Abstract
]The microcirculation of bone and marrow is vital for bone development, maintenance, and repair. In addition to the well-known function of transporting oxygen, nutrients, systemic hormones, precursor cells, waste, etc., the bone vascular network plays a role in the mechanical induction of bone formation. In addition, arteries and marrow sinusoids are critical components of hematopoietic stem cell niches. This review discusses the various roles of the bone and marrow microcirculation in regard to (1) bone development, remodeling, and fracture repair; (2) the regulation of bone intramedullary pressure and interstitial fluid flow; and (3) the mobilization of mature blood cells into the peripheral circulation. Age-associated dysfunction of the microcirculation is discussed in relation to how it may disturb bone and marrow homeostasis, fracture repair, and organismal vitality. Finally, the review invites the reader to consider the efficacy of treatments designed to alleviate bone and marrow pathologies in the face of a compromised vascular network.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Highways and transit systems permit the passage of people and commerce, with lane closures, road closures, and road blocks slowing this transport. The vascular network is equivalent to the highways and transit systems in that it carries white blood cells, red blood cells, platelets, systemic hormones, nutrients, oxygen, carbon dioxide, waste products, precursor cells, etc. (i.e., goods and services) along its route. Cells and factors are carried to and from the peripheral circulation, originating in certain tissues and traveling to target tissues. As we age and/or develop disease, vascular function declines [1,2,3]. Age- and disease-related pathologies of bone blood vessels, in particular, include diminished endothelium-dependent vasodilation [4,5,6,7,8], vascular rarefaction [9, 10], augmented vascular calcification [9], and arteriosclerosis/atherosclerosis [11,12,13].
In regard to the transit of good and services, declines in vasodilator function, vascular rarefaction and calcification, and arteriosclerosis/atherosclerosis of bone blood vessels are corollary to lane closures, road closures, and road blocks. All of these vascular ailments serve to reduce and/or impede the passage of blood and, in essence, diminish or obstruct the delivery of blood cells (i.e., white and red blood cells, platelets, and precursor cells), systemic hormones, nutrients, and oxygen, and attenuate the removal of tissue waste. In fact, diminished bone and marrow blood flow and perfusion are a common occurrence in old age and has been demonstrated in animal models and humans [4, 6, 14,15,16]. From this perspective, we can appreciate how bone vascular dysfunction contributes to bone and marrow pathology. Under this premise, we must further appreciate how bone vascular dysfunction may encumber efforts to treat bone and marrow ailments. In other words, medications and treatments designed to correct or assuage bone and marrow pathologies often rely upon the vascular system for its transport into the skeletal system. This begs the question as to how effective is the transport of goods and services (i.e., medications and treatments) if the highways and transit systems are in disrepair. This review will highlight three main facets as to why we should investigate the bone vascular system, how blood vessels change with advancing age, and how this impacts fracture repair and marrow function.
Bone Development and Repair Require a Vascular Supply
The interaction between osteoblasts, vascular endothelial cells, and other cells within the bone microenvironment modulates development and remodeling [17]. The theory that vascular endothelial cells and/or pericytes convert into pre-osteoblasts or osteoblasts suggests the direct involvement of blood vessels in bone formation [17]. During bone development, remodeling, and regeneration, the formation of new blood vessels (i.e., angiogenesis) is a key regulating factor [18, 19]. In fact, vascular ingrowth is necessary for both endochondral and intramembranous ossification [20]. The presence or absence of a pre-existing cartilage template and the site of bone development distinguishes the two types of ossification. During embryogenesis, intramembranous and endochondral ossification typically occur in the axial and appendicular skeleton, respectively, and form the flat and tubular bones, respectively [21]. Both, however, rely upon a vascular supply.
