Abstract
Austin spectrum disorder (ASD) is a complex neurodevelopmental disorder that can include impairments in communication skills and social interaction as well as behavioral challenges. Recent research has evaluated bone health and bone mineral density (BMD) in cohorts of pediatric, adolescent, and young adult participants. Consistent findings across publications indicate that individuals with ASD have decreased BMD when compared to non-ASD age-matched peers. Factors raised in the literature for consideration of impact on BMD status include dietary intake, feeding behavior, nutrient status, gastrointestinal (GI) symptoms and diagnoses, physical activity, and prescription medication usage. This review aims to provide a comprehensive overview of published research evaluating BMD in those with ASD, analyze potential issues of correlation with lowered BMD in this population, offer perspective for future research consideration, and propose evaluation and intervention strategies to address and potentially ameliorate both the short-term and long-term impact of decreased BMD in children and adolescents.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Autism spectrum disorder (ASD) is a neurodevelopmental disorder characterized by persistent deficits in social communication and social interaction as well as restricted, repetitive patterns of activities, behavior, and interests [1]. The most recent Centers for Disease Control (CDC) ASD prevalence rate is 1 in 59 based on 8–11 year old children in 11 diverse populations across the USA [2]. Prevalence rates for ASD continue to increase worldwide, with estimates of 1.5% or greater in developed countries. This increase reflects changes in prevalence for those primarily without intellectual disability comorbidity [3].
In the past decade, research publications have highlighted complex medical comorbidities that could impact behavioral diagnostic criteria for ASD such as self-injurious behavior (SIB), aggression, and stereotypy, which includes repetitive behaviors such as hand flapping, rocking, and vocal sounds [4,5,6]. The presence of specific medical comorbidities such as gastrointestinal and sleep concerns has been associated with an increase in self-injurious and aggressive behaviors [7, 8]. Anxiety and ADHD symptoms may aggravate certain ASD symptoms, such as social withdrawal and increased ritual/stereotypic behaviors, which may affect overall functioning [9]. Those with ASD also have an increased risk of feeding concerns, food selectivity, and food neophobia resulting in challenging behaviors over non-ASD peers [10,11,12]. Other specific comorbid medical diagnoses such as inflammatory bowel disease directly impact bone health [13, 14].
Within the field of bone health research, seminal work published in 2008 evaluated bone development for males with ASD aged 4–8 years, noting that participants with ASD had significantly lower bone cortical thickness than normative values [15]. Since that publication, several cohorts of pediatric, adolescent, and young adults with ASD have been evaluated for overall bone health markers in comparison with non-ASD peers. Evaluation included bone cortical thickness, bone mineral density (BMD), and bone microarchitecture, as well as nutritional status and dietary intake [16,17,18].
An increasing prevalence of ASD coupled with the long-term health implications of potentially decreased BMD and overall bone health speaks to the need for proactive identification and intervention for those diagnosed with ASD and at-risk for lowered BMD status. In this review, we will speak to the current body of research literature on the topic of pediatric and adolescent BMD in ASD, offer potential direction for research consideration in the future, and propose potential clinical identification and evaluation strategies in this population. We aim also to speak to ongoing challenges that remain in this endeavor.
Current Status in the Research Literature
It has been established across several cohorts of participants that pediatric, adolescent, and young adult males with ASD have lower BMD than non-ASD peers (Table 1) [15,16,17,18,19,20]. This represents consistent data across multiple research study cohorts and sites.
The 2008 work of Hediger and colleagues established decreased bone cortical thickness in males 4–8 years of age with ASD versus normative values. In this cohort, bone cortical thickness reflected a slowing of appositional bone development in participants, with older males showing greater deviations from normative values than those 4–5 years of age [15]. The authors suggested that this concern could be a function of a number of issues, including decreased calcium and vitamin D intake potentially associated with adherence to gluten and casein free dietary protocols (GFCF), gastrointestinal disorders, limited physical activity, and decreased access to sunlight.
Research on a second cohort followed by Neumeyer and colleagues expanded the work in bone health in males with ASD, finding that 18 males aged 8–14 years old had significantly lower BMD z-scores than 19 age-matched non-ASD peers [16]. The authors also documented decreased dietary intake of vitamin D and lower levels of physical activity. This work supported the earlier conclusions that decreased dietary intake of vitamin D3, decreased endogenous vitamin D synthesis, and reduced activity levels could contribute to decreased BMD in males with ASD.
A 4-year follow-up study on this population evaluating bone accrual rates concluded decreases in bone mineralization occur early in life for those with ASD, with no difference in pubertal bone accrual for ASD and non-ASD participants [21]. Additional findings included significantly lower physical activity levels for those with ASD, but no significant difference in serum 25(OH) vitamin D between ASD and non-ASD participants. Further published work on this cohort [22] concluded that ASD participants consumed less animal protein, calcium, and phosphorus than non-ASD participants, and these values were positively associated with BMD results.
Neumeyer and colleagues also evaluated bone microarchitecture in participants from this cohort, concluding that males with ASD have lower cortical and trabecular thickness at the ultradistal radius and lower strength estimates both the ultradistal radius and the distal tibia in 16 ASD and 18 non-ASD peers [19]. Findings in this population indicated that decreased physical activity levels contributed significantly to this difference [19]. Through this contribution to the literature, the authors also suggested the influence of insulin-like growth factor (IGF-1) was protective of bone accrual in puberty and adolescence, with early decreased bone mass the likely primary factor in overall decreased BMD in those participants with ASD.
It is of note that these papers [19, 21, 22] evaluated 25 males with ASD and 24 age-matched non-ASD peers, including 14 males with ASD and 13 non-ASD peers included in the original 2012 paper. An additional 13 participants (6 ASD, 7 non-ASD) were also included at the follow up time point in 2015.
