Abstract
Hypopituitarism is a common complication of TBI in long-term survivors, more frequent than previously realized. It may be partial or complete, sometimes very subtle without visible lesions in hypothalamo-pituitary region and is diagnosed only by biochemical means. Neuroendocrine abnormalities caused by TBI may have significant implications for the recovery and rehabilitation of these patients. The subjects at risk are those who have suffered moderate to severe trauma, although mild intensity trauma may precede hypopituitarism also. Particular attention should be paid to this problem in children and adolescents. We describe a patient with hypopituitarism thought to be idiopathic due to mild head trauma which caused diabetes insipidus in childhood, gradual failure of pituitary hormones during the period of growth and development, and metabolic (dyslipidemia), physical (obesity), and cognitive impairments in the adult period.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Recent studies have demonstrated that hypopituitarism, in particular growth hormone (GH) deficiency, is common among survivors of traumatic brain injury (TBI) tested several months or years following head trauma [1–7]. In addition, it has been shown that post-traumatic neuroendocrine abnormalities occur early and with high frequency [8, 9]. These findings may have significant implications for the recovery and rehabilitation of patients with TBI. Although data emerging after 2000 demonstrate the relevance of the problem, in general there is a lack of awareness in the medical community about the incidence and clinical repercussions of the pathology. Most, but not all, head trauma associated with hypopituitarism is the result of motor vehicle accidents. The subjects at risk are those who have suffered moderate to severe head trauma although mild intensity trauma may precede hypopituitarism also. Particular attention should be paid to this problem in children and adolescents. We report a young female who at age 7 years was diagnosed as idiopathic diabetes insipidus, at age 12 years was investigated for short stature and diagnosed isolated growth hormone deficiency (GHD). Later she developed central hypogonadism and further central hypothyroidism. Finally at age of 26 years, with more in-depth questioning of parents she was diagnosed as TBI-induced hypopitutarism and diabetes insipidus.
Case report
Female patient, age 26 years was referred for endocrine evaluation. She complained of weakness, mild headache, periorbital and ankle swellings, weight gain and hypertension. At age 7 years diabetes insipidus was diagnosed and considered idiopathic. At the age of 12, due to the short stature, she was investigated by pediatric endocrinologist and GHD was diagnosed, without any other anterior pituitary hormone deficiency. She was not treated with GH replacement, grew spontaneously 2–3 cm per year and reached adult height of 151 cm. At age 13 years she was staged Tanner 2 yet she did not proceed through puberty and remained amenorrhoeic till age 18 years when she was diagnosed as hypogonadotropic hypogonadism. Ultrasound appearance of ovaries and uterus was normal. She was replaced with gonadal steroids, but she suffered severe weight gain. At age 19 years, low levels of T4 with normal TSH were found, central hypothyroidism was diagnosed and she was replaced with l-thyroxine. Family history was positive for obesity, arterial hypertension and dyslipidemia (both parents).
When this time at age 26, past history was taken with more in-depth questioning. Patient’s father recollected that at age 7 years (prior to diabetes insipidus), his daughter fell on head from bed. Parents took her to see the doctor who considered this to be mild head trauma (concussion) with no need for further follow-ups. This event was since considered non-relevant and not mentioned before.
Clinical characteristics and body composition
On admission, she was of 151 cm height, extremely obese with body weight of 92 kg and BMI 40 kg/m2, with central type of obesity (WHR 0.92) and mild periorbital and ankle edemas. Other physical findings were unremarkable.
Clinical characteristics of the patient are shown in Table 1. Combined dyslipidemia was found and leptin level was increased according to the BMI. Body composition was estimated by bioelectrical impendance tests (BIA) with predominant adipose tissue.
Endocrine evaluation
Hormones at baseline are shown in Table 2. Low levels of IGF-I and IGF-BP3 were compatible with the diagnosis of GHD. Low-gonadotropin levels at baseline with low-serum estradiol level in our amenorrhoeic patient were in favor of central hypogonadism. Low level of FT4 with normal TSH level confirmed central hypothyroidism. ACTH level was normal, as well as morning cortisol level, which confirmed normal adrenocortical function. Mild hyperprolactinemia was found increased. Central diabetes insipidus was confirmed.
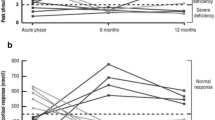
She was retested now (at age 26 years) for GH secretion by two provocative tests namely, the insulin tolerance test (ITT), with peak GH level 0.1 μg/l (Fig. 1) and potent combined test GHRH+GHRP-6 with peak GH level 4.9 μg/l (Fig. 2). Both tests confirmed severe GHD. GnRH test confirmed central hypogonadism (peak LH response 4.25 IU/l).