Intramembranous ossification occurs without a pre-existing cartilage template and commences with an avascular mesenchymal condensation [21]. While the role of blood vessels during this type of ossification is not frequently studied [18], the differentiation of pre-osteoblasts into secretory osteoblasts occurs concomitant with blood vessel invasion of the bone anlage [22]. In models of distraction osteogenesis, which transpires primarily via intramembranous mechanisms [17], bone formation occurs only at sites close to blood vessels [23]. In the distraction gap, bone formation occurred in close proximity to the recently developed and large (150–200 μm in diameter) vascular sinuses [17]. It has been postulated that vascular cells or cells close to blood vessels have osteogenic properties or secret osteogenic factors [24,25,26,27]. In elderly patients following vertical distraction of the mandible, instances of scarce vascularization were coupled with poor bone formation [18]. As anticipated, blood supply to the site of bone formation is augmented during distraction osteogenesis, as evidence of this measure via various techniques (e.g., vascular corrosion casts [28] and scintigraphic investigations [29, 30]). In addition, regional blood flow to bone-forming sites has been demonstrated to increase 10-fold above control conditions [17], remaining 3-fold higher vs. control up to 17 weeks post-corticotomy [31,32,33].
During endochondral ossification, bone replaces an existing calcified cartilage template [34], only after invasion by new capillaries [35]. The cartilage template is established by chondrocytes that eventually hypertrophy and secrete an extracellular matrix containing collagen X [36]. Hypertrophic chondrocytes release vascular endothelial cell growth factor (VEGF) to initiate blood vessel development and the newly established blood supply brings in nutrients [37] and presumably brings in osteogenic cells [18, 37]. Pre-osteoclasts and chondroclasts remove the extracellular matrix secreted by the hypertrophic chondrocytes [36, 37] and blood vessel invasion delivers osteoblasts to lay down the extracellular matrix of bone [36]. In concert with VEGF, osteoclasts and matrix metalloproteinase-9 work to ensure proper bone development [36]. Inactivation of VEGF during endochondral ossification in a murine model altered the vascular morphology within the growth plate, eliminating proper growth and invasion of the metaphyseal vascular supply [37]. Thus, vascular invasion of the cartilage template, as mediated by VEGF, serves as the linchpin between resorption of the template and formation of bone [37]. Further, it has been recently speculated that bone blood vessels serve as a guide for (1) the collagen template, (2) the replacement of the template with bone, and (3) bone morphogenesis [38]. For example, calcein labeling (i.e., bone formation) was observed in close proximity to blood vessels during endochondral ossification in developing mice, leading the authors to conclude that the blood vessels served as a template for mineral deposition [38]. In the same series of investigations, VEGF overexpression in osteoblasts altered the vascular pattern, increased the number of blood vessels, and caused vascular over-sprouting at the bone circumference [38]. Interestingly, enhanced bone formation was observed at the bone circumference, next to the blood vessels [38].
Recent investigations have shed more light on the interrelationship between bone blood vessels and bone cells. For example, co-invasion of pre-osteoblasts and blood vessels into the cartilage template during bone development and into the cartilaginous callus during fracture repair has been observed [39]. Further, angiogenesis related to the development of long bones in mice was associated with Notch signaling [40]. When Notch signaling was experimentally disrupted, vascular defects in morphology and growth occurred, as well as bone abnormalities (i.e., diminished osteogenesis and bone length, chondrocyte deficiencies, a decreased number of trabeculae, and reduced bone mass) [40]. Restoration of Noggin, an angiocrine factor secreted from endothelial cells and regulated by Notch, reversed the vascular defects and bone abnormalities [40]. In addition, marrow capillaries and sinusoids in genetically modified and aged mice were distinguished based upon their expression of CD31 and endomucin (Emcn) [41]. Based upon this categorization, endothelial cells associated with capillaries stained high for CD31 and Emcn, while those in sinusoids stained low for CD31 and Emcn [41]. Thus, endothelial cells in bone were termed type H for the CD31hi/Emcnhi subpopulation (i.e., capillaries) and type L for the CD31lo/Emcnlo subpopulation (i.e., sinusoids) [41]. Interestingly, osteoprogenitor cells (i.e., Osterix+, Runx2+, and collagen 1α+), which can eventually differentiate into osteoblasts, were preferentially located next to the type H as opposed to the type L blood vessels, despite the low prevalence of type H endothelial cells in relation to the total population within the marrow [41]. These data suggest a direct relationship between endothelial cells and pre-osteoblasts during bone growth. Further, 7 days following irradiation in C57BL/6J mice, tibiae had a high and low number of type H and type L endothelial cells, respectively, suggestive of a role for H type blood vessels in neo-angiogenesis [41].
