Abstract
Purpose
Gamma Knife Radiosurgery(GKRS) is an established modality for treatment of non-functioning pituitary adenomas(NFPA). The objective of the study was to assess long-term hormonal and imaging outcomes after adjunctive GKRS in patients with NFPA.
Methods
A retrospective review of records of 109 patients with NFPA, from 1996 to 2020, who received adjunctive GKRS, was performed. Patients who had received GKRS as the primary modality of treatment for NFPA were not included.
Results
Sixty-three (57.8%) patients were available for follow up at our institute. The median follow-up period was 47 months (range, 6–260). At a median time of 38 months (range, 8–97), 25 (39.7%) patients developed ≥ 1 new pituitary hormone deficiency. Median time to cortisol deficiency was 38 months (range, 8–55), thyroid hormone deficiency was 45.5 months (range, 12–97) and gonadotropin deficiency was 45 months (range, 21–75). The actuarial risk of developing a new pituitary hormone deficit at 1, 3, 5, 7, and 10 years was 2.5%, 11%, 26.3%, 28% and 29.7%, respectively. Adenoma size decreased in 36 (57.1%) patients, remained unchanged in 19 (30.2%) patients, and increased in 8 (12.7%) patients. Overall tumor control rate was 87.3%. Endocrinopathy-Free Survival was 47.1%, and tumor Progression-Free Survival was 93.3%, at 5 years. Five (4.6%) patients required additional treatment after GKRS. One (1.6%) patient each had worsening of headache, optic atrophy and cerebellar infarct after GKRS therapy.
Conclusion
GKRS offers a safe adjunctive treatment modality, with satisfactory long-term preservation of hormone functions and a high rate of tumor control, in patients with NFPA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Adenomas of the pituitary gland comprise 10–20% of all intracranial tumors, 30% of which are non-functioning pituitary adenomas (NFPA) [1]. Trans-sphenoidal surgery remains the first line of treatment in NFPA, as it provides rapid resolution of tumor mass-effect, and a likelihood of complete tumor excision [2, 3]. However, various studies have reported that 33–85% of patients with NFPA have residual tumors after surgical resection due to various tumor, technique and/or surgeon-related factors [1,2,3,4,5,6]. The recurrence rates in surgically treated NFPA vary from 10 to 20% in gross total resections, and 50 to 60% in subtotal resections [1, 4, 7, 8]. Thus, patients with NFPA may require additional surgery and/or adjuvant radiotherapy for the treatment of residual or recurrent disease.
Repeat surgery on the pituitary fossa is associated with higher perioperative and postoperative complications than those encountered during initial resection [9, 10]. Gamma Knife Radiosurgery (GKRS) is a safe and effective treatment modality that can be used as an adjunct for patients who undergo subtotal resection of NFPA, and for those with recurrent NFPA [5, 11, 12]. Less commonly, GKRS can also be used as a primary treatment modality in NFPA for patients with surgical comorbidities, advanced age, or for those with a personal preference [13, 14]. GKRS is associated with a lesser risk of hypopituitarism, radiation-induced secondary tumors, carotid stenosis, stroke, as well as neurocognitive side effects as compared to conventional radiotherapy [13].
There is relatively modest data on the long-term outcomes of GKRS on NFPA. In the present study, we retrospectively evaluated our experience with adjunctive GKRS in the management of patients with NFPA, with emphasis on hormonal and radiologic outcome. To the best of our knowledge, the present publication is the first of its kind from South Asia. We believe this evaluation of post-operative adjunctive GKRS in a sizable cohort of patients with NFPA will be a valuable addition to the literature.
Methods and materials
Study design
This retrospective study was conducted using a database of 109 patients with NFPA, who underwent adjunctive GKRS between 1996 and 2020, at the P. D. Hinduja Hospital and Medical Research Centre, Mumbai, India. Information related to the clinico-imaging characteristics, surgical data, laboratory parameters, follow-up, and outcomes of all the patients was collected by reviewing the individual case files in the medical record section. Patients with a post-GKRS follow-up of at least 6 months at our institute, and having had at least one neuroimaging in the ‘follow-up cohort’ were included in the study. Inclusion criteria were satisfied by 63 patients. Data was collated, tabulated, and analyzed statistically.
