Abstract
Gamma Knife radiosurgery is often used in pituitary adenomas. Aim of our study is to describe the characteristics and long-term outcome of patients with adenoma recurrence after Gamma Knife radiosurgery. We conducted a retrospective analysis of patients with pituitary adenoma treated by Gamma Knife radiosurgery between 1994 and 2014. Tumor recurrence was labeled as “in field” when the tumor growth occurred adjacent or within the prescribed isodose, whereas it was classified as “out of field” when the tumor growth occurred outside the prescribed isodose. Five hundred forty-three patients were included, 272 (50.1 %) had a nonfunctioning pituitary adenoma (NFPA) and 271 (49.9 %) patients had a hormone secreting-pituitary adenoma. The median follow-up after GKRS was 78 months (IQR, 36-125 months). Thirty-nine patients (7.2 %) had recurrence of disease and it was more frequent in patients with NFPA than in patients with hormone secreting adenomas (9.6 % vs. 4.8 %). The 10-yr progression-free survival in patients with NFPA was 78.7 % (95 % CI 69.5 – 87.9 %), as compared with 93.3 % (95 % CI 89.3 – 97.3 %; p < 0.01) in hormone secreting adenomas. Tumor recurrence was “in field” in 17 cases (43.6 %) and “out of field” in 22 cases (56.4 %). Seven of the 39 patients with recurrence died despite further treatments. Six of these patients had an “in field” recurrence. Recurrence of a pituitary adenoma after GKRS may occur several years after initial treatment. Distinction between “in field” and “out of field” tumor recurrence probably reflects two different pathophysiological mechanisms and may have prognostic importance.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In neurosurgical practice, Gamma Knife radiosurgery (GKRS) permits a highly precise and focused delivery of radiation to a biological target in a single session. Despite some limitations, such as the requirement of target lesions smaller than 3 cm and a distance from critical nervous structures of at least 2 mm, GKRS has gained widespread popularity as an adjuvant treatment for both nonfunctioning pituitary adenoma (NFPA) and hormone secreting pituitary tumors [1, 2].
Several large series of patients treated by GKRS at a single center for residual or recurrent NFPA [3–5] or hormone secreting pituitary adenoma [6–9] have been published in recent years, demonstrating both the efficacy and safety of this treatment. In particular, the control of tumor growth during long-term follow-up has been reported to range between 90–100 % of cases. Much emphasis has been devoted to quantify and unraveling the factors associated with the occurrence of tumor shrinkage after GKRS. On the contrary, there are few data on the patients who developed tumor recurrence after GKRS.
In the present study, we report the long-term tumor control rate in a large series of more than 500 patients with either NFPA or a hormone secreting pituitary adenoma treated consecutively at our center. We particularly focus on the characteristics of recurring tumors and provide information on further treatments and outcome for patients with tumor recurrence.
Patients and methods
Patients
We conducted a retrospective analysis of a prospectively maintained database of patients with a residual pituitary adenoma treated by GKRS in our department. Diagnosis of residual or recurrent pituitary adenoma was based on magnetic resonance imaging (MRI). The database was reviewed for patients treated for pituitary adenoma between 1994 and 2014 and was updated with available clinical information collected up to April 2015. Patients were included into the study if they had at least one post GKRS control visit, including MRI, at our center.
Standard informed consent relating to the procedure was obtained from all patients.
Clinical evaluation
Imaging and hormonal follow-up data were typically obtained at baseline, 6-month intervals after GKRS for the first year and yearly thereafter for 5 years. After this period, clinical control, including neuroimaging, was usually scheduled at two years interval. Whenever possible, patients underwent follow-up examination at our center. Otherwise, clinical information, laboratory tests and neuroimaging studies were sent and reviewed at our center.
Neuroimaging studies consisted in all patients, except one bearing a pacemaker, of a standard T1-weighted pre- and post-contrast coronal and sagittal MRI images.