Following intramembranous and endochondral ossification during development, the skeletal system becomes highly vascularized [42]. Beyond skeletal maturity, bone resorption and formation continues under a regulated fashion to maintain strength and integrity. This is achieved by bone remodeling, which is under the control of basic multicellular units of cortical bone [43] or bone remodeling compartments of trabecular bone [44]. Basic multicellular units and bone remodeling compartments consist of osteoclasts, osteoblasts, and an always present blood vessel [45,46,47]. The blood vessel provides nutrients, oxygen, and precursor cells to the bone-remodeling site, illustrating the importance of the vascular supply for bone homeostasis.
Partial or complete breaks cause a discontinuity in bone and represent fracture. Often there is a disruption in the vascular supply surrounding the fracture site. Bone healing commences in an attempt to restore homeostasis and this occurs via mechanisms of endochondral ossification [17]. Under these circumstances, a callus forms over the fracture site where angiogenesis will occur [18] and blood vessel formation at the callus allows for the replacement of cartilage with bone [48]. When blood delivery reaches its nadir, the transports of products necessary for bone repair are enhanced [49]. For example, blood flow was enhanced 0–14 days following osteotomy and returned to baseline by 21–28 days, coinciding with mineral deposition at the fracture site [49, 50]. A review of the literature in regard to the vascularization of human long bones revealed disparate zones of blood vessel density in femoral and tibial shafts [51]. When divided into three zones (i.e., the upper third, middle, and lower third), femora had moderate, high, and poor vascularization, respectively, and tibiae had high, moderate, and poor vascularization, respectively [51]. The authors speculated that these disparate zones of vascularization could contribute to fracture non-union [51], which occurs at high rates in femora and tibiae [52]. Once again, the vascular supply is key. Under all four paradigms (i.e., intramembranous ossification, endochondral ossification, bone remodeling, and fracture healing), the vascular supply is requisite.
The Fluidic Nature of Bone Ensures Its Dependence upon the Vascular System
The skeletal system is highly vascularized and porous, lending itself to a high fluid content. The vascular system of bone contains afferent vessels, capillaries and sinusoids, and efferent vessels [53, 54]. Ionic exchange [53] and filtration [55] occur in the capillaries and sinusoids, with capillaries located in yellow (fatty) marrow and the sinusoids located in red (hematopoietic) marrow [21]. The distinction between capillaries and sinusoids based solely on this characteristic should be done with caution, since fat cells are present in hematopoietic marrow [53] and sinusoids are observed in fatty marrow. The filtrate from the capillaries and sinusoids enter into the interstitial spaces of marrow and the porosities of bone. The porosities comprise of the Volkmann and Haversian canals, the lacunar-canalicular network of osteocytes and their dendritic processes, and the spaces within the mineral hydroxyapatite [56]. In mature animals, ~ 1/3rd of cortical bone contains fluid located in blood vessels (~ 6%), cells (~ 25%), and the interstitial space (~ 69%) [54]. In immature animals, the fluid spaces in cortical bone are larger [54].
Interstitial fluid is sourced by the marrow capillaries [57], which are supplied by bone and marrow arterioles and arteries. The nutrient arteries serve as bridges between the circulation of bone and that of the periphery, regulating blood flow and intramedullary pressure [58]. Recent investigations have revealed interesting findings in relation to newly discovered transcortical blood vessels, which are suspected to play the major role in blood supply to the skeleton [59]. In regard to flow, ~ 5–7% of cardiac output in male rats 2, 6, and 24 months of age went to the skeleton, which represented ~ 7–8% of the total body mass [60]. Blood flow to bone is on par with blood flow to some resting skeletal muscles [61] and, relative to cell mass, the skeleton receives a significant blood supply [54]. In regard to intramedullary pressure, recorded values have demonstrated high variability. For example, mean intramedullary pressures of ~ 16 mmHg were observed in C57BL/6 mice [62] vs. ~ 33 mmHg in adult New Zealand White rabbits [63]. The recorded variability no doubt reflects the diversity in the experimental protocols. Since intramedullary pressure is usually lower than blood pressure (i.e., a pressure differential), plasma can filtrate and allow for exchange of fluid between the vascular system, the interstitial space, and the lacunar-canalicular network [56].