Imaging study
MRI revealed the absence of TW1 hyperintensity in the posterior part of the pituitary, as well as hypothalamo-pituitary disconnection. The pituitary is atrophic (Fig. 3).
Neuropsychological testing
On psychological testing mild anxiety with compulsive structure, with emotional and social immaturity were found. Cognitive functioning was slightly impaired, with disturbances in efficacy and concentration.
In conclusion, TBI-induced hypopitutarism with diabetes insipidus was diagnosed. In this case, auto-immune pituitary alteration or idiopathic cause should be considered in differential diagnosis of hypopituitarism, but with low probability. Metabolic syndrome (central obesity, dyslipidemia, hyperuricemia, and arterial hypertension) was also present and could be due to long standing GHD or to the TBI itself. The patient was treated with low-calorie diet, statins and was replaced with L-thyroxine. Gonadal steroids were withheld due to severe weight gain and hypertension.
Discussion
A number of patients who suffered brain trauma are currently without specific care while TBI-mediated hypopituitarism is underdiagnosed and untreated. When such patients or their families are asked specific questions with the aim to facilitate to recollect previous head trauma, then a history of head trauma is obtained. Thus, our patient was diagnosed only after in-depth questioning of her family. This has also been recently shown for several patients with unexplained central hypothyroidism [10].
The evolving hypopituitarism in our patient was also interesting. She was diagnosed as isolated GH deficiency at age 12 years. Patients with childhood-onset GH deficiency should be retested as adults before committing them to long-term GH replacement and our patient upon retesting was GH deficient [11–13]. Nevertheless she achieved adult height without GH replacement. Growth without GH has been attributed to obesity (hyperinsulinemia). Since our patient became severely obese, and because of the difficulty in assessing GH/IGF-I axis in morbid obesity, she was retested using the most potent provocative test, the combined GHRH plus GHRP-6 shown to be effective provocative stimulus for GH secretion in obesity [14]. The stimulated GH level was significantly lower than the cut-off for this test, GH < 10 μg/l. Accordingly with the insulin tolerance test, the stimulated GH level was significantly lower than the cut-off, GH < 3 μg/l, and together with IGF-I values below 2 SDS, severe GHD was reconfirmed. For the assessment of the GH–IGF axis in TBI patients, plasma IGF-I concentration, plus dynamic testing with GHRH + arginine [15], GHRH + GHRP-6 [16–19] or glucagon test [20] is indicated. Insulin-induced hypoglycemia testing can be used when not contraindicated [21]. The cut-off response that defines severe GHD based on glucagon stimulation alone is not well established [8]. Testing for growth hormone deficiency in adults has been recently reviewed [22].
Our patient did not complete puberty and remained amenorrhoeic till diagnosis of hypogonadotropic hypogonadism when she was replaced. In recent studies in long-term survivors of TBI between 9 and 17% of subjects are hypogonadal [23].
Mild hyperprolactinemia is in favor of hypothalamic-pituitary disconnection confirmed by the MRI. In a recent study, prolactin concentrations were elevated in 52% of patients in the acute phase of TBI [8]. The frequency of hyperprolactinemia in long-term survivors of TBI ranged from 3 to 12% in reported series [24]. Some authors found that PRL, a stress hormone, correlates positively with severity of the head injury.
Subsequently, our patient was diagnosed as having central hypothyroidism and was replaced. Other patients with unexplained central hypothyroidism were later diagnosed as TBI-induced hypopituitarism [10]. Lower free thyroxine (FT4) levels with TSH concentrations within reference range are not unusual in TBI survivors [25].
In conclusion, pituitary failure can occur even in minor head injuries, can evolve in time and is poorly recognized. Occasional case reports have called attention to this entity and reported significant cognitive improvement by replacement with rhGH [26]. We suggest that rhGH replacement may improve the well-being, physical, metabolic, and cognitive abilities of our patient. Thus not only patients with pituitary adenomas, but those with the history of head trauma need endocrine evaluation.