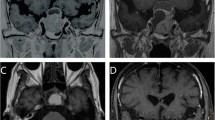
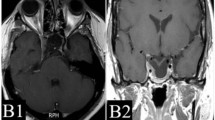
Recurrence of pituitary adenoma during follow-up was defined as the evidence on repeated MRI of pathological tissue not previously identified or further growth of residual adenomatous tissue in comparison with its appearance on the earlier study. Two distinct types of tumor recurrence have been defined in the present study, as the underlying pathogenesis might be different. Growth was labeled as “in field” when the tumor growth occurred adjacent or within the prescribed isodose (Fig. 1). Growth was labeled as “out of field” when the tumor growth occurred outside the prescribed isodose (i.e., in the controlateral cavernous sinus; Fig. 2).
Axial (upper panels) and coronal (lower panels), gadolinium-enhanced, T1–weighted, MRI of a pituitary adenoma before a, c and 105 months after b, d GKRS. a and c images show residual NFPA in the right cavernous sinus before GKRS treatment. b and d images show a recurrence of the tumor that is located outside the first treatment area (“out of field” recurrence)
Axial (upper panels) and coronal (lower panels), gadolinium-enhanced, T1–weighted, MRI of a pituitary adenoma before a, c and 80 months after b, d GKRS. a and c images show residual NFPA in the left cavernous sinus before GKRS treatment. b and d images show a recurrence of the tumor that is located within the first treatment area (“in field” recurrence)
Radiosurgical treatment
Patients underwent placement of the Leksell stereotactic headframe (model G; Elekta Instruments, Stockholm, Sweden) under mild sedation and after application of a local anesthetic agent. High-resolution gadolinium-enhanced stereotactic MRI, which is performed as thin slice (1 mm) axial and coronal T1-weighted pre- and post-contrast sequences, was then carried out on a Siemens 1,5 Tesla machine to obtain precise information on the three-dimensional coordinates of the tumor target. The Leksell Gamma Unit Model B was used until December 2001 and was then replaced by the C Model. On September 2007, the Leksell Perfexion unit replaced the C Model. Kula software was used for dose planning software until 1995 and was then replaced by the GammaPlan software (Elekta instruments, Atlanta, GA). Treatment plans were collaboratively formulated by the treating neurosurgeon, a medical physicist, and a radiation oncologist. The main therapeutic goal was the complete tumor coverage to arrest any further adenoma growth and to reverse hormone hypersecretion in case of hormone secreting tumors. The radiation dose delivered to the adenoma margins was fixed, when possible, to at least 15 Gy for NFPA and 25 Gy for hormone secreting adenomas. Multiple isocenters were distributed throughout the target volume to conform the dose to the tumor margins. To this aim, small collimator sizes (4 and 8 mm) were used and frequent source blocking was applied to obtain a sharper dose decrease toward the optic nerves, chiasm, and pituitary stalk. Prescription dose to the target volume was decreased, when necessary, to keep a maximal dose of 10 Gy to the optical pathway. All patients were discharged the day after GKRS treatment.
Statistical analysis
Continuous variables were examined for homogeneity of variance by the Kolmogorov–Smirnov test. For continuous variables with a normal distribution, the mean (±SEM) is reported. For variables not normally distributed, the median and interquartile ranges (IQR) are reported. Statistical analyses of categorical variables were carried out using the chi-square test or Fisher exact test as appropriate. Statistics of means were carried out using the unpaired Student t-test and Wilcoxon rank sum tests when variables were not normally distributed. Estimates of the cumulative event rate were calculated by the Kaplan–Meier method and differences in subgroups of patients were tested by the Log Rank test. Data for patients who were lost at follow-up, died, or refused further controls were censored at the time of the last MRI. Adjusted analysis of the primary outcome, i.e., recurrence of the pituitary tumor, was performed with the use of a Cox proportional-hazards regression model with the factors that had a P < 0.10 in the univariate analysis plus preidentified covariates of interest.
A probability value less than 0.05 was considered to indicate statistical significance and all reported probability values are two-tailed. All calculations were performed using the statistical package Stat View 5.0 (SAS Institute, Cary, NC).
Results
Patient characteristics
Between January 1994 and December 2014, 543 patients with pituitary adenoma were treated at our Institution with GKRS and had undergone at least one clinical and radiological follow-up visit at our center.