Patient characteristics
The follow-up cohort consisted of 39 male and 24 female patients, with a mean age of 47.3 ± 11.3 years at the time of GKRS, and a median follow-up period of 47 months (range, 6–260 months). All 63 patients received GKRS as an adjunctive therapy for residual or recurrent tumors. Forty (63.5%) patients had undergone adenoma resection surgery once and 23 (36.5%) patients had two surgeries prior to being subjected to GKRS. Median time to GKRS was 293 days (range, 69–1659 days) after the last surgery. Headache (25.4%), vision loss (23.8%) and double vision (6.3%) were the common symptoms prior to GKRS in the study group. Of the 15 patients with complaint of vision loss, 7 had bilateral partial loss of vision, 3 had partial loss of vision in the right eye, 4 had partial loss of vision in the left eye, and 1 patient had complete vision loss in the left eye. Thirty seven of the 63 patients (58.7%) had visual field defects prior to undergoing GKRS. 26 (70.3%) had bi-temporal hemianopia, 8 (21.6%) had unilateral temporal defects and 1 (1.6%) patient each had left homonymous hemianopia, bilateral severely constricted visual fields and bilateral few scattered defects. Two (3.2%) patients had third cranial nerve palsy, while 1 (1.6%) patient had optic nerve involvement (Table 1).
Endocrine evaluation & characteristics
All the patients had a periodic evaluation of adrenal (baseline serum cortisol with/without serum adrenocorticotropic hormone), thyroid (serum total or free T4, and serum thyroid stimulating hormone), and gonadal (serum follicle-stimulating hormone and serum luteinizing hormone, and serum testosterone in men) axes. Diagnosis of secondary hypocortisolism was established if 8 a.m. serum cortisol < 5 mcg/dL (137.9 nmol/L), or an inappropriately low/normal serum adrenocorticotropic hormone (ACTH) value (< 20 pg/ml, < 4.4 pmol/L) accompanying a decreased serum cortisol level, or a 1 h post-standard ACTH (using intravenous tetracosactide acetate 250 μg) serum cortisol of < 18 mcg/dL (496.5 nmol/L), or patient already receiving glucocorticoid replacement therapy. Patients with serum total T4 < 4.5 mcg/dL (57.9 nmol/L) or serum free T4 < 0.8 ng/dL (10.3 pmol/L), with serum thyroid stimulating hormone (TSH) < 4.5 mIU/L, or those on levothyroxine replacement therapy were diagnosed to have hypothyroidism. Premenopausal women were diagnosed with hypogonadism if they had amenorrhea and both serum follicle-stimulating hormone (FSH) and serum luteinizing hormone levels (LH) were < 10 mIU/mL; while post-menopausal women were defined as gonadotropin deficient when serum LH was < 10 mU/L and serum FSH was < 30 mU/L. In men, gonadotropin deficiency was diagnosed if serum total testosterone level was < 3 ng/ml (10.4 nmol/L). Diagnosis of diabetes insipidus was made if the patient was receiving desmopressin. Sufficient data was not available regarding prolactin and growth hormone axes. Hypopituitarism was defined if the patient had any one of hypocortisolism, hypothyroidism, or hypogonadism. Panhypopituitarism was diagnosed if the patient had hypocortisolism, hypothyroidism, and hypogonadism.
Pre-GKRS, 25 (39.7%) patients did not have any hormonal deficiency. Among the remaining 38 (60.3%) patients, single endocrine axis was involved in 16 (25.4%) patients and various combinations of multiple axes involvement were seen in 22 (34.9%) patients. Panhypopituitarism was present in 11 (17.5%) patients (Table 2).
Imaging characteristics
The NFPA imaging patterns were assessed by Magnetic Resonance Imaging (MRI, all available T1-weighted sequences), in axial, coronal, and sagittal planes. Tumor volumes were calculated from the volume of an oblate spheroid, using the Di Chiro and Nelson formula: (anteroposterior dimension × vertical dimension × horizontal dimension in cm) × (π/6). Given the irregular shape of some tumors, volume measurement should be considered only a rough estimate of the actual tumor volume. Tumor shrinkage was defined as tumor reduction > 50% of the volume, and tumor progression was defined as a > 25% increase in the volume of the tumor at the last follow-up. Otherwise, the tumors were classified as having no change [15].
Pituitary adenomas bulging into the chiasmatic cistern or beyond were classified as supra-sellar extending (Type A or higher as per Hardy classification) [16]. Adenomas extending beyond the intercarotid/median carotid line laterally were labeled as having cavernous sinus extension (Grade 2 or higher as per Knosp classification) [17]. Those which had eroded the sellar floor and had come to lie in the sphenoid sinus were classified as inferior extending. Pituitary adenomas extending over the planum sphenoidale into the frontal lobe were classified as anterior extending. Posterior growth was reported when the tumor extended into the interpeduncular cistern and/or prepontine area or beyond. In the follow-up cohort, majority tumors extended into cavernous sinus (42 of 63, 66.7%), followed by suprasellar extension (21 of 63, 33.3%); 7 (11.1%) adenomas were intra-sellar. The mean pre-GKRS volume of NFPAs was 3.1 ± 2.5 cm3 (Table 2).