A third cohort retrospectively assessed BMD status in adolescents and young adults with and without ASD. In this study, Ehklaspour and colleagues included evaluation of a case-control cohort offering data on an older population of 9 individuals (8 male/1 female) with ASD and 9 non-ASD peers (8 male/1 female) aged 14–21 years [17]. The study identified on chart review participants with DXA assessment, finding adolescents and young adults with ASD have lower BMD z-scores than non-ASD peers. The study did not evaluate dietary intake, nutritional status, and physical activity of participants and the majority (8 of 9) of ASD participants were on medications known to impact bone density.
Barnhill and colleagues provided BMD data in a cohort of 40 males aged 4–8 years with ASD and 40 age-matched non-ASD participants. BMD was significantly lower in boys with ASD as opposed to peers [18]. Contrary to prior findings, in this study, dietary intake of vitamin D as well as serum vitamin 25(OH) D was significantly higher in males with ASD than that of non-ASD peers. This finding was likely a function of professional care and appropriate supplementation of both calcium and vitamin D for many participants with ASD included in the study. When comparing those participants with ASD who were following a dietary protocol limiting casein to those who were on unrestricted diets indicated no difference in BMD status in this population. This finding suggests that vitamin D and calcium intake in children on a specialized diet is not a contributing factor to decreased BMD in this population.
In summary, this body of work suggests that prepubescent males with ASD have decreased bone accrual when compared to non-ASD peers, though bone accrual in puberty is consistent in both ASD and non-ASD populations [21]. Additionally, adolescents with ASD have weaker bones than age-matched non-ASD peers [19]. Finally, research also suggests that individuals with ASD have a higher risk of bone fracture than non-ASD individuals in both pediatric and adult populations [23, 24]. Taken together, these findings are concerning as they establish reduced BMD in pediatric males which tracks through adolescence and adulthood.
Additional original research in this body of literature includes work from Roke et al. evaluating the use of antipsychotic medications in males with ASD aged 10–20 years [25]. The authors conclude that BMD in the study population was similar for participants with and without antipsychotic treatment. An important finding here was decreased volumetric BMD for those experiencing hyperprolactinemia with antipsychotic treatment versus those without hyperprolactinemia. This result suggests that antipsychotic-induced hyperprolactinemia may be an influence on bone health in this population.
An evaluation of bone health in 21 male and 6 female ASD participants aged 10–18 years indicated that those with ASD coupled with low body mass index, low caloric, and low calcium intake were at risk for low BMD [20]. Serum vitamin D (OH)25 was insufficient in more than 50% of participants. The findings also indicated a high screen time to physical activity ratio, supporting the influence of exercise upon BMD in this population.
Finally, a 2012 study assessed the impact of weight-bearing exercise and calcium supplementation on BMD in 60 males with ASD aged 8–10 years old [26]. Study participants were randomly assigned to research groups with or without calcium supplementation and then also those with or without an exercise regime, resulting in possible enrollment in 1 of 4 cohorts. Results of this work indicated that weight-bearing exercise and calcium supplementation had a synergistic effect on bone health, with a 22.68% greater increase in BMD than in the control population.
Potential Factors Influencing BMD in Individuals with ASD
Multiple factors are known to play a role in BMD and bone health. A number of specific variables have been identified to influence BMD in children with ASD, including physical activity levels [21], gastrointestinal disorders [27], medications [28], food selectivity and restrictive eating behavior [29], and dietary intake and nutritional status, with particular focus on calcium and vitamin D [30, 31].
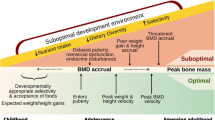
Bone accrual and bone mineral status in childhood, puberty, and adolescence plays a critical role in long-term bone health. Peak bone mass is a determinant of future bone health as 90% of peak bone mass is gained in the first 20 years of life, with 40% of lifetime bone mass accrued in puberty [32]. Low BMD affects 1 in 4 females and 1 in 20 males over the age of 65 [33] presenting a significant health challenge as bone tissue is altered with age and the risk of bone fractures increases over time.
Physical Activity and Exercise
Exercise and physical activity are established important determinants of BMD. Exercise is associated with increases in skeletal mass in children, and physical activity is particularly important in the pediatric and prepubertal population, as weight-bearing activity is noted to make a significant difference in bone accrual in this population [34,35,36]. Weight-bearing activity leads to an increase in overall bone formation rate through the stimulation of osteoblasts in the tissue [37, 38]. Decreased physical activity (including weight bearing activity) is established for pediatric males with ASD vs non-ASD peers in multiple research publications [21]. This decreased activity is a risk factor for compromised BMD in those with ASD [16, 19, 20, 22, 39]. Finally, lean muscle mass is positively correlated with BMD in the pediatric population; thus, children with greater muscle mass have greater BMD [40]. Decreased muscle mass associated with decreased physical activity in children with ASD is therefore a concern.
Hypotonia
With regard to decreased muscle mass and tone, children with ASD can also experience comorbid hypotonia [41], a disorder defined by low or abnormal muscle tone [42]. Mechanical loading of the skeleton is a key factor in bone mineral accrual in peripubertal and adolescent children [43]. Many children with ASD present with idiopathic toe-walking in which they walk on the balls of their feet and their heels do not touch the ground [44]. Idiopathic toe-walking is a diagnosis of exclusion, and while there are several potential comorbid diagnoses which could lead to toe-walking, in the majority of those with ASD, this is likely a function of decreased core and lower body tone or sensory processing disorder [41, 45, 46]. This hypotonia contributes to this decreased mechanical loading of muscle/bone interaction, leading to diminished overall bone health [47, 48].
Gastrointestinal Concerns
Gastrointestinal (GI) symptoms have been very well documented in the literature for children with ASD [49]. These can include constipation, diarrhea, gastroesophageal reflux disease (GERD), bloating, flatulence, and pain [50], but noted symptoms vary widely across multiple studies [51]. Symptoms can be difficult to recognize in children with ASD and often go undiagnosed and untreated [52]. One recent BMD study evaluated GI symptoms for all participants through a standardized questionnaire across 5 separate domains. Males with ASD reported significantly greater GI concerns across all domains [18].