Two hundred and seventy-two patients (50.1 %) had a NFPA, whereas the remaining 271 (49.9 %) patients had a hormone secreting-pituitary adenoma. In the latter group there were 148 cases of growth hormone (GH)-secreting pituitary adenoma (27.3 %), 90 cases of adrenocorticotropin (ACTH)-secreting pituitary adenoma (16.6 %), 22 cases of prolactin (PRL)-secreting-pituitary adenoma (4.1 %), and 11 cases of thyrotropin (TSH)-secreting pituitary adenoma (2.0 %). ACTH-secreting pituitary adenomas included 75 patients with active Cushing’s disease and 15 patients with Nelson’s syndrome. The main clinical and demographic characteristics of the patients, grouped according to the secretory status of the tumor, are summarized in Table 1. Patients with NFPA were older than patients with hormone secreting adenomas (p < 0.01). In the latter group, there were more females than in the NFPA group (p < 0.01). Only three patients, two with acromegaly and one with Cushing’s disease had not undergone surgery before GKRS. The remaining 540 patients received GKRS because of residual or recurring tumor. The majority of patients (90.9 %) underwent the last surgical procedure at our center. The number of surgical procedures before GKRS varied: 392 patients (72.6 %) had received one procedure, 128 patients (23.7 %) had received two procedures, 16 patients (3.0 %) had received three procedures, and 4 patients (0.7 %) had received four procedures. Nine patients (1.7 %) had previously received fractionated radiation therapy. In all these cases, there was evidence of tumor progression despite radiation therapy. The main characteristics of GKRS treatment also changed according to the type of pituitary adenoma (Table 1): patients with NFPA had larger tumor volume (p < 0.01) and received a lower marginal (p < 0.01) and maximum median dose (p < 0.01) than patients with hormone secreting adenomas. The conformity index was similar in the two groups.
Tumor recurrences
All patients had at least one MRI six months after GKRS and were, therefore, included in the analysis of progression-free survival. The median follow-up after GKRS treatment was 78 months (IQR, 36–125 months, range 6–233 months) and it was similar in patients with NFPA (79 months; IQR, 33–118 months) and in those with hormone secreting adenoma (78 months; IQR, 38–133 months).
During follow-up 39 patients (7.2 %) had recurrence of disease. The recurrence rate was higher in patients with NFPA than in patients with hormone secreting adenomas (9.6 % vs. 4.8 %). Fig. 3 shows the progression-free survival according to the secretory status of the tumor. The 5-yr and 10-yr progression-free survivals in patients with NFPA were 94.5 % (95 % CI 91.1–97.9 %) and 78.7 % (95 % CI 69.5–87.9 %), as compared with 95.2 % (95 % CI 92.2–98.2 %) and 93.3 % (95 % CI 89.3–97.3 %; p < 0.01), respectively, in the hormone secreting adenoma group. The difference in the risk of tumor recurrence became evident 8–9 years after GKRS because of an increasing number of events in the NFPA group (Fig. 3). Among patients with a secreting-pituitary adenoma, recurrence occurred in 6 of 75 patients with Cushing’s disease (8.0 %), one of 15 patients with Nelson’s syndrome (6.7 %), 4 of 148 patients with acromegaly (2.7 %), and 2 of 22 patients with prolactinoma (9.1 %). None of the 11 patients with a TSH-secreting adenoma experienced recurrence of disease. Both patients with prolactinoma were receiving high-dose dopamine agonists at the time of recurrence, while the four acromegalic patients were not receiving medical therapy when regrowth of the tumor occurred.
Kaplan–Meier analysis showing the recurrence-free survival in 272 patients with a nonfunctioning pituitary adenoma (continuous line) and 271 patients with a hormone secreting pituitary adenoma treated with Gamma Knife radiosurgery because of residual or recurrent tumor. The risk of tumor recurrence was significantly higher (p < 0.01) in patients with nonfunctioning pituitary adenoma than in patients with a hormone secreting pituitary adenoma
According to our definition, tumor recurrence was considered “in field” in 17 cases (43.6 %) and “out of field” in 22 cases (56.4 %). Patients with NFPA had more “out of field” recurrences than patients with hormone secreting adenomas (69.2 % vs. 30.8 %; p < 0.05). Among the 39 patients with a recurrent tumor, the 5-yr and 10-yr progression-free survivals in patients with “in field” recurrence were 35.3 % (95% CI 12.1–58.5 %) and 11.8 % (95 % CI 0–27.4 %), as compared with 59.1 % (95 % CI 38.1–80.1 %) and 13.6 % (95 % CI 0–28.2 %), respectively, in patients with “out of field” recurrence (p = ns).