GKRS technique
Stereotactic radiosurgery was performed with Leksell® Gamma Knife (Model C till 2016 and Perfexion from 2017 onwards). Leksell® stereotactic frame was applied to patient’s head under local scalp anesthesia. The patient was transferred to the magnetic resonance imaging (MRI) room and high-resolution contrast-enhanced axial images of the brain were obtained in the three-dimensional spoiled gradient recalled sequence, and the data was transferred to the gamma knife planning computer via ethernet. The Leksell Gamma Plan® software was used to perform the dose planning (Fig. 1). The goal of GKRS dose planning for NFPA was to achieve a prescribed marginal dose ≥ 10 Gy while keeping the radiation reaching the anterior visual pathway ≤ 8 Gy, and covering the whole tumor within 50% isodose lines. Exposure to the normal gland and pituitary stalk was not always accounted for due to the inconsistency in the clear-cut delineation of the normal structures from the tumor. In the subset of cases with clear delineated stalk, the radiation exposure was kept ≤ 9 Gy.
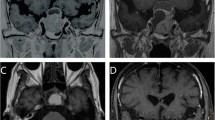
Serial contrast enhanced MRI Brain images (including GKRS planning) in a case of stable residual NFPA with bilateral (left > right) cavernous sinus extension treated with GKRS. A1-2 GKRS planning MRI images showing contrast enhancing residual lesion in the bilateral (left > right) cavernous region in axial (A1) & coronal (A2) plane. Patient received a margin dose of 13 Gy at 50% isodose (yellow line) through 6 shots. B1-2 Axial (B1)&coronal (B2) fat suppressed contrast images, at 1-year post-GKRS, showing reduction in the residual tumor volume. C1-2 5-year follow-up fat suppressed contrast MRI scan in axial (C1) & coronal plain (C2) showing near complete resolution of the residual tumor in both the cavernous sinuses
The median prescription dose delivered to the tumor margin was 13 Gy (range, 8 to 15 Gy), while the maximum dose varied from 16 to 30 Gy, with a median dose of 26 Gy. The prescription isodose was 50% in 59 patients, 40% in 3 patients, and 30% in 1 patient. The median number of isocenters used was 14 (range, 6–31), and the maximum dose to the anterior visual pathway varied from 3.2 to 9 Gy (median 6.4 Gy). (Table 3).
Statistical analysis
Survival analysis was carried out using the Kaplan–Meier technique and compared by using a log-rank test. 5-year progression free survival (PFS) and overall survival (OS) were calculated, along with (GKRS induced) endocrinopathy free survival (EFS), with either the last follow-up or death as the primary endpoints. Possible prognostic factors like patient’s age, gender, margin dose, tumor volume etc. were examined using statistical tests (Chi-square; Univariate analysis). Chi-square test with Yates correction was applied to test differences between immunohistochemistry positivity, extra-sellar tumor extension, supra-sellar tumor extension etc., and Mann–Whitney U test was used to compare difference in time after GKRS, between the patients with tumor control and those with tumor progression. Results were considered significant if the P-value was < 0.05. Statistical analysis was performed using Statistical Package for the Social Sciences (SPSS) software, version 23 for Windows®.
Results
Endocrine outcome
In the follow-up cohort, 11 patients already had panhypopituitarism (hypocortisolism, hypothyroidism and hypogonadism) pre-GKRS. Of the 52 patients with residual pituitary hormonal function at the time of GKRS, 25 (48.1%) developed ≥ 1 new pituitary hormone deficiency at median time of 38 months (range, 8–97 months). Only hypocortisolism, hypothyroidism and hypogonadism developed in 4, 7 and 5 patients respectively. Three patients developed hypocortisolism and hypothyroidism, 2 developed hypocortisolism and hypogonadism, and 2 developed hypothyroidism and hypogonadism. Two patients developed panhypopituitarism. Out of 25 patients who did not have any pre-GKRS endocrinopathy, 2 patients developed only hypocortisolism, 4 developed only hypothyroidism, 3 developed hypocortisolism and hypothyroidism, 1 developed hypocortisolism and hypogonadism, and 2 developed panhypopituitarism.
Median time to development of hypocortisolism, hypothyroidism and hypogonadism was 38 months (range, 8–55 months), 45.5 months (range, 12–97 months), and 45 months (range, 21–75 months), respectively. The actuarial risk of developing a new pituitary hormone deficit at 1, 3, 5, 7, and 10 years was 2.5%, 11%, 26.3%, 28% and 29.7% (Table 4). One patient with secondary cortisol deficiency experienced an improvement of adrenal function at 14 months after GKRS, leading to cessation of glucocorticoid replacement.