References
D. Kelly, I. Gonzalo, P. Cohan, N. Berman, R. Swerdloff, C. Wang, J. Neurosurg. 93, 743–752 (2000)
S. Benvenga, A. Campenni, R.M. Ruggeri, F. Trimarrchi, J. Clin. Endocrinol. Metabol. 85, 1353–1361 (2000)
S.A. Lieberman, A.L. Oberoi, C.R. Gilkinson, B.E. Masel, R.J. Urban, J. Clin. Endocrinol. Metabol. 86, 2752–2756 (2001)
G. Aimaretti, M.R. Ambrosio, C. Di Somma, A. Fusco, S. Cannavo, M. Gasperi, C. Scaroni, L. De Marinis, S. Benvenga, E.C.D. Uberti, G. Lombardi, F. Mantero, E. Martino, G. Giordano, E. Ghigo, Clin. Endocrinol. 61, 320–326 (2004)
M.D.E. Bondanelli, L. Marinis, M.R. Ambrosio, M. Monesi, D. Valle, M.C. Zatelli, A. Fusco, A. Bianchi, M. Farneti, E.C. degli Uberti, J. Neurotrauma 21, 685–696 (2004)
V. Popovic, S. Pekic, D. Pavlovic, N. Maric, M. Jasovic-Gasic, B. Djurovic, M. Medic-Stojanoska, V. Zivkovic, M. Stojanovic, M. Doknic, N. Milic, M. Djurovic, C. Dieguez, F.F. Casanueva, J. Endocrinol. Invest. 27, 1048–1054 (2004)
A. Leal-Cerro, J.M. Flores, M. Rincon, F. Murillo, M. Pujol, F. Garcia-Pesquera, C. Dieguez, F.F. Casanueva, Clin. Endocrinol. 62, 525–532 (2005)
A. Agha, B. Rogers, D. Mylotte, F. Taleb, W. Tormey, J. Philips, C. Thompson, Clin. Endocrinol. 60, 584–591 (2004)
F. Tanriverdi, H. Senyurek, K. Unluhizarci, A. Selcuklu, F.F. Casanueva, F. Kelestimur, J. Clin. Endocrinol. Metabol. 91, 2105–2111 (2006)
S. Benvenga, T. Vigo, R. Ruggeri, D. Lapa, B. Almoto, F. LoDuidice, M. Longo, A. Blandino, A. Campenni, S. Cannavo, F. Trimarchi, Am. J. Med. 116, 767–771 (2004)
Growth Hormone Research Society: consensus guidelines for the diagnosis and treatment of adults with growth hormone deficiency: summary statement of the growth hormone research society workshop on adult growth hormone deficiency, J. Clin. Endocrinol. Metabol. 83, 379–381 (1998)
Growth Hormone Research Society Critical evaluation of the safety of recombinant human growth hormone administration: statement from the Growth Hormone Research Society, J. Clin. Endocrinol. Metabol. 86, 1868–1870 (2001)
G. Aimaretti, B. Bellone, L. Di Vito, G. Corneli, E. Arvat, I. Benso, F. Camanni, E. Ghigo, J. Clin. Endocrinol. Metabol. 85, 3693–3699 (2000)
F. Kelestimur, V. Popovic, A. Leal, P.S. Van Dam, E. Torrens, L.F. Perez Mendez, Y. Greenman, P.F.H. Koppeschaar, C. Dieguez, F.F. Casanueva, Clin. Endocrinol. 64, 667–671 (2006)
E. Ghigo, H.G. Aimaretti, L. Gianotti, J. Bellone, E. Arvat, F. Cammani, Eur. J. Endocrinol. 134, 352–356 (1996)
V. Popovic, A. Leal, D. Micic, H.P.F. Kooppeschaar, E. Torrens, C. Paramo, S. Obradovic, C. Dieguez, F.F. Casanueva, Lancet 356(9236), 1137–1142 (2000)
K.K.Y. Ho, Lancet 356(9236), 1125–1126 (2000)
V. Popovic, S. Pekic, M. Doknic, D. Micic, S. Damjanovic, M. Zarkovic, G. Aimaretti, G. Corneli, E. Ghigo, C. Dieguez, F.F. Casanueva, Clin. Endocrinol.0 59, 251–257 (2003)
V. Popovic, Pituitary 8, 239–243 (2005)
J. Cain, G. Williams, R. Dluhy, Can. Med. Assoc. J. 107, 617–622 (1972)
D. Hoffman, A. O’Sullivan, R. Baxter, K. Ho, Lancet 343, 1065–1068 (1994)
J.O. Jorgensen, J.S. Christiansen, Clin. Endocrinol. 58, 18–19 (2003)
A. Agha, C.J. Thompson, Pituitary 8, 245–249 (2005)
A. Agha, C.J. Thompson, Clin. Endocrinol. 64, 481–488 (2006)
M.O. van Aken, S.W.J. Lamberts, Pituitary 8, 183–191 (2005)
J. Springer, A. Chollet, Lancet 357, 1848 (2001)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Medic-Stojanoska, M., Pekic, S., Curic, N. et al. Evolving hypopituitarism as a consequence of traumatic brain injury (TBI) in childhood—call for attention. Endocr 31, 268–271 (2007). https://doi.org/10.1007/s12020-007-0037-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-007-0037-4