Among the 9 patients who had received radiotherapy in the past, two cases (22.2 %), one patient with NFPA and one patient with prolactinoma, had another recurrence after GKRS and in both cases it was an “in field” recurrence.
In the whole group of patients, a multivariate Cox analysis that included age at GKRS, secretory status of the tumor, number of previous surgical procedures, previous radiotherapy and marginal dose to the tumor, showed that the risk of tumor recurrence was negatively associated with the marginal dose to the tumor (HR 0.85; 95 % CI 0.78–0.93; p < 0.001) and positively associated with the number of previous surgical procedures (HR 1.67; 95 % CI 1.06–2.61; p < 0.05). Other characteristics, such as sex, target irradiated volume, number of shots, and conformity index were not included in the model because they did not reach a p value lower than 0.10 in the univariate analysis.
We next repeated the same analysis separately in the group of patients with NFPA and patients with hormone secreting adenoma because some indications to and characteristics of GKRS treatment vary according to the secretory status of the tumor. In patients with NFPA, the final multivariate model indicated that the number of previous surgical procedures was the only variable significantly associated with the risk of tumor recurrence (HR 1.82; 95 % CI 1.08–3.09; p < 0.05).
In patients with hormone secreting adenoma, age at GKRS (HR 1.08; 95 % CI 1.04–1.13; p < 0.001), and marginal dose to the tumor (HR 0.73; 95 % CI 0.63–0.84; p < 0.001) were the only variables significantly associated with the risk of tumor recurrence.
Further treatments and long-term outcome after recurrence
The median follow-up after tumor recurrence was 50 months (IQR, 14–85 months, range 0–167 months). One patient with NFPA was lost to follow-up soon after detection of an “out of field” tumor recurrence 9 years after GKRS and an indication to receive another GKRS treatment.
During follow-up, 7 of the 38 patients (18.4 %) died (Table 2). Six of these patients, three with an ACTH-secreting adenoma, two with a PRL-secreting adenoma (one of which had received fractionated radiotherapy before GKRS), and one with a NFPA died because of tumor progression. The remaining case with an ACTH-secreting adenoma died of ischemic heart disease. Six of these patients had an “in field” recurrence. The only patient who had an “out of field” recurrence was initially treated with another GKRS treatment but, eventually, she had a relapse of the originally treated residual tumor that was unresponsive to medical treatment with temozolomide and pasireotide. Five patients (13.2 %), four with NFPA and one with an ACTH-secreting adenoma, showed progression of the tumor at last follow-up (Table 2). Two cases had an “in field” recurrence. One other patient with NFPA (2.6 %) had recurrence of disease after GKRS but responded well to salvage therapy with temozolomide. He initially had an “out of field” recurrence that was well controlled by GKRS but later developed a recurrence of the originally treated tumor (Table 2). Fifteen patients (39.5 %), all with NFPA, except one with an ACTH-secreting adenoma, have stable disease at last follow-up. Most of them (11 cases) had an “out of field” recurrence. GKRS alone was used in all cases except one who received debulking surgery to decompress the optic pathway (Table 2). Five patients (12.8 %), four with a GH-secreting adenoma and one with NFPA were considered in remission at last follow-up (Table 2). The remaining five patients (12.8 %) cannot be evaluated because they are still awaiting the planned treatment or have received it less than six months ago (Table 2).
Discussion
Our study is the largest single-center series reporting the long-term rate of tumor control in patients treated with GKRS for residual or recurring pituitary adenoma.