By Kaplan–Meir survival analysis, 5-year endocrinopathy free survival (EFS) in NFPA post-GKRS was 47.1% (Fig. 2B). Though age ≥ 45 years, female gender, marginal radiation dose of < 13 Gy, and pre-GKRS tumor volume > 5 cm3 were the parameters that predicted better endocrinopathy free survival, none of these reached statistical significance. (Table 5).
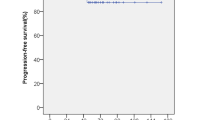
Kaplan–Meier survival analysis in the follow-up cohort. A Kaplan–Meier plots estimating the progression free survival (PFS) in the patients of NFPA who underwent adjunctive GKRS. The 5-year PFS was estimated to be 93.3%. B Kaplan–Meier curves estimating the (GKRS induced) endocrinopathy free survival (EFS). The 5-year (GKRS induced) EFS was estimated to be 47.1%
Tumor control
Overall tumor control rate in the follow-up cohort was 87.3% at the last follow-up. Adenoma size decreased in 36 (57.1%) patients and remained stable in 19 (30.2%) patients. The median follow-up time of patients with tumor reduction was 86 months after GKRS (range, 6 to 260 months). Tumor progression was seen in 8 patients (12.7%), and the median follow-up time was 55.5 months after GKRS (range, 6 to 163 months) (Table 6). Of the 8 patients with increase in the tumor size, 5 underwent additional surgical resection, and 1 amongst them underwent repeat GKRS post-resurgery after 163 months of initial GKRS. (Table 7).
By Kaplan-Meir survival analysis, the 5-year progression free survival (PFS) and overall survival (OS) for patients after GKRS were 93.3% and 100%, respectively (Fig. 2A). On subgroup analysis, the PFS appeared higher for patients aged < 45 years, female patients, patients receiving marginal radiation dose ≥ 13 Gy, and patients with tumor volume ≤ 5 cm3, but none were statistically significant (Table 5).
There was no difference between the tumors that were controlled after GKRS (N = 55), from those that progressed (N = 8) when compared with respect to immunohistochemistry (IHC) staining for any of LH, FSH, TSH, ACTH, GH or prolactin (77.4% vs 87.5%, P = 0.85), gonadotroph (LH/FSH) positivity on IHC (43.4% vs 37.5%, P = 0.945), extra-sellar extension (89.1% vs 87.5%, P = 0.643), supra-sellar extension (30.9% vs 50%, P = 0.504) and cavernous sinus extension (69.1% vs 50%, P = 0.504). Median time to GKRS after last surgery did not significantly differ between the controlled and the progressed groups (307 vs 259 days, P = 0.726).
Complications
The complications noticed in the follow-up cohort are listed in Table 7. One patient, who had received a maximum radiation dose of 28 Gy, developed severe headache on day 2 post-GKRS. He improved over the next 2 days with only symptomatic management. Another patient had worsening of vision and deterioration of visual fields on follow-up at 16 months, and on MRI was diagnosed to have developed bilateral optic atrophy. She had received a maximum radiation dose of 24 Gy, and 5.2 Gy to the anterior visual pathway.
A male patient with posterior extension of NFPA, who had undergone GKRS at 71 years of age, had left cerebellar infarct after 60 months of GKRS. His tumor size had remained stable over the period (3.3–2.8 cm3). He had received a maximum radiation dose of 26 Gy, with a marginal dose of 13 Gy. He had not received any additional therapy after GKRS.
No patient experienced any diplopia, cranial nerve palsy or radiation necrosis as immediate or short-term complication. None of the patients had hydrocephalus or second neoplasm till last follow-up.
Discussion
Though surgical adenomectomy remains the treatment of choice for NFPA, the chance for residual or recurrent disease remains high. Adjunctive treatment modalities, like GKRS, have a major role to play in managing such residual or recurrent NFPA, and averting the need for resurgery. Long-term tumor control rates of NFPA patients are shown to be higher with GKRS in comparison to conventional fractionated radiotherapy [13, 18]. The evidence-based knowledge about the GKRS outcomes in NFPA is limited and needs expanding, especially the endocrine outcome. We retrospectively analysed the long-term endocrine and imaging outcome and the influencing factors in our study group. This is the first such report in the south Asian region in treating NFPA with adjunctive GKRS.
Endocrine outcome
Delayed hypopituitarism is the single most important complication seen in the post-GKRS NFPA patients. There is a wide spectrum of reported adverse endocrine outcomes in the current literature from large cohorts, ranging from 4 to 32% [5, 9, 11, 12, 14, 19,20,21,22,23,24]. Hayashi et al. reported 0% new hypopituitarism in patients who received GKRS for NFPA in cavernous sinus location only [25]. Incidence of GKRS induced hypopituitarism was comparatively higher (25 of 63, 39.7%) in our study, with ≥ 1 new pituitary hormone deficiency developing at median time of 38 months. 2 patients developed panhypopituitarism. In our study, deficiency of thyroid axis (22.2%) was most commonly seen, followed by adrenal and gonadal axes (17.5% each). Liscak et al. and Kara et al. reported hypocortisolism being the most common, followed by hypothyroidism and hypogonadism [24, 26]; while gonadal, thyroid and adrenal axes were involved in 9%, 7% and 5% patients in the study by Sun et al. [20], in NFPA patents after GKRS. These differences in hormonal axes involvement may be due to small number of actual outcomes.