Individuals with ASD are also noted to be at greater risk for comorbid inflammatory bowel disease (IBD) [53, 54]. It has been established in the literature that pediatric patients with IBD have decreased BMD [55]. The GI tract is responsible for digestion and absorption of key bone nutrients including calcium, vitamin D, and magnesium, and phosphorus [56]. If the GI tract is compromised in some fashion, there is ongoing concern for digestion, absorption, and assimilation of key bone building nutrients. Of note, several dietary micronutrient deficiencies reported for those with IBD, such as vitamins B1, B6, B12, D and iron are also found as serum deficiencies in studies of those with ASD [18, 57].
A body of literature now points to the role of the microbiota in bone health and mineral metabolism. A number of studies have identified changes to the gut microbiome in children with ASD (reviewed by [58] leading to an imbalance in intestinal flora [59, 60]. This imbalance can be addressed through the use of both prebiotic and probiotic foods and nutritional supplementation [61,62,63,64,65,66].
Of note, prebiotics were first fully described in the literature in 1995 as non-digestible food ingredients that beneficially affect the host by selectively stimulating the growth and/or activity of one or a limited number of bacteria in the colon, thus improving host health [67]. As knowledge in this area expanded, the definition has been widened to “a non-digestible compound that, through its metabolization by microorganisms in the gut, modulates composition and/or activity of the gut microbiota, thus conferring a beneficial physiologic effect on the host” [68]. A growing body of research has established that prebiotics are essential for improving the digestion and absorption of calcium and other minerals and enhancing skeletal health [69, 70]. It has also been suggested that the use of prebiotics to maximize mineral accretion and facilitate bone accrual in childhood, puberty, and adolescence will contribute to greater bone mass later in life [71].
Hormones: Insulin-Like Growth Factor-1 and Serotonin
There are many factors that regulate bone growth and turnover including hormones, neurotransmitters, growth factors, and cytokines [72]. Disruption of this process and the resulting imbalance between bone resorption and formation can lead to osteoporosis [73].
Insulin-Like Growth Factor-1 (IGF-1)
Important determinants of pubertal skeletal development include rising levels of growth hormone, IGF-1, and the gonadal steroids [74]. IGF-1 crosses the blood brain barrier where it stimulates DNA synthesis, cell production, and neurite outgrowth, and enhances secretion of several neurotransmitters [75]. Low levels of serum IGF-1 in both newborns and young children point to a disruption of the normal neurobiological mechanisms in this period, and are associated with an increased risk for ASD [76]. Both animal and human cell culture studies suggest a beneficial effect of IGF-1 on synaptic development by promoting neuronal cell survival, synaptic maturation, and synaptic plasticity [77, 78]. In recent years, IGF-1 has been explored as a potential treatment for the core symptoms of ASD. One pilot study investigating the effects of IGF-1 on behavior in children with Phelan-McDermid syndrome (PMS), a monogenic disorder closely associated with ASD, reported improvements in social impairment and restrictive behaviors establishing preliminary evidence of benefit of IGF-1 on ASD symptomatology [79]. However, the precise mechanisms by which IGF-1 exerts its effects on the CNS in ASD remain an active area of study.
Serotonin
Serotonin, a neurotransmitter that is known to influence the balance between bone formation and resorption [80], is frequently elevated in ASD [81]. Selective serotonin reuptake inhibitors (SSRIs) are a class of antidepressants that are sometimes given to reduce anxiety or obsessive-compulsive behaviors in children with ASD, although there is limited evidence as to their effectiveness [82]. Clinical studies have reported an association of SSRIs with increase in fracture and decrease in bone mineral density (reviewed by [80] raising concerns regarding the effect of blocking serotonin reuptake on bone metabolism.
Medication
Use of anticonvulsants, such as diphenylhydantoin, phenobarbital, topiramate carbamazepine, and valproic acid, is reported to reduce bone density [83]. Furthermore, lower bone turnover has been reported in those receiving lithium [84]. Stimulant medications, frequently used to treat attention-deficit/hyperactivity disorder and ASD, increase sympathetic tone and may affect bone remodeling [85]. Additionally, antidepressants which are used to address anxiety and obsessive-compulsive behaviors are suggested to affect BMD, though the research is mixed [86, 87].
Feeding Behavior
Research indicates that children with ASD and GI concerns are at a higher risk of developing problem feeding behaviors than children with ASD who do not have these symptoms [88,89,90], although a clear understanding of etiology for children with ASD has yet to be clearly established [91]. Children with ASD often have restrictive eating habits, such as picky or problem eating and they are more likely to have food selectivity and feeding issues resulting in challenging behaviors surrounding food intake than their typically developing peers [10, 12, 92]. Those with ASD are 5 times as likely to experience feeding problems than non-ASD peers [93]. This food selectivity, defined as consumption of an abnormally limited variety of food, leaves children with ASD at elevated risk for altered nutritional status and growth [94,95,96,97,98,99], and is likely to have detrimental effects on bone health.
Diet and Nutritional Status
Various micronutrient deficiencies for those with ASD have been identified in the research literature with conflicting results [93, 100]. Several studies have shown that children with ASD are more likely to have inadequate intake of protein, vitamin D, pantothenic acid, vitamin B6, folic acid, vitamin K, and calcium compared to children without ASD, but that data is not always consistent across the literature [29, 97, 101,102,103]. Of particular interest in bone health are calcium, magnesium, phosphorus, and vitamin D.
One consistent finding across multiple publications is a deficiency in both calcium and vitamin D intake [15, 18, 22, 96, 102,103,104,105]. The research literature establishes that lower calcium and vitamin D intake affects bone accrual in the pediatric population [106]. Further, decreased vitamin D intake is one of the most consistent findings in the literature where not only a large majority of ASD participants had inadequate intake of vitamin D, but non-ASD participants also presented with inadequate intake as well [12, 18, 29, 107].