Combined with the pressure differential between the vascular system and interstitial space, mechanical loading also drives the movement of interstitial fluid, allowing for the transport of nutrients and factors to and the removal of waste products from osteocytes embedded within bone [56]. Mechanical compression of bone and release of that compression expels and reuptakes interstitial fluid, respectively [64]. The porous nature of the skeleton permits the passage of interstitial fluid throughout bone [64] and shifts in interstitial fluid activate bone cells [56]. As a result, bone tissue is able to adapt to mechanical loading [65] via this indirect stimulus. As proof of concept, experimental conditions that eliminated or reduced mechanical loading, but altered bone intramedullary pressure and fluid flow, initiated bone formation [62, 66,67,68,69].
Bone adaptation to these alterations in interstitial fluid flow results from the creation of shear stress on bone cell surfaces, which stimulates bone anabolism or catabolism [56]. Mechanisms by which bone anabolism or catabolism occur are via release of shear stress-related factors such as nitric oxide and prostaglandins (e.g., PGE2) from bone cells [70,71,72,73]. Nitric oxide participates in osteoblast differentiation [74, 75] and impairs osteoclast activity [76]. In addition, PGE2 has been shown to inhibit bone loss with hind limb immobilization, such that trabecular bone was higher vs. age-matched controls [77]. In fact, vascular endothelial cells release a variety of factors (e.g., adenosine triphosphate, adenosine diphosphate, adenosine monophosphate, adenosine, prostacyclin, interleukin-11, insulin-like growth factor-1, endothelin-1, RANKL) that modulate bone cellular activity [78,79,80,81,82,83,84,85,86,87], indicating a regulatory function of bone blood vessels on bone in this regard. In conclusion, the delivery of blood flow to the skeleton via the bone vascular network ensures the fluidity of bone. As a result of pressure differentials created by the vascular system and mechanical loading, interstitial fluid flow throughout bone serves as the stimulus to augment or depress bone remodeling.
Reliance on the Bone Vascular Network for Egress and Ingress of Bone Marrow Cells to and from the Peripheral Circulation
Marrow, located in the diaphyseal shaft and the intratrabecular spaces of the metaphyses, is classified as either hematopoietic or fatty. The distinction between the two is minimal, however, since hematopoietic marrow contains fat cells [53]. In the adult skeleton, hematopoiesis occurs primarily in the marrow [88]. Hematopoiesis is the development and formation of all blood cells, which develop from a single precursor cell; i.e., the hematopoietic stem cell (HSC) [89]. HSCs have several unique properties that distinguish them from other cell types; e.g., (1) they can survive up to the entire lifespan of the organism, (2) they have self-replicative capacities, and (3) can proliferate broadly, producing all lineages of myeloid and lymphoid blood progenies [90] [89]. HSCs grow and mature on a lattice of non-hematopoietic stromal cells (e.g., fat cells, fibroblasts, endothelial cells, and macrophages), which assist in the development and differentiation of HSCs by providing a hematopoietic-inducing environment [89]. Thus, the infrastructure capable of hematopoietic cell renewal (i.e., a constant production of the various blood cells per unit volume of blood) is located within the marrow of the bones throughout the skeleton [91]. Marrow is therefore important for the integrity of the whole organism. Due to these lifelong duties, immense cell turnover rates occur, with ~ 500 billion cells being produced daily [91]. In states of pathology, hematopoiesis can occur in extramedullary tissues (i.e., the spleen, liver, and lymph nodes); however, this occurs at the cost of reduced efficiency [91].