We observed better 5-year (GKRS induced) EFS rates in patients aged ≥ 45 years (52.7% vs 40.3%) and female patients (63.3% vs 40.9%). Similarly, patients with lesser median marginal dose of radiation (< 13 Gy) had a better EFS (51.3% vs 45%). Leenstra et al. in their study of adjuvant GKRS in NFPA noted 30% pituitary deficits for doses < 15 Gy, compared with 45% who received a mean dose > 15 Gy [27]. In our study, patients with tumor volume of > 5 cm3 had a better EFS (48.1% vs 36.6%). This reported relationship of the tumor volume and endocrinopathy status is in disagreement with the findings of a systematic review and meta-analysis published by Pollock BE et al. [12]. Another large cohort by Kara et al. also made observations in agreement with that of Pollock et al.’s review [26]. In our cohort, the patients with a tumor volume of > 5 cm3 (N = 10) had received significantly lesser maximum dose (23.6 ± 3.8 vs 26.3 ± 2.4 Gy, P = 0.005) and marginal dose (11.1 ± 1.9 vs 13.1 ± 1.2, P < 0.001) of radiation as compared to patients with adenoma volume ≤ 5cm3 (N = 53), which may explain our observation regarding tumor volume and endocrinopathy status.
Imaging outcome
Tumor control was seen in 87.3% patients, which has been consistent with other studies reporting 85 to 91% tumor control with GKRS in NFPA [9, 11, 19, 20, 22, 28]. A few studies have reported 95 to 100% tumor control post-GKRS [21, 23,24,25]. Long term radiologic outcomes have been excellent with all large cohorts reporting 5-year PFS in excess of 90% [5, 9, 11, 12, 14, 19,20,21,22,23, 27, 28], and our results of 5-year PFS of 93.3% are in accordance with them. The 5-year PFS was better for patients aged < 45 years, female patients, patients receiving marginal radiation dose ≥ 13 Gy, and patients with tumor volume ≤ 5 cm3. Park et al. and Narayan et al. reported worse control in tumors > 5 cm3 [9, 11], while Sheehan et al. observed poorer GKRS outcomes in tumors with suprasellar extensions [5]. Though minimal effective dose of radiation for tumor control remains debatable, Gopalan et al. observed that NFPA inclined to progress with GKRS doses < 12 Gy [29], while Mingione et al. reported that doses > 20 Gy did not significantly better tumor control rates [30]. A recent meta-analysis studying GKRS outcomes in pituitary adenomas by Albano et al. did not find significant differences in tumor control rates with increasing prescription doses [18]. There is conflicting data regarding tumor control with early GKRS (within 6 months) after surgery, with studies by Kara et al. and Promeraniec et al. observing a significantly lower rate of NFPA progression [26, 31], while Sadik et al. detected no such significant difference in tumor progression with the timing of GKRS [21]. In their systematic review, Albano et al. recommend early radiosurgical therapy in patients who undergo adenomectomy and have a clear residual mass [18].
Grave complications
Several gamma knife centers have reported GKRS to be a safe treatment in patients with NFPA. Many studies have reported 0% incidence of optic neuropathy after GKRS for NFPA [11, 12, 14, 21, 24, 28], others have reported development of optic neuropathy in 1 to 4% patients [9, 19, 20, 22, 23], while Sheehan et al. had an incidence of 7% in their study [5]. In our study, 1 patient (1.6%) developed optic atrophy after GKRS. The optic chiasm typically lies 10 mm above the pituitary gland, and in 80% of the population rests immediately above the sella. It may rest above tuberculum sellae in 10% (having short optic nerves and long optic tracts), and above dorsum sellae in the remaining 10% of the population (having long optic nerves and short optic tracts) [32]. Therefore, accurate stereotactic localization and head-frame placement is critically important not only for precise delivery of ionizing radiation to the adenoma, but also to prevent inadvertent exposure of radiation to the anterior visual pathway and other structures at risk.