In the whole group, 7.2 % of patients experienced recurrence of the pituitary adenoma. Despite the similar length of follow-up, more patients with NFPA than with a hormone secreting pituitary adenoma had a recurrence (9.6 % vs. 4.8 %). Visual inspection of the progression-free curves (Fig. 3) clearly shows that the risk of tumor regrowth is similar up to 7–8 years after GKRS but then diverges because of an increased number of tumor recurrences in the NFPA group only. Therefore, maintaining a distinction between NFPA and hormone secreting pituitary adenoma when analyzing the tumor control rate after GKRS seems appropriate.
Our data in NFPA are similar to those reported by Sheehan and coworkers [5] in a pooled analysis of data from 9 Gamma Knife centers in North America. During a shorter median follow-up period of 36 months, they found regrowth of NFPA in 31 of 469 patients (6.6 %) and the actuarial progression-free survivals were 95 % and 85 % at 5 and 10 years post GKRS, respectively [5]. In multivariate analysis, tumor volume at GKRS was the only characteristic positively associated with the risk of tumor recurrence [5]. In our analysis restricted to NFPA only, tumor volume did not correlate with clinical outcome of GKRS. A likely explanation for this discrepancy may lie in the much smaller median tumor volume in our series (1.50 cm3) as compared with that in the Sheehan’s study (3.3 cm3) [5], reflecting the fact that almost all patients in our study received surgery at our center before GKRS with the goal to obtain maximal tumor debulking [10]. Moreover, it has always been our standard policy to advise GKRS early after demonstration of residual tumor after surgery or in case of tumor recurrence during follow-up to minimize the risk of side effects [3]. Interestingly, Pomeraniec and coworkers recently showed that early vs. late GKRS for NFPA resulted in a lower risk not only of hypopituitarism but also of tumor progression [11]. The number of surgical procedures was the only significant characteristic positively associated with the risk of tumor recurrence in our study. It is likely that repeat surgery may serve as a proxy for an increased biological aggressiveness of NFPA. Most other series of GKRS in NFPA report recurrence of the tumor in 0–7.8 % of treated patients [4, 12–17] but a reliable analysis of prognostic factors could not be performed because of the small number of events and/or a short median follow-up. It should be underscored that the relationship between growth and aggressiveness of NFPA is central to understand the natural history of disease and optimize the therapeutic strategy in these patients [18].
Recurrence of a secreting pituitary adenoma occurred in 4.8 % of our patients with no clear predilection according to tumor type, even though GH- and TSH-secreting subtypes had a very low risk or tumor regrowth. Most series of GKRS in hormone secreting pituitary adenomas focus on remission of the specific endocrine disease rather than on control of tumor growth. However, the apparent lack of tumor control in large series of patients with acromegaly, Cushing’s disease, or prolactinoma treated by GKRS varies between 0 and 13 % [6, 9, 19–22]. Because of the small number of events, analyses of prognostic factors associated with tumor growth after GKRS for hormone secreting pituitary adenomas are lacking. We found that both lower dose to the tumor margin and increasing age at GKRS were associated with an increased risk of tumor recurrence. While, the first factor is biologically plausible, age at GKRS must be considered with great caution and seems to be driven almost uniquely by the advanced age of all patients with recurrence of Cushing’s disease (Table 2). We feel that further analyses in a larger dataset of patients receiving GKRS because of an ACTH-secreting tumor are necessary to strengthen or refuse our observation.