In the 1970s, the HSC niche was first described as a three-dimensional space that housed HSCs and provided the regulatory environment for self-renewal and proliferation [92], which is critical for tissue homeostasis [93]. The HSCs located at the bone endosteum were referred to as the osteoblast niche, since HSCs adhere to bone-lining osteoblasts [94]. Early experimental evidence suggests that as progenitor cells (originating from the endosteal HSC pool) mature and differentiate, they migrate towards the marrow sinusoids [95,96,97]. The perivascular space surrounding the marrow sinusoids was coined the vascular niche. Thus, the osteoblast niche represented HSC quiescence, while the vascular niche represented HSC proliferation and mobilization [98]. Current evidence suggests that the osteoblast and vascular niches are both related to blood vessels and they have been coined the arteriolar-pericyte niche and the sinusoidal-megakaryocyte niche [99]. Both reside at the endosteal surface, maintaining the postulate that osteoblasts are still a component of the niche [99]. Further, subset niches have been demonstrated in the central marrow [100, 101], countering long-held beliefs of an endosteal preference.
The establishment of marrow hematopoiesis and osteogenesis is highly coordinated [102,103,104] and bone and marrow are linked by the vascular system [105]. During development, hematopoiesis and vascularization occur in tandem [93] and it is theorized that bone and marrow are organized according to the spatial distribution of blood vessels [38, 106, 107]. For example, there is a long-held belief that the preponderance of metabolically active marrow (i.e., hematopoietic) is in close proximity to the endosteal surface of bone [104], with a sparse amount of activity occurring in the central area of the marrow cavity [105]. However, recent data suggest that the majority of HSC are perivascular, located in highly vascularized regions at the endosteal surface [108, 109]. This corresponds to the spatial arrangement of the bone microcirculation, whereby a rich plexus of sinusoids line the bone endosteum [55], providing collection sites for mature blood cells and greater flow to areas of the marrow with higher metabolic rates. The endosteal sinusoids extend branches into the adjacent bone and also extend branches into the central marrow area, towards the central sinus [55]. Following suit, non-pathological blood flow follows a centrifugal direction; i.e., from the center of the marrow towards the endosteal surface [53, 55]. It has been demonstrated in the long bones of mice that arterial blood flows through the H-type capillaries, then the L-type sinusoids, and finally into the large central vein of the diaphysis for egress [110]. Further, blood velocity and shear stress are higher through the H-type capillaries vs. the L-type sinusoids [110]. Once blood enters the sinusoidal plexus at the endosteal surface [55], it flows back towards the central sinus in a centripetal direction [55, 110]. Most recently, however, discovery of transcortical blood vessels suggests a large volume of blood leaves the skeleton via these routes [59]. Nevertheless, the amalgamation of the vascular, skeletal, and hematopoietic systems at the endosteal surface may modulate bone formation as well as hematopoiesis [88].
The marrow sinusoids, consisting of a single layer of endothelial cells with an incomplete basal membrane and discontinuous adventitial layer of perivascular cells [111], allow for the migration of mature blood cells into the peripheral circulation [55, 112,113,114,115,116,117]. In fact, passage of mature leukocytes and immature hematopoietic stem and progenitor cells in a murine model demonstrated access into the peripheral circulation exclusively via a sinusoidal route [118]. This occurs via a transendothelial route [114], with the formation of temporary pores in which the blood cells can pass via diapedesis [111]. The newly formed blood cells are collected into the sinusoids and the central sinus for release into the blood stream [91]. Vascular endothelial cells also play a more direct role in stimulating and regulating myelopoiesis (i.e., the production of marrow and cells) via manufacture of myeloid growth factors (i.e., colony-stimulating factors) when subjected to interleukin-1 [21]. Further, the vascular endothelium participates in the homing of cells to the marrow. Transplanted hematopoietic stem and progenitor cells in Has3−/− mice exhibited delayed transendothelial migration across the sinusoids in the femoral metaphysis in comparison to wild-type controls [119]. Has3 is the synthase that produces hyaluronic acid, which provides a scaffold of support and localizes, retains, and binds hematopoietic stem cells [120]. Thus, the function of marrow is reliant upon an intact microvascular system [121]; i.e., hematopoietic stem cells home to marrow and to other organs via the bone sinusoidal network [91, 122].