In our cohort, a diabetic and hypertensive male patient with posterior extension of NFPA, who had undergone GKRS at 71 years of age, had cerebellar infarction after 60 months of GKRS. He had received a maximum radiation dose of 26 Gy, with a marginal dose of 13 Gy. Van Westrhenen A et al. in their systemic review of ischemic stroke after radiation therapy for pituitary adenomas reported incidence ranging from 0 to 11.6%, with no reports of ischemic stroke in patients receiving stereotactic radiosurgery [33]. Irradiation to the arteries in the field of radiation (cavernous segment of internal carotid artery in parasellar extending adenomas, or circle of Willis in suprasellar extending adenomas) may predispose them to accelerated atherosclerosis, thereby increasing the risk of ischemic infarction. There is no data to suggest if occurrence of stroke is prescription dose dependent. However, Shin et al. recommend < 30 Gy dose restriction to cavernous part of internal carotid artery to prevent postradiosurgery stenosis [34]. More recent study has however observed that there are no increased anatomical brain abnormalities (cerebral atrophy, brain infarctions, abnormalities of the temporal lobes and hippocampi) in patients receiving radiotherapy for NFPA, as compared to surgery alone [35]. Another study reported pre-existing coronary artery disease, peripheral artery disease, and hypertension as the primary risk factors for occurrence of stroke in patients with pituitary adenomas, irrespective of treatment with radiation therapy [36]. In our study, the patient who suffered from cerebellar infarction post-GKRS, likely represents an independent event unrelated to radiotherapy.
In patients with NFPA, post-GKRS new cranial nerve palsies have been reported in a few studies, with incidence varying from 1.6 to 9% [5, 9, 19, 22]. Only one study reported hydrocephalus (1.7%, 1 patient) [22], while none of the referenced studies have reported radiation necrosis or secondary tumors in patients receiving GKRS for NFPA. No patient developed cranial nerve palsy, radiation necrosis, hydrocephalus or second neoplasm in our cohort.
Limitations
Relatively small sample size and questionable representativeness of the study population act as limitations for the sub-group analysis. Though Pomeraniec et al. reported that the dose ratio to pituitary stalk over the normal gland of ≥ 0.8 was an independent indicator for GKRS induced endocrinopathy, a similar analysis was not possible in our retrospective analysis [37]. We had other significant differences in few of our findings with that of the available literature. However, it is important to highlight that such comparisons between retrospective studies are limited by differences in the patient/tumor/treatment characteristics that can significantly affect outcomes. No statistically significant results regarding parameters affecting outcomes were obtained. The inability to standardize MRI slice thickness and magnet strength during the follow-up visits adds to the limitations of our study.
Conclusion
Adjunctive GKRS in NFPA has an excellent 5-year progression free survival of 93.3%. Risk of GKRS induced endocrinopathy exists, and we report a 5-year (GKRS induced) endocrinopathy free survival of 47.1%. GKRS offers a high rate of tumor control, with satisfactory long-term preservation of hormone functions in patients with NFPA. GKRS is an effective and safe adjunctive treatment modality for patients with NFPA.
References
Ferrante E, Ferraroni M, Castrignanò T, Menicatti L, Anagni M, Reimondo G et al (2006) Non-functioning pituitary adenoma database: a useful resource to improve the clinical management of pituitary tumors. Eur J Endocrinol 155(6):823–829. https://doi.org/10.1530/eje.1.02298
Chang EF, Sughrue ME, Zada G, Wilson CB, Blevins LS Jr, Kunwar S (2010) Long term outcome following repeat transsphenoidal surgery for recurrent endocrine-inactive pituitary adenomas. Pituitary 13(3):223–229. https://doi.org/10.1007/s11102-010-0221-z
Turner HE, Stratton IM, Byrne JV, Adams CB, Wass JA (1999) Audit of selected patients with nonfunctioning pituitary adenomas treated without irradiation—a follow-up study. Clin Endocrinol (Oxf) 51(3):281–284. https://doi.org/10.1046/j.1365-2265.1999.00865.x