We found two clearly distinct patterns of tumor recurrence after GKRS: regrowth of tissue that had been included in the radiation field and growth of tissue that was clearly outside the original radiation field. The frequency of the two different patterns of tumor recurrence in the whole group of patients was roughly similar but “out of field” recurrence seemed to be more prevalent in patients with NFPA than in patients with hormone secreting tumors. Such a distinction has occasionally been reported in previous series [3, 4, 9, 16, 17, 22] but never discussed in deep. We feel that the pattern of recurrence is not a mere curiosity but may carry important prognostic and therapeutic consequences for the patient, which relate to the different pathophysiology of the two situations. Indeed, “out of field” recurrence may simply represent an incomplete demarcation of the biological target at GKRS, especially when the tumor has an infiltrating nature into surrounding structures. It should be reminded that contouring of the tumor target is performed on MRI, which may miss very small tumor rests located away from the main residue. The steep decrease of radiation dose, typical of GKRS, may leave the hidden tumor rest exposed to an insufficient amount of radiation. The advantage of a highly focused radiation, i.e., sparing of the normal surrounding tissue, may, thus, revert to a disadvantage when the tumor has a pronounced infiltrating behavior. On the other hand, growth of a tumor that was initially covered by an adequate radiation dose (“in field” recurrence) usually means that neoplastic cells are or have become radioresistant, which limits the therapeutic options for controlling further tumor growth and may signal the acquisition of an aggressive phenotype by the tumor. The prognostic importance of the type of tumor recurrence seems corroborated by our findings: further progression of the tumor despite multiple therapeutic attempts, including temozolomide [23], occurred almost exclusively in patients who had an “in field” recurrence, while most of the patients with an “out of field” recurrence responded well to further treatments and had stable disease at last follow-up.
In conclusion, recurrence of a pituitary adenoma after GKRS may occur several years after initial treatment, underlying the need for long-term surveillance of the patients, especially those with NFPA. Distinction between “in field” and “out of field” tumor recurrence probably reflects two different pathophysiological mechanisms and should be considered both for selecting further treatment options and for prognostic issues.
References
J.P. Sheehan, A. Niranjan, J.M. Sheehan, J.A. Jane Jr, E.R. Laws, D. Kondziolka et al., Stereotactic radiosurgery for pituitary adenomas: an intermediate review of its safety, efficacy, and role in the neurosurgical treatment armamentarium. J. Neurosurg. 102(4), 678–691 (2005)
G. Minniti, D.C. Gilbert, M. Brada, Modern techniques for pituitary radiotherapy. Rev. Endocr. Metab. Disord. 10(2), 135–144 (2009). doi:10.1007/s11154-008-9106-0
M. Losa, M. Valle, P. Mortini, A. Franzin, C. Ferrari da Passano, M. Cenzato et al., Gamma knife surgery for treatment of residual nonfunctioning pituitary adenomas after surgical debulking. J. Neurosurg. 100(3), 438–444 (2004)
V. Mingione, C.P. Yen, M.L. Vance, M. Steiner, J. Sheehan, E.R. Laws et al., Gamma surgery in the treatment of nonsecretory pituitary macroadenoma. J. Neurosurg. 104(6), 876–883 (2006)
J.P. Sheehan, R.M. Starke, D. Mathieu, B. Young, P.K. Sneed, V.L. Chiang et al., Gamma Knife radiosurgery for the management of nonfunctioning pituitay adenomas: a multicenter study. J. Neurosurg. 119(2), 446–456 (2013). doi:10.3171/2013.3.JNS12766
M. Losa, L. Gioia, P. Picozzi, A. Franzin, M. Valle, M. Giovanelli et al., The role of stereotactic radiotherapy in patients with growth hormone-secreting pituitary adenoma. J. Clin. Endocrinol. Metab. 93(7), 2546–2552 (2008). doi:10.1210/jc.2008-0135