For blood cells incapable of accessing the peripheral circulation via diapedesis (i.e., red blood cells), growth pressure and pliability of their membranes are key factors. Red blood cell passage into the peripheral circulation relies upon alterations in intramedullary pressure induced by blood flow through bone [123]. The investigation of intramedullary pressure within bone has not been given proper attention in regard to its role in regulating the release of morphotic blood elements [123], but should be considered as a factor potentially contributing to the overall health of an organism. Red blood cell release into the circulation results from extravascular and vascular factors [123]. Within the rigid bone capsule, two forces opposed one another; i.e., the pressure generated by blood flowing through the sinusoids opposes the pressures generated by proliferating cells in the surrounding marrow parenchyma [91]. As proliferation pressure in the marrow parenchyma augments, it causes the nearby sinusoids to close [91]. Under circumstances that increase local blood flow, closed sinusoids can reopen [91]. At this time, mature cells that line the sinusoids are captured into the blood steam and carried into the peripheral circulation [91]. Thus, blood flow through the sinusoids is a key determinant of blood cell release from the marrow [117]. The sinusoids are in constant flux [55] and oscillations between the open or closed state permit a consistent exchange of parenchymal cells into the collecting sinuses [91]. In addition, recent finding demonstrated that neutrophil mobilization from the marrow can occur through the transcortical vessels [59]. Thus, the vascular system is at the forefront of the proper regulation of marrow function; i.e., it is essential for the mobilization and egress of blood cells (i.e., white and red blood cells and platelets) [95, 124, 125].
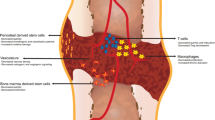
In total, vascular function is important for bone and marrow homeostasis as well as organismal health. Figure 1 summarizes how the bone and marrow microcirculation, via its regulation of blood flow, contributes to adequate nutrition of bone and marrow cells, supports hematopoiesis, and is requisite for bone development and repair. In addition, via the regulation of interstitial fluid flow and intramedullary pressure, the bone and marrow microcirculation provide factors that stimulate or depress bone cellular activity, allow for the transference of mechanical stimuli into chemical signals, and permit the collection and egress of mature blood cells into the peripheral circulation.
The Aging Blood Vessel
In association with age-related declines in bone mass, the cardiovascular system develops multiple pathologies. Recent studies have associated cardiovascular ailments (i.e., peripheral arterial disease, cardiovascular disease) and osteoporosis [126, 127]. More specifically, development of diseases in the bone vascular system have been reported [9, 10, 12, 13, 41], which have a more direct and profound influence on bone and marrow homeostasis. The end result of bone vascular pathology is reduced blood flow [4, 6] and marrow ischemia [128].
Vascular involvement in bone disease has been theorized since the early part of the twentieth century. In an article examining senile osteoporosis, Spencer, Hausinger, and Laszlo theorized that “a decline in blood supply due to degenerative vascular changes, impairment of capillary distribution and altered permeability of the capillary wall affecting the exchange of nutrients and waste products, may also be contributing factors” [129]. In the subsequent decades, many investigators followed along these lines of thinking and provided a multitude of experimental data in support [4, 6, 7, 10, 14,15,16, 53, 110, 128, 130,131,132,133]. Despite their efforts, investigations examining the vascular involvement of bone disease were limited by available technologies. While remaining a difficult subject to examine due to the necessity of penetrating bone to observe the vascular network, modern technologies have permitted more advanced visualization and investigative techniques. Over the years, experimental data has proven that blood delivery to bone with advancing age diminishes in both animal models [4, 6, 16, 110, 133] and humans [14, 15]. Various techniques (e.g., radiolabeled microspheres [4, 6, 16], magnetic resonance imaging [133], proton magnetic resonance spectroscopy [14, 15], intravital microscopy [110], and dynamic contrast-enhanced magnetic resonance [14, 15]) have been employed to examine this age-related phenomenon. Further, the observed decrements in bone blood flow have coincided with several vascular abnormalities (e.g., vascular rarefaction, reduced angiogenic capabilities, diminished vasodilator capacity, vascular calcification, arteriosclerosis, and atherosclerosis). Vascular rarefaction (i.e., a reduced number of blood vessels) has been reported in bone and marrow [9, 10, 128, 134]. Coupled with a reduced capacity to create new blood vessels, as demonstrated during fracture repair in elderly mice [135], once vascular rarefaction has occurred, restoration of an adequate vascular density is not easily achieved. Impaired angiogenesis may be reflective of the loss of H-type blood vessels recently discovered in a murine model and suspected to be highly associated with angiogenesis [41]. For example, H-type blood vessels were diminished in 70-week-old vs. 4-week-old mice, which corresponded with a loss of osteoprogenitor cells and bone mass in advanced age [41].