With regard to calcium, more recent studies examining dietary status in ASD demonstrated no significant differences in calcium intake when comparing children who were on a well-supported gluten free and casein free (GFCF) diet versus those that did not report any dietary intervention [18, 108, 109]. Therefore, dietary intervention in ASD, such as a GFCF diet, does not necessarily result in deficiencies in calcium and vitamin D [105, 110] or decreased bone density, as long as it is implemented and well supported by a clinician [18]. For example, a child on a nutrient-dense, casein-free diet may receive more than adequate levels of calcium and vitamin D compared to a child receiving the standard American diet [96]. It is also worth noting here that children on a supported GFCF diet are more likely to be taking a vitamin D supplement [111] and meet their vitamin D intake requirements [112].
The most recent work on the Neumeyer cohort [22] suggests that higher calcium, phosphorus, and animal protein intake is positively associated with greater BMD. Further, calcium and phosphorus intake were both consistent predictors of all BMD measures in those with ASD.
Sleep Status
Sleep disruption is correlated with gastrointestinal symptoms, anxiety, sensory sensitivity, and ASD severity per a 2013 evaluation of Autism Treatment Network participants [113]. Further disrupted sleep in children with ASD is associated with daytime behavioral dysregulation, and night waking has the strongest correlation with daytime behavioral concerns in this population [8]. While previous clinical studies have failed to reach a consensus on the association between sleep duration and BMD due to the many variables in conducting such studies, sleep disruption in a rat model was found to markedly affect bone mass and bone metabolism by lowering BMD, deteriorating bone microarchitecture, and decreasing bone formation and resorption markers [114].
Limitations of Current Data
There are several limitations of the current bone density literature in children with ASD that should be considered. These include the study design (some studies were retrospective or cross-sectional and did not include a control group; the study size was likely underpowered in some datasets; several papers did not include a control group; and the age of cohorts included children as young as 4, as well as prepubescent and pubescent participants, which represent quite distinct periods of bone development). Some studies did not confirm an ASD diagnosis and relied on parental report. Similarly, nutrient intake and use of medication and supplements could not always be verified. This is particularly important in studies looking at the effects of diet on bone health as a GFCF diet was not shown to be deleterious to bone density when it was well-supported with the appropriate clinical oversight. Finally, several of the papers included in this review are based on the same cohorts, or additional subjects were added to the original cohorts in subsequent papers to expand the analyses, which complicated the interpretation of the data. Larger prospective studies would be very helpful in assessing the many variables that can impact bone metabolism in children with ASD.
Direction on Future Research and Current Clinical Assessment
The literature is clear that physical activity and weight-bearing exercise specifically are positively correlated with bone health and BMD. Researchers have demonstrated that those with ASD are less active than age-matched non-ASD peers. Future research can be directed toward investigation of the impact of hypotonia early in life on BMD and establishing a better understanding of appropriate exercise opportunities for those with ASD.
Research has recently demonstrated the safety and efficacy of IGF-1 treatment in children with Phelan-McDermid syndrome, particularly in the treatment of the core symptoms of ASD. Therefore, these studies should be expanded to include the idiopathic autism population. Further, there is no work published to-date on the impact of serotonin levels and bone health in this population. Finally, given the presence of significant sleep disturbance in children and adolescents with autism and the known impact of sleep on bone health, research evaluating sleep and bone health status in this population may be warranted.
It is known that diets higher in calcium, vitamin D, and amino acids appear important in overall bone health of those with ASD, and that these nutrients coupled with physical activity appear to have a synergistic effect on bone health. For primary care practitioners, this translates to early assessment for dietary intake and nutritional status. This would include the recommendation of a comprehensive anthropometric, dietetic, and physical assessment for all children with ASD in a primary care setting as a baseline measurement to evaluate the need for referral for more specialized evaluation and potential intervention on a case-by-case basis. Additionally, it is recommended that PCPs follow current guidelines for serum vitamin D assessment in at-risk populations [30] for all patients with ASD.
Abbreviations
- ASD:
-
autism spectrum disorder
- BMC:
-
bone mineral content
- BMD:
-
BMD
- DEXA:
-
dual-energy X-ray absorptiometry
- GFCF:
-
gluten free casein free
- IBD:
-
inflammatory bowel disease
- PCP:
-
primary care practitioner
References
American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. Arlington: American Psychiatric Association; 2013.
Centers for Disease Control. Prevalence of autism spectrum disorder among children aged 8 years — Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2014. Autism and Developmental Disabilities Monitoring Network Surveillance Year 2010 Principal Investigators 2018.
Lyall K. The changing epidemiology of autism spectrum disorders. Annu Rev Public Health. 2017:81–102.
Schieve LA, Gonzalez V, Boulet SL, Visser SN, Rice CE, Van Naarden BK, et al. Concurrent medical conditions and health care use and needs among children with learning and behavioral developmental disabilities, National Health Interview Survey, 2006-2010. Res Dev Disabil. 2012;33(2):467–76. https://doi.org/10.1016/j.ridd.2011.10.008.
Bauman ML. Medical comorbidities in autism: challenges to diagnosis and treatment. Neurotherapeutics. 2010:320–7.
Tye C. Characterizing the interplay between autism spectrum disorder and comorbid medical conditions: an integrative review. Front Psychiatry. 2019.
Cohen H. The relationship between sleep and behavior in autism spectrum disorder (ASD): a review. J Neurodev Disord. 2014.
Mazurek MO. Sleep and behavioral problems in children with autism spectrum disorder. J Autism Dev Disord. 2016:1906–15.
Avni E, Ben-Itzchak E, Zachor DA. The presence of comorbid ADHD and anxiety symptoms in autism spectrum disorder: clinical presentation and predictors. Front Psychiatry. 2018;9:717. https://doi.org/10.3389/fpsyt.2018.00717.
Curtin C, Hubbard K, Anderson SE, Mick E, Must A, Bandini LG. Food selectivity, mealtime behavior problems, spousal stress, and family food choices in children with and without autism spectrum disorder. J Autism Dev Disord. 2015;45(10):3308–15. https://doi.org/10.1007/s10803-015-2490-x.
Wallace GL. Autism spectrum disorder and food neophobia: clinical and subclinical links. Am J Clin Nutr. 2018:701–7.