B.E. Pollock, P.D. Brown, T.B. Nippoldt, W.F. Young Jr, Pituitary tumor type affects the chance of biochemical remission after radiosurgery of hormone-secreting pituitary adenomas. Neurosurgery 62(6), 1271–1276 (2008). doi:10.1227/01.neu.0000333298.49436.0e discussion 1276-1278
F. Castinetti, M. Nagai, I. Morange, H. Dufour, P. Caron, P. Chanson et al., Long-term results of stereotactic radiosurgery in secretory pituitary adenomas. J. Clin. Endocrinol. Metab. 94(9), 3400–3407 (2009). doi:10.1210/jc.2008-2772
C.C. Lee, M.L. Vance, Z. Xu, C.P. Yen, D. Schlesinger, B. Dodson et al., Stereotactic radiosurgery for acromegaly. J. Clin. Endocrinol. Metab. 99(4), 1273–1281 (2014). doi:10.1210/jc.2013-3743
M. Losa, P. Mortini, R. Barzaghi, P. Ribotto, M.R. Terreni, S. Bianchi Marzoli et al., Early results of surgery in patients with nonfunctioning pituitary adenoma and analysis of the risk of tumor recurrence. J. Neurosurg. 108(3), 525–532 (2008). doi:10.3171/JNS/2008/108/3/0525
I.J. Pomeraniec, R.F. Dallapiazza, Z. Xu, J.A. Jane Jr, J.P. Sheehan, Early versus late Gamma Knife radiosurgery following transsphenoidal resection for nonfunctioning pituitary adenomas: a matched cohort study. J. Neurosurg. Published online October 30, 2015; doi: 10.3171/2015.5.JNS15581
B. Wowra, W. Stummer, Efficacy of gamma knife radiosurgery for nonfunctioning pituitary adenomas: a quantitative follow up with magnetic resonance imaging-based volumetric analysis. J. Neurosurg. 97(5 Suppl), 429–432 (2002)
Z. Petrovich, C. Yu, S.L. Giannotta, C.S. Zee, M.L. Apuzzo, Gamma knife radiosurgery for pituitary adenoma: early results. Neurosurgery 53(1), 51–59 (2003). discussion 59–61
T. Kobayashi, Long-term results of stereotactic gamma knife radiosurgery for pituitary adenomas. Specific strategies for different types of adenoma. Prog. Neurol. Surg. 22, 77–95 (2009). doi:10.1159/000163384
R. Liscak, V. Vladyka, J. Marek, G. Simonova, J. Vymazal, Gamma knife radiosurgery for endocrine-inactive pituitary adenomas. Acta Neurochir. (Wien) 149(10), 999–1006 (2007). discussion 1006
B.E. Pollock, J. Cochran, N. Natt, P.D. Brown, D. Erickson, M.J. Link et al., Gamma knife radiosurgeryfor patients with nonfunctioning pituitary adenomas: results from a 15-year experience. Int. J. Radiat. Oncol. Biol. Phys. 70(5), 1325–1329 (2008)
C. Hoybye, T. Rahn, Adjuvant Gamma Knife radiosurgery in non-functioning pituitary adenomas; low risk of long-term complications in selected patients. Pituitary 12(3), 211–216 (2009). doi:10.1007/s11102-008-0163-x
K.A. Øystese, J.A. Evang, J. Bollerslev, Non-functioning pituitary adenoma: growth and aggressiveness. Endocrine 53(1), 28–34 (2016). doi:10.1007/s12020-016-0940-7
F. Castinetti, M. Nagai, H. Dufour, J.M. Kuhn, I. Morange, P. Jacquet et al., Gamma knife radiosurgery is a successful adjunctive treatment in Cushing’s disease. Eur. J. Endocrinol. 156(1), 91–98 (2007)
H. Wan, O. Chihiro, S. Yuan, MASEP gamma knife radiosurgery for secretory pituitary adenomas: experience in 347 consecutive cases. J. Exp. Clin. Cancer Res. 28, 36 (2009). doi:10.1186/1756-9966-28-36
X. Liu, H. Kano, D. Kondziolka, K.J. Park, A. Iyer, S. Shin et al., Gamma knife stereotactic radiosurgery for drug resistant or intolerant invasive prolactinomas. Pituitary 16(1), 68–75 (2013)
J.P. Sheehan, Z. Xu, D.J. Salvetti, P.J. Schmitt, M.L. Vance, Results of gamma knife surgery for Cushing’s disease. J. Neurosurg. 119(6), 1486–1492 (2013). doi:10.3171/2013.7.JNS13217
M. Losa, F. Bogazzi, S. Cannavo, F. Ceccato, L. Curtò, L. De Marinis et al., Temozolomide therapy in patients with aggressive pituitary adenomas or carcinomas. J. Neurooncol. 126(3), 519–525 (2016). doi:10.1007/s11060-015-1991-y
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Losa, M., Spatola, G., Albano, L. et al. Frequency, pattern, and outcome of recurrences after gamma knife radiosurgery for pituitary adenomas. Endocrine 56, 595–602 (2017). https://doi.org/10.1007/s12020-016-1081-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-016-1081-8