Shen CC, You WC, Sun MH, Lee SD, Tsou HK, Chen YJ et al (2018) Outcome of partially irradiated recurrent nonfunctioning pituitary macroadenoma by gamma knife radiosurgery. J Neurooncol 139(3):767–775. https://doi.org/10.1007/s11060-018-2925-2
Sheehan JP, Starke RM, Mathieu D, Young B, Sneed PK, Chiang VL et al (2013) Gamma knife radiosurgery for the management of nonfunctioning pituitary adenomas: a multicenter study. J Neurosurg 119(2):446–456. https://doi.org/10.3171/2013.3.JNS12766
Peker S, Kurtkaya-Yapicier O, Kiliç T, Pamir MN (2005) Microsurgical anatomy of the lateral walls of the pituitary fossa. Acta Neurochir 147(6):649. https://doi.org/10.1007/s00701-005-0513-7
Brochier S, Galland F, Kujas M, Parker F, Gaillard S, Raftopoulos C et al (2010) Factors predicting relapse of nonfunctioning pituitary macroadenomas after neurosurgery: a study of 142 patients. Eur J Endocrinol 163(2):193–200. https://doi.org/10.1530/EJE-10-0255
Trifiletti DM, Dutta SW, Lee CC, Sheehan JP (2019) Pituitary tumor radiosurgery. Prog Neurol Surg 34:149–158. https://doi.org/10.1159/000493059
Park KJ, Kano H, Parry PV, Niranjan A, Flickinger JC, Lunsford LD et al (2011) Long-term outcomes after gamma knife stereotactic radiosurgery for nonfunctional pituitary adenomas. Neurosurgery 69(6):1188–1199. https://doi.org/10.1227/NEU.0b013e318222afed
Laws ER Jr, Fode NC, Redmond MJ (1985) Transsphenoidal surgery following unsuccessful prior therapy. An assessment of benefits and risks in 158 patients. J Neurosurg 63(6):823–9. https://doi.org/10.3171/jns.1985.63.6.0823
Narayan V, Mohammed N, Bir SC, Savardekar AR, Patra DP, Bollam P et al (2018) Long-term outcome of nonfunctioning and hormonal active pituitary adenoma after gamma knife radiosurgery. World Neurosurg 114:e824–e832. https://doi.org/10.1016/j.wneu.2018.03.094
Pollock BE, Cochran J, Natt N, Brown PD, Erickson D, Link MJ et al (2008) Gamma knife radiosurgery for patients with nonfunctioning pituitary adenomas: results from a 15-year experience. Int J Radiat Oncol Biol Phys 70(5):1325–1329. https://doi.org/10.1016/j.ijrobp.2007.08.018
Lee MH, Lee JH, Seol HJ, Lee JI, Kim JH, Kong DS et al (2016) Clinical concerns about recurrence of non-functioning pituitary adenoma. Brain Tumor Res Treat 4(1):1–7. https://doi.org/10.14791/btrt.2016.4.1.1
Lee CC, Kano H, Yang HC, Xu Z, Yen CP, Chung WY et al (2014) Initial gamma knife radiosurgery for nonfunctioning pituitary adenomas. J Neurosurg 120(3):647–654. https://doi.org/10.3171/2013.11.JNS131757
Di Chiro G, Nelson KB (1962) The volume of the sella turcica. Am J Roentgenol Radium Ther Nucl Med 87:989–1008
Hardy J, Vezina JL (1976) Transsphenoidal neurosurgery of intracranial neoplasm. Adv Neurol 15:261–273
Knosp E, Steiner E, Kitz K, Matula C (1993) Pituitary adenomas with invasion of the cavernous sinus space: a magnetic resonance imaging classification compared with surgical findings. Neurosurgery 33(4):610–7. https://doi.org/10.1227/00006123-199310000-00008
Albano L, Losa M, Barzaghi LR, Niranjan A, Siddiqui Z, Flickinger JC et al (2021) Gamma knife radiosurgery for pituitary tumors: a systematic review and meta-analysis. Cancers 13(19):4998. https://doi.org/10.3390/cancers13194998
Deng Y, Li Y, Li X, Wu L, Quan T, Peng C et al (2020) Long-term results of gamma knife radiosurgery for postsurgical residual or recurrent nonfunctioning pituitary adenomas. Int J Med Sci 17(11):1532–1540. https://doi.org/10.7150/ijms.47168
Sun S, Liu A, Zhang Y (2019) Long-term follow-up studies of gamma knife radiosurgery for postsurgical nonfunctioning pituitary Adenomas. World Neurosurg S1878–8750(19):30078–30086. https://doi.org/10.1016/j.wneu.2019.01.009
Sadik ZHA, Voormolen EHJ, Depauw PRAM, Burhani B, Nieuwlaat WA, Verheul J et al (2017) Treatment of nonfunctional pituitary adenoma postoperative remnants: adjuvant or delayed gamma knife radiosurgery? World Neurosurg 100:361–368. https://doi.org/10.1016/j.wneu.2017.01.028
Bir SC, Murray RD, Ambekar S, Bollam P, Nanda A (2015) Clinical and radiologic outcome of gamma knife radiosurgery on nonfunctioning pituitary adenomas. J Neurol Surg B Skull Base 76(5):351–357. https://doi.org/10.1055/s-0035-1549309
Zeiler FA, Bigder M, Kaufmann A, McDonald PJ, Fewer D, Butler J, Schroeder G et al (2013) Gamma knife in the treatment of pituitary adenomas: results of a single center. Can J Neurol Sci 40(4):546–552. https://doi.org/10.1017/s0317167100014645