Mari-Bauset S, Zazpe I, Mari-Sanchis A, Llopis-Gonzalez A, Morales-Suarez-Varela M. Food selectivity in autism spectrum disorders: a systematic review. J Child Neurol. 2014;29(11):1554–61. https://doi.org/10.1177/0883073813498821.
Muskens JB. Medical comorbidities in children and adolescents with autism spectrum disorders and attention deficit hyperactivity disorders: a systematic review. Eur Child Adolesc Psychiatry. 2017:1093–103.
Aldinger KA, Lane CJ, Veenstra-VanderWeele J, Levitt P. Patterns of risk for multiple co-occurring medical conditions replicate across distinct cohorts of children with autism Spectrum disorder. Autism Res. 2015;8(6):771–81. https://doi.org/10.1002/aur.1492.
Hediger ML, England LJ, Molloy CA, Yu KF, Manning-Courtney P, Mills JL. Reduced bone cortical thickness in boys with autism or autism spectrum disorder. J Autism Dev Disord. 2008;38(5):848–56. https://doi.org/10.1007/s10803-007-0453-6.
Neumeyer AM, Gates A, Ferrone C, Lee H, Misra M. Bone density in peripubertal boys with autism spectrum disorders. J Autism Dev Disord. 2013;43(7):1623–9. https://doi.org/10.1007/s10803-012-1709-3.
Ekhlaspour L, Baskaran C, Campoverde KJ, Sokoloff NC, Neumeyer AM, Misra M. Bone density in adolescents and young adults with autism spectrum disorders. J Autism Dev Disord. 2016;46(11):3387–91. https://doi.org/10.1007/s10803-016-2871-9.
Barnhill K, Ramirez L, Gutierrez A, Richardson W, Marti CN, Potts A, et al. Bone mineral density in boys diagnosed with autism spectrum disorder: a case-control study. J Autism Dev Disord. 2017;47(11):3608–19. https://doi.org/10.1007/s10803-017-3277-z.
Neumeyer AM, Cano Sokoloff N, McDonnell E, Macklin EA, McDougle CJ, Misra M. Bone microarchitecture in adolescent boys with autism spectrum disorder. Bone. 2017;97:139–46. https://doi.org/10.1016/j.bone.2017.01.009.
Soden SE. Nutrition, physical activity, and bone mineral density in youth with autistic spectrum disorders. J Dev Behav Pediatr. 2012:618–24.
Neumeyer AM, Cano Sokoloff N, McDonnell E, Macklin EA, McDougle CJ, Misra M. Bone accrual in males with autism spectrum disorder. J Pediatr. 2017;181:195–201 e6. https://doi.org/10.1016/j.jpeds.2016.10.080.
Neumeyer AM. Nutrition and bone density in boys with autism spectrum disorder. J Acad Nutr Diet. 2018:865–77.
Neumeyer AM, O'Rourke JA, Massa A, Lee H, Lawson EA, McDougle CJ, et al. Brief report: bone fractures in children and adults with autism spectrum disorders. J Autism Dev Disord. 2015;45(3):881–7. https://doi.org/10.1007/s10803-014-2228-1.
Furlano RI. Bone fractures in children with autistic spectrum disorder. J Dev Behav Pediatr. 2014:353–9.
Roke Y, van Harten PN, Buitelaar JK, Tenback DE, Quekel LG, de Rijke YB, et al. Bone mineral density in male adolescents with autism spectrum disorders and disruptive behavior disorder with or without antipsychotic treatment. Eur J Endocrinol. 2012;167(6):855–63. https://doi.org/10.1530/EJE-12-0521.
Goodarzi M. Bone mineral density accrual in students with autism spectrum disorders: effects of calcium intake and physical training. Res Autism Spectr Disord. 2012:690–5.
Thangarajah D, Hyde MJ, Konteti VK, Santhakumaran S, Frost G, Fell JM. Systematic review: body composition in children with inflammatory bowel disease. Aliment Pharmacol Ther. 2015;42(2):142–57. https://doi.org/10.1111/apt.13218.
Calarge CA, Schlechte JA, Burns TL, Zemel BS. The effect of psychostimulants on skeletal health in boys co-treated with risperidone. J Pediatr. 2015;166(6):1449–54 e1. https://doi.org/10.1016/j.jpeds.2015.03.005.
Hyman SL, Stewart PA, Schmidt B, Cain U, Lemcke N, Foley JT, et al. Nutrient intake from food in children with autism. Pediatrics. 2012;130(Suppl 2):S145–53. https://doi.org/10.1542/peds.2012-0900L.
Golden NH, Abrams SA. Optimizing bone health in children and adolescents. Pediatrics. 2014;134(4):e1229–43. https://doi.org/10.1542/peds.2014-2173.
Palacios C. The role of nutrients in bone health, from A to Z. Crit Rev Food Sci Nutr. 2006;46:621–8.
Bachrach LK. Acquisition of optimal bone mass in childhood and adolescence. Trends Endocrinol Metab. 2001:22–8.
Looker A. Percentage of adults aged 65 and over with osteoporosis or low bone mass at the femur neck or lumbar spine. United States, 2005–2010, CDC/NCHS, National Health and Nutrition Survey, Hyattsville, MD: National Center for Health Statistics. 2015.
Siemenda CW. Role of physical activity in the development of skeletal mass in children. J Bone Miner Res. 1991:1227–31.
Tan VP. Influence of physical activity on bone strength in children and adolescents: a systematic review and narrative synthesis. J Bone Miner Res. 2014:2161–81.
Behringer M. Effects of weight-bearing activities on bone mineral content and density in children and adolescents: a meta-analysis. J Bone Miner Res. 2014:467–78.
Willems HME. Diet and exercise: a match made in bone. Curr Osteoporos Rep. 2017:555–63.
Bonewald LF. Osteocytes, mechanosensing, and Wnt signaling. Bone. 2008:606–15.
Tyler K. Physical activity and physical fitness of school-aged children and youth with autism spectrum disorders. Autism Res Treat. 2014;312163.
Dorsey KB. Greater lean tissue and skeletal muscle mass are associated with higher bone mineral content in children. Nutr Metab. 2010:41–51.