In addition to reduced vascular density and impaired angiogenesis, pathologies related to the function of blood vessels have also been observed. Similar to other tissue beds, bone blood vessels demonstrate age-related diminished vasodilator capacity. For example, decrements (27–55%) in endothelium-dependent vasodilation of the femoral principal nutrient artery (PNA) have been observed in 22–24-month-old vs. 4–6-month-old female and male rats [4,5,6,7,8]. In addition, arteriosclerosis and atherosclerosis have been observed in the bone vasculature [11,12,13], with arteriosclerosis being linked to diminished marrow arterial pressure and marrow ischemia [128]. Arteriosclerosis is suspected to occur ~ 10 years in advance of the same disease in blood vessels from other tissue beds [12] and atherosclerosis was observed in bone marrow blood vessels from human subjects, such that the cross-sectional lumen area was reduced by 18–26% in 50-year-old vs. 30-year-old individuals [13]. Interestingly, induction of atherosclerosis in rhesus monkeys fed a high-fat diet resulted in diminished skeletal blood flow, even though the lesions were observed in blood vessels outside of the skeleton [136].
A recent discovery demonstrated a pathology more severe than arteriosclerosis and atherosclerosis. For example, severe mineralization and calcification was observed on bone marrow blood vessels from the medullary cavity of young and old rats and human patients [9]. Morphologically, these vessels appear ossified and resembled bone, such that osteocyte lacunae and osteoid seams were visible on the abluminal surfaces [9]. Analysis with micro-CT demonstrated progressive ossification of the bone marrow vasculature as a function of advancing age in male rats [9]. The presumed conversion of blood vessels into bone (i.e., ossification) represents “microvascular dead space”; i.e., diminished vasodilation, vasoconstriction, and patency. To date, it is unknown which blood vessels (i.e., arteries, arterioles, capillaries, sinusoids, venules, and/or veins) undergo ossification. If the sinusoids suffer such pathology, another functional consequence may include reduced egress of mature blood cells from the marrow.
The Aging Bone Marrow
Anemia is a common and underestimated condition in the elderly [137], can increase mortality rates [138], diminish physical performance, and attenuate activities of daily living [125, 139]. Anemia is a reduced red blood cell count and is defined by the World Health Organization as hemoglobin levels of < 12 g/dL for women and < 13 g/dL for men [137]. Mahlknecht and Kaiser reported age-related declines in hemoglobin and hematocrit for both genders [137]. The cases of anemia in the elderly can be attributed to several factors (e.g., chronic disease, infection, iron or vitamin B12 deficiency) [140, 141]. However, ~ 36% of cases in the elderly have an unknown origin [137, 140, 141] and are referred to as senile anemia [142]. In such instances, reduced blood counts are linked to reduced hematopoietic stem cells [143], progenitor cell differentiation anomalies [144], inability to mobilize progenitor cells [145], and attenuated responses to hormonal stimulation [146,147,148,149]. An impaired ability to generate and mobilize blood cells into the peripheral circulation would also include those of the lymphoid lineage. Thus, on a broader scale, pancytopenia is clinically diagnosed as a reduction in the number of white and red blood cells and platelets. Of particular note, some elderly individuals present with an attenuated host defense mechanisms and a diminished neutrophil response to infection [150]. While many factors contribute to reduce blood cell counts in the elderly, few researchers have examined a vascular etiology in relation to these symptoms. This review highlighted the importance of the marrow microcirculation in maintaining the HSC niches and for the mobilization and egress of blood cells. Further, this review demonstrated several age-related pathologies in the bone and marrow microcirculation that can have a dramatic impact on bone and marrow blood flow. Thus, the possibility exists that the etiologies contributing to senile anemia and/or pancytopenia may be partially related to vascular decline.
