Subclinical hypothyroidism and its association with increased cardiovascular mortality: call for action. Cardiol. Res. 8, 31–35 (2017)
Y. Ning, Y.J. Cheng, L.J. Liu, J.D. Sara, Z.Y. Cao, W.P. Zheng, T.S. Zhang, H.J. Han, Z.Y. Yang, Y. Zhang, F.L. Wang, R.Y. Pan, J.L. Huang, L.L. Wu, M. Zhang, Y.X. Wei, What is the association of hypothyroidism with risks of cardiovascular events and mortality? A meta-analysis of 55 cohort studies involving 1,898,314 participants. BMC Med. 15(1), 21 (2017)
N.M.Y. Journy, M.O. Bernier, M.M. Doody, B.H. Alexander, M.S. Linet, C.M. Kitahara, Hyperthyroidism, hypothyroidism, and cause-specific mortality in a large cohort of women. Thyroid 27, 1001–1010 (2017)
K. Inoue, T. Tsujimoto, J. Saito, T. Sugiyama, Association between serum thyrotropin levels and mortality among euthyroid adults in the United States. Thyroid 26, 1457–1465 (2016)
A. Akirov, H. Gimbel, A. Grossman, T. Shochat, I. Shimon, Elevated TSH in adults treated for hypothyroidism is associated with increased mortality. Eur. J. Endocrinol. 176, 57–66 (2017)
S.M. Grundy, J.I. Cleeman, S.R. Daniels, K.A. Donato, R.H. Eckel, B.A. Franklin, D.J. Gordon, R.M. Krauss, P.J. Savage, S.C. Smith Jr et al.. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation 112, 2735–2752 (2005)
S. Genuth, K.G. Alberti, P. Bennett, J. Buse, R. Defronzo, R. Kahn, J. Kitzmiller, W.C. Knowler, H. Lebovitz, A. Lernmark, D. Nathan, J. Palmer, R. Rizza, C. Saudek, J. Shaw, M. Steffes, M. Stern, J. Tuomilehto, P. Zimmet, Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care 26, 3160–3167 (2003)
M. Cellini, M.G. Santaguida, C. Virili, S. Capriello, N. Brusca, L. Gargano, M. Centanni, Hashimoto’s thyroiditis and autoimmune gastritis. Front. Endocrinol. 26(8), 92 (2017).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical appral
All procedures performed in studies involving human participants were in accordance with the ethical standards of our institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Rights and permissions
About this article
Cite this article
Morini, E., Catalano, A., Lasco, A. et al. l-thyroxine malabsorption due to calcium carbonate impairs blood pressure, total cholesterolemia, and fasting glycemia. Endocrine 64, 284–292 (2019). https://doi.org/10.1007/s12020-018-1798-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-018-1798-7