Ming X, Brimacombe M, Wagner GC. Prevalence of motor impairment in autism spectrum disorders. Brain and Development. 2007;29(9):565–70. https://doi.org/10.1016/j.braindev.2007.03.002.
Provost B. A comparison of motor delays in young children: autism spectrum disorder, developmental delay, and developmental concerns. J Autism Dev Disord. 2007:321–8.
Dowthwaite JN. Site-specific advantages in skeletal geometry and strength at the proximal femur and forearm in young female gymnasts. Bone. 2012:1173–83.
Valagussa G, Trentin L, Signori A, Grossi E. Toe walking assessment in autism spectrum disorder subjects: a systematic review. Autism Res. 2018;11(10):1404–15. https://doi.org/10.1002/aur.2009.
Shulman LH, Sala DA, Chu ML, McCaul PR, Sandler BJ. Developmental implications of idiopathic toe walking. J Pediatr. 1997;130(4):541–6. https://doi.org/10.1016/s0022-3476(97)70236-1.
Stricker SJ, Angulo JC. Idiopathic toe walking: a comparison of treatment methods. J Pediatr Orthop. 1998;18(3):289–93.
Delgado-Calle J. Osteocytes and skeletal pathophysiology. Curr Mol Biol Rep. 2015:157–67.
Robling AG. Mechanical signaling for bone modeling and remodeling. Crit Rev Eukaryot Gene Expr. 2009:319–38.
McElhanon BO. Gastrointestinal symptoms in autism spectrum disorder: a meta-analysis. Pediatrics. 2014:2304–8.
Buie T, Campbell DB, Fuchs GJ 3rd, Furuta GT, Levy J, Vandewater J, et al. Evaluation, diagnosis, and treatment of gastrointestinal disorders in individuals with ASDs: a consensus report. Pediatrics. 2010;125(Suppl 1):S1–18.
Holingue C, Newill C, Lee LC, Pasricha PJ, Daniele Fallin M. Gastrointestinal symptoms in autism spectrum disorder: a review of the literature on ascertainment and prevalence. Autism Res. 2018;11(1):24–36. https://doi.org/10.1002/aur.1854.
Kushak RI, Buie TM, Murray KF, Newburg DS, Chen C, Nestoridi E, et al. Evaluation of intestinal function in children with autism and gastrointestinal symptoms. J Pediatr Gastroenterol Nutr. 2016;62(5):687–91. https://doi.org/10.1097/MPG.0000000000001174.
Doshi-Velez F. Prevalence of inflammatory bowel disease among patients with autism spectrum disorders. Inflamm Bowel Dis. 2015:2281–8.
Lee M. Association of Autism Spectrum Disorders and Inflammatory Bowel Disease. J Autism Dev Disord. 2018:1523–9.
Lopes LH, Sdepanian VL, Szejnfeld VL, de Morais MB, Fagundes-Neto U. Risk factors for low bone mineral density in children and adolescents with inflammatory bowel disease. Dig Dis Sci. 2008;53(10):2746–53. https://doi.org/10.1007/s10620-008-0223-0.
Christakos S, Veldurthy V, Patel N, Wei R. Intestinal regulation of calcium: vitamin D and bone physiology. Adv Exp Med Biol. 2017;1033:3–13.
Weisshof R, Chermesh I. Micronutrient deficiencies in inflammatory bowel disease. Curr Opin Clin Nutr Metab Care. 2015;18(6):576–81. https://doi.org/10.1097/MCO.0000000000000226.
Berding K, Donovan SM. Microbiome and nutrition in autism spectrum disorder: current knowledge and research needs. Nutr Rev. 2016;74(12):723–36. https://doi.org/10.1093/nutrit/nuw048.
De Angelis M, Piccolo M, Vannini L, Siragusa S, De Giacomo A, Serrazzanetti DI, et al. Fecal microbiota and metabolome of children with autism and pervasive developmental disorder not otherwise specified. PLoS One. 2013;8(10):e76993. https://doi.org/10.1371/journal.pone.0076993.
Kang DW, Park JG, Ilhan ZE, Wallstrom G, Labaer J, Adams JB, et al. Reduced incidence of Prevotella and other fermenters in intestinal microflora of autistic children. PLoS One. 2013;8(7):e68322. https://doi.org/10.1371/journal.pone.0068322.
Grimaldi R, Gibson GR, Vulevic J, Giallourou N, Castro-Mejia JL, Hansen LH, et al. A prebiotic intervention study in children with autism spectrum disorders (ASDs). Microbiome. 2018;6(1):133. https://doi.org/10.1186/s40168-018-0523-3.
Doenyas C. Dietary interventions for autism spectrum disorder: new perspectives from the gut-brain axis. Physiol Behav. 2018;194:577–82. https://doi.org/10.1016/j.physbeh.2018.07.014.
Doenyas C. Gut microbiota, inflammation, and probiotics on neural development in autism spectrum disorder. Neuroscience. 2018;374:271–86. https://doi.org/10.1016/j.neuroscience.2018.01.060.
Sanctuary MR, Kain JN, Angkustsiri K, German JB. Dietary considerations in autism spectrum disorders: the potential role of protein digestion and microbial putrefaction in the gut-brain Axis. Front Nutr. 2018;5:40. https://doi.org/10.3389/fnut.2018.00040.
Sanctuary MR, Kain JN, Chen SY, Kalanetra K, Lemay DG, Rose DR, et al. Pilot study of probiotic/colostrum supplementation on gut function in children with autism and gastrointestinal symptoms. PLoS One. 2019;14(1):e0210064. https://doi.org/10.1371/journal.pone.0210064.
Patusco R, Ziegler J. Role of probiotics in managing gastrointestinal dysfunction in children with autism spectrum disorder: an update for practitioners. Adv Nutr. 2018;9(5):637–50. https://doi.org/10.1093/advances/nmy031.
Gibson GR, Roberfroid MB. Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J Nutr. 1995;125(6):1401–12. https://doi.org/10.1093/jn/125.6.1401.