Hematopoietic marrow is progressively replaced by fatty marrow with advancing age, whereby for each decade of life, there is a 10% reduction in cellularity [151]. A study examining iliac crest biopsies of human subjects revealed an age-related diminution of the number of marrow sinusoids that corresponded with a reduced hematopoietic compartment and fat cell augmentation [10]. Clinically, osteoporosis and osteopenia in men were associated with augmented vertebral marrow fat vs. individuals with normal bone density [15] and marrow perfusion was reduced in the osteoporotic vs. osteopenic and control subjects [15]. Animal models have demonstrated similar trends, whereby declines in hematopoietic marrow coincided with augmented adiposity in the proximal tibia of 42-month-old vs. < 6-month-old rabbits [152]. Accordingly, reduced marrow cellularity corresponded with diminished peripheral blood counts in individuals > 60 years of age [144, 153]. Additionally, arteriolar and capillary densities were reduced in 65–70-week-old vs. 4-week-old mouse tibiae and corresponded with diminished presence of perivascular mesenchymal cells and stromal cell factor [154]. Mesenchymal cells are linked to HSC regulation [155] and stromal cell factor participates in HSC maintenance and homing [156, 157]. Interestingly, experimental enhancement of vascular niche function (i.e., augmenting arterioles, capillaries, perivascular cells, stromal cell factor, and HSC number) in old mice tibiae could not restore the age-related declines of HSC function [154], which is suspected to result from abnormalities intrinsic to the aged HSC [158, 159].
Since marrow function is reliant upon an intact microvascular system [121], one has to speculate if the age-related changes in hematopoietic and fatty marrow are related to pathologies that would ultimately serve to reduce skeletal blood flow. In fact, blood flow to areas of high hematopoietic activity (i.e., marrow and trabecular bone) is augmented in comparison with areas of low hematopoietic activity (i.e., the cortical shell) [4, 6, 160]. Further, blood flow to bone and marrow is lower in advanced age [4, 6, 128], coinciding with reduced hematopoiesis and increased marrow adiposity [9, 10, 152]. Blood flow through the marrow sinusoids allows for the capture and egress of mature blood cells [91, 117, 122, 161]. Thus, one should rightly speculate that diseases such as arteriosclerosis [12], atherosclerosis [11, 13], and ossification [9] will impair this process. In fact, the relationship between atherosclerosis and senile anemia has been previously considered [13]. In addition, ossification of the marrow microcirculation may attenuate diapedesis of blood cells through the sinusoidal wall, if it contains bone and/or calcium deposits. Therefore, in addition to senile anemia, altered marrow morphology may have consequences on immune system ontogeny [162].
Aging and Fracture Repair
Fracture in the elderly coincides with elevated rates of morbidity and mortality [163] and age-related delays in fracture repair have been documented [164,165,166]. For example, during the inflammatory phase (day 3) following fracture of the tibia, periosteal cells differentiated into collagen X-expressing chondrocytes in juvenile (4 weeks of age) vs. middle-aged (6 months of age) and elderly (18 months of age) mice [166]. By day 5, juvenile mice displayed large volumes of cartilage in the tibial callus as opposed to smaller volumes of cartilage in the calluses of the middle-aged and elderly groups [166]. Further, at this time point, new bone and robust osteocalcin expression were observed in the periosteum of juvenile mice, while middle-aged and elderly mice demonstrated much smaller quantities of both [166]. Vascularization at the fracture site follows the same sequential pattern. For example, while PECAM+ blood vessels were observed in fracture calluses of juvenile (4 weeks of age), middle-aged (6 months of age), and elderly (18 months of age) 129J/B6 male mice, their presence was less numerous in the middle-aged and elderly groups [135]. This coincided with a higher surface density of blood vessels in the juveniles vs. the elderly [135]. Further, important regulators of angiogenesis (i.e., hypoxia-inducible factor-1α [HIF-1α] and VEGF) were also detected at early time points in juvenile calluses. For example, HIF-1α and VEGF transcripts were detected in the callus at 3 days following fracture in juveniles vs. 5 days following fracture in middle-aged and elderly mice [135