Liscák R, Vladyka V, Marek J, Simonová G, Vymazal J (2007) Gamma knife radiosurgery for endocrine-inactive pituitary adenomas. Acta Neurochir 149(10):999–1006. https://doi.org/10.1007/s00701-007-1253-7
Hayashi M, Chernov M, Tamura N, Nagai M, Yomo S, Ochiai T et al (2010) Gamma knife robotic microradiosurgery of pituitary adenomas invading the cavernous sinus: treatment concept and results in 89 cases. J Neurooncol 98(2):185–194. https://doi.org/10.1007/s11060-010-0172-2
Kara M, Yılmaz M, Şengöz M, Peker S (2021) Hormonal and radiologic outcomes after gamma knife radiosurgery for nonfunctioning pituitary adenomas. Br J Neurosurg 1:1–7. https://doi.org/10.1080/02688697.2021.1903388
Leenstra JL, Tanaka S, Kline RW, Brown PD, Link MJ, Nippoldt TB et al (2010) Factors associated with endocrine deficits after stereotactic radiosurgery of pituitary adenomas. Neurosurgery 67(1):27–32. https://doi.org/10.1227/01.NEU.0000370978.31405.A9
Iwai Y, Yamanaka K, Yoshioka K (2005) Radiosurgery for nonfunctioning pituitary adenomas. Neurosurgery 56(4):699–705. https://doi.org/10.1227/01.neu.0000156836.42945.28
Gopalan R, Schlesinger D, Vance ML, Laws E, Sheehan J (2011) Long-term outcomes after gamma knife radiosurgery for patients with a nonfunctioning pituitary adenoma. Neurosurgery 69(2):284–293. https://doi.org/10.1227/NEU.0b013e31821bc44e
Mingione V, Yen CP, Vance ML, Steiner M, Sheehan J, Laws ER et al (2006) Gamma surgery in the treatment of nonsecretory pituitary macroadenoma. J Neurosurg 104(6):876–883. https://doi.org/10.3171/jns.2006.104.6.876
Pomeraniec IJ, Dallapiazza RF, Xu Z, Jane JA Jr, Sheehan JP (2016) Early versus late gamma knife radiosurgery following transsphenoidal resection for nonfunctioning pituitary macroadenomas: a matched cohort study. J Neurosurg 125(1):202–212. https://doi.org/10.3171/2015.5.JNS15581
Gala F (2015) Magnetic resonance imaging of optic nerve. Indian J Radiol Imaging 25(4):421–38. https://doi.org/10.4103/0971-3026.169462
van Westrhenen A, Muskens IS, Verhoeff JJC, Smith TRS, Broekman MLD (2017) Ischemic stroke after radiation therapy for pituitary adenomas: a systematic review. J Neurooncol 135(1):1–11. https://doi.org/10.1007/s11060-017-2530-9
Shin M, Kurita H, Sasaki T, Tago M, Morita A, Ueki K et al (2000) Stereotactic radiosurgery for pituitary adenoma invading the cavernous sinus. J Neurosurg 93(Suppl 3):2–5. https://doi.org/10.3171/jns.2000.93.supplement_3.0002
Sattler MG, Meiners LC, Sluiter WJ, van den Berg G, Langendijk JA, Wolffenbuttel BH et al (2015) Brain abnormalities on MRI in non-functioning pituitary adenoma patients treated with or without postoperative radiotherapy. Radiother Oncol 114(2):239–244. https://doi.org/10.1016/j.radonc.2015.01.003
Sattler MG, Vroomen PC, Sluiter WJ, Schers HJ, van den Berg G, Langendijk JA et al (2013) Incidence, causative mechanisms, and anatomic localization of stroke in pituitary adenoma patients treated with postoperative radiation therapy versus surgery alone. Int J Radiat Oncol Biol Phys 87(1):53–59. https://doi.org/10.1016/j.ijrobp.2013.05.006
Pomeraniec IJ, Taylor DG, Cohen-Inbar O, Xu Z, Lee Vance M, Sheehan JP (2019) Radiation dose to neuroanatomical structures of pituitary adenomas and the effect of gamma knife radiosurgery on pituitary function. J Neurosurg 132(5):1499–1506. https://doi.org/10.3171/2019.1.JNS182296
Acknowledgements
Nil
Funding
This research did not receive any specific grant or financial contribution from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Dr. ANM and Dr. SP. The first draft of the manuscript was written by Dr. ANM, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interest or personal relationships that could have appeared to influence the work reported in this paper.
Ethical approval
Ethical approval was waived by the local Ethics Committee of P. D. Hinduja Hospital in view of the retrospective nature of the study and all the procedures being performed were part of the routine care.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Maldar, A.N., Pattankar, S., Misra, B.K. et al. Long-term hormonal and imaging outcomes of adjunctive gamma knife radiosurgery in non-functioning pituitary adenomas: a single center experience. J Neurooncol 158, 423–433 (2022). https://doi.org/10.1007/s11060-022-04029-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-022-04029-0