Bindels LB, Delzenne NM, Cani PD, Walter J. Towards a more comprehensive concept for prebiotics. Nat Rev Gastroenterol Hepatol. 2015;12(5):303–10. https://doi.org/10.1038/nrgastro.2015.47.
Weaver CM. Diet, gut microbiome, and bone health. Curr Osteoporos Rep. 2015;13(2):125–30. https://doi.org/10.1007/s11914-015-0257-0.
McCabe L, Britton RA, Parameswaran N. Prebiotic and probiotic regulation of bone health: role of the intestine and its microbiome. Curr Osteoporos Rep. 2015;13:363–71. https://doi.org/10.1007/s11914-015-0292-x.
Whisner CM, Weaver CM. Prebiotics and bone. Adv Exp Med Biol. 2017;1033:201–24. https://doi.org/10.1007/978-3-319-66653-2_10.
Lavoie B, Lian JB, Mawe GM. Regulation of bone metabolism by serotonin. Adv Exp Med Biol. 2017;1033:35–46. https://doi.org/10.1007/978-3-319-66653-2_3.
Kenkre JS, Bassett J. The bone remodelling cycle. Ann Clin Biochem. 2018;55(3):308–27. https://doi.org/10.1177/0004563218759371.
Theintz G, Buchs B, Rizzoli R, Slosman D, Clavien H, Sizonenko PC, et al. Longitudinal monitoring of bone mass accumulation in healthy adolescents: evidence for a marked reduction after 16 years of age at the levels of lumbar spine and femoral neck in female subjects. J Clin Endocrinol Metab. 1992;75(4):1060–5. https://doi.org/10.1210/jcem.75.4.1400871.
Riikonen R. Insulin-like growth factors in the pathogenesis of neurological diseases in children. Int J Mol Sci. 2017;18(10). https://doi.org/10.3390/ijms18102056.
Vanhala R, Turpeinen U, Riikonen R. Low levels of insulin-like growth factor-I in cerebrospinal fluid in children with autism. Dev Med Child Neurol. 2001;43(9):614–6. https://doi.org/10.1017/s0012162201001116.
Marchetto MC, Carromeu C, Acab A, Yu D, Yeo GW, Mu Y, et al. A model for neural development and treatment of Rett syndrome using human induced pluripotent stem cells. Cell. 2010;143(4):527–39. https://doi.org/10.1016/j.cell.2010.10.016.
Bozdagi O, Tavassoli T, Buxbaum JD. Insulin-like growth factor-1 rescues synaptic and motor deficits in a mouse model of autism and developmental delay. Mol Autism. 2013;4(1):9. https://doi.org/10.1186/2040-2392-4-9.
Kolevzon A, Bush L, Wang AT, Halpern D, Frank Y, Grodberg D, et al. A pilot controlled trial of insulin-like growth factor-1 in children with Phelan-McDermid syndrome. Mol Autism. 2014;5(1):54. https://doi.org/10.1186/2040-2392-5-54.
Wadhwa R, Kumar M, Talegaonkar S, Vohora D. Serotonin reuptake inhibitors and bone health: a review of clinical studies and plausible mechanisms. Osteoporos Sarcopenia. 2017;3(2):75–81. https://doi.org/10.1016/j.afos.2017.05.002.
Muller CL, Anacker AMJ, Veenstra-VanderWeele J. The serotonin system in autism spectrum disorder: from biomarker to animal models. Neuroscience. 2016;321:24–41. https://doi.org/10.1016/j.neuroscience.2015.11.010.
Williams K, Brignell A, Randall M, Silove N, Hazell P. Selective serotonin reuptake inhibitors (SSRIs) for autism spectrum disorders (ASD). Cochrane Database Syst Rev. 2013;8:CD004677. https://doi.org/10.1002/14651858.CD004677.pub3.
Lee RH, Lyles KW, Colon-Emeric C. A review of the effect of anticonvulsant medications on bone mineral density and fracture risk. Am J Geriatr Pharmacother. 2010;8(1):34–46. https://doi.org/10.1016/j.amjopharm.2010.02.003.
Zamani A, Omrani GR, Nasab MM. Lithium’s effect on bone mineral density. Bone. 2009;44(2):331–4. https://doi.org/10.1016/j.bone.2008.10.001.
Feuer AJ, Thai A, Demmer RT, Vogiatzi M. Association of stimulant medication use with bone mass in children and adolescents with attention-deficit/hyperactivity disorder. JAMA Pediatr. 2016;170(12):e162804. https://doi.org/10.1001/jamapediatrics.2016.2804.
Rizzoli R, Cooper C, Reginster JY, Abrahamsen B, Adachi JD, Brandi ML, et al. Antidepressant medications and osteoporosis. Bone. 2012;51(3):606–13. https://doi.org/10.1016/j.bone.2012.05.018.
Schweiger JU, Schweiger U, Huppe M, Kahl KG, Greggersen W, Jauch-Chara K, et al. The use of antidepressive agents and bone mineral density in women: a meta-analysis. Int J Environ Res Public Health. 2018;15(7). https://doi.org/10.3390/ijerph15071373.
Emond A, Emmett P, Steer C, Golding J. Feeding symptoms, dietary patterns, and growth in young children with autism spectrum disorders. Pediatrics. 2010;126(2):e337–42. https://doi.org/10.1542/peds.2009-2391.
Chaidez V, Hansen RL, Hertz-Picciotto I. Gastrointestinal problems in children with autism, developmental delays or typical development. J Autism Dev Disord. 2014;44(5):1117–27. https://doi.org/10.1007/s10803-013-1973-x.
Erickson CA, Stigler KA, Corkins MR, Posey DJ, Fitzgerald JF, McDougle CJ. Gastrointestinal factors in autistic disorder: a critical review. J Autism Dev Disord. 2005;35(6):713–27. https://doi.org/10.1007/s10803-005-0019-4.
Vissoker RE, Latzer Y, Gal E. Eating and feeding problems and gastrointestinal dysfunction in autism spectrum disorders. Res Autism Spectr Disord. 2015;12:10–21.
Volkert VM, Vaz PC. Recent studies on feeding problems in children with autism. J Appl Behav Anal. 2010;43(1):155–9. https://doi.org/10.1901/jaba.2010.43-155.
Sharp WG, Berry RC, McCracken C, Nuhu NN, Marvel E, Saulnier CA, et al. Feeding problems and nutrient intake in children with autism spectrum disorders: a meta-analysis and comprehensive review of the literature. J Autism Dev Disord. 2013;43(9):2159–73. https://doi.org/10.1007/s10803-013-1771-5.
Barnhill K, Gutierrez A, Ghossainy M, Marediya Z, Marti CN, Hewitson L. Growth status of children with autism spectrum disorder: a case-control study. J Hum Nutr Diet. 2017;30(1):59–65. https://doi.org/10.1111/jhn.12396.
Evans EW, Must A, Anderson SE, Curtin C, Scampini R, Maslin M, et al. Dietary patterns and body mass index in children with autism and typically developing children. Res Autism Spectr Disord. 2012;6(1):399–405. https://doi.org/10.1016/j.rasd.2011.06.014.
Herndon AC, DiGuiseppi C, Johnson SL, Leiferman J, Reynolds A. Does nutritional intake differ between children with autism spectrum disorders and children with typical development? J Autism Dev Disord. 2009;39(2):212–22. https://doi.org/10.1007/s10803-008-0606-2.
Mari-Bauset S, Llopis-Gonzalez A, Zazpe-Garcia I, Mari-Sanchis A, Morales-Suarez-Varela M. Nutritional status of children with autism spectrum disorders (ASDs): a case-control study. J Autism Dev Disord. 2015;45(1):203–12. https://doi.org/10.1007/s10803-014-2205-8.
Dovey TM. Food neophobia and ‘picky/fussy’ eating in children: a review. Appetite. 2008:181–93.
Schwarz SM. Feeding disorders in children with developmental disabilities. Infants Young Child. 2003:317–30.
Mari-Bauset S, Llopis-González A, Zazpe I, Mari-Sanchís A, Morales-Suárez-Varela M. Anthropometric measures of Spanish children with autism spectrum disorder. Res Autism Spectr Disord. 2015;9:26–33.
Bandini LG, Anderson SE, Curtin C, Cermak S, Evans EW, Scampini R, et al. Food selectivity in children with autism spectrum disorders and typically developing children. J Pediatr. 2010;157(2):259–64. https://doi.org/10.1016/j.jpeds.2010.02.013.
Xia W, Zhou Y, Sun C, Wang J, Wu L. A preliminary study on nutritional status and intake in Chinese children with autism. Eur J Pediatr. 2010;169(10):1201–6. https://doi.org/10.1007/s00431-010-1203-x.
Zimmer MH, Hart LC, Manning-Courtney P, Murray DS, Bing NM, Summer S. Food variety as a predictor of nutritional status among children with autism. J Autism Dev Disord. 2012;42(4):549–56. https://doi.org/10.1007/s10803-011-1268-z.
Barnhill K. Dietary status and nutrient intake of children with autism spectrum disorder: a case-control study. Res Autism Spectr Disord. 2018:51–9.
Cornish E. A balanced approach towards healthy eating in autism. J Hum Nutr Diet. 1998;11:501–9.
Davies JH, Evans BA, Gregory JW. Bone mass acquisition in healthy children. Arch Dis Child. 2005;90(4):373–8. https://doi.org/10.1136/adc.2004.053553.
Attlee A, Kassem H, Hashim M, Obaid RS. Physical status and feeding behavior of children with autism. Indian J Pediatr. 2015;82(8):682–7. https://doi.org/10.1007/s12098-015-1696-4.
Adams SJ, Burton N, Cutress A, Adamson AJ, McColl E, O’Hare A, et al. Development of double blind gluten and casein free test foods for use in an autism dietary trial. J Hum Nutr Diet. 2008;21(4):374.
Johnson CR, Handen BL, Zimmer M, Sacco K, Turner K. Effects of a gluten free/casein free diet in young children with autism: a pilot study. J Dev Phys Disabil. 2011;23:213–25. https://doi.org/10.1007/s10882-010-9217-x.
Whiteley P, Shattock P, Knivsberg AM, Seim A, Reichelt KL, Todd L, et al. Gluten- and casein-free dietary intervention for autism spectrum conditions. Front Hum Neurosci. 2012;6:344. https://doi.org/10.3389/fnhum.2012.00344.
Srinivasan S, O'Rourke J, Bersche Golas S, Neumeyer A, Misra M. Calcium and vitamin D supplement prescribing practices among providers caring for children with autism spectrum disorders: are we addressing bone health? Autism Res Treat. 2016;2016:6763205. https://doi.org/10.1155/2016/6763205.
Stewart PA, Hyman SL, Schmidt BL, Macklin EA, Reynolds A, Johnson CR, et al. Dietary supplementation in children with autism spectrum disorders: common, insufficient, and excessive. J Acad Nutr Diet. 2015;115(8):1237–48. https://doi.org/10.1016/j.jand.2015.03.026.
Holloway JA. Correlates and risk markers for sleep disturbance in participants of the autism treatment network. J Autism Dev Disord. 2014:2830–43.
Xu X, Wang L, Chen L, Su T, Zhang Y, Wang T, et al. Effects of chronic sleep deprivation on bone mass and bone metabolism in rats. J Orthop Surg Res. 2016;11(1):87. https://doi.org/10.1186/s13018-016-0418-6.
Author information
Authors and Affiliations
Contributions
KB designed and organized this review. MD completed literature searches, the first draft of the manuscript was written by KB, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
This article does not contain any studies with human participants performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Barnhill, K.M., Devlin, M. & Hewitson, L. Bone Health and BMD Research in Pediatric and Adolescent Individuals with ASD: Current Data, Evaluation, and Next Steps. Clinic Rev Bone Miner Metab 17, 160–169 (2019). https://doi.org/10.1007/s12018-019-09268-w
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12018-019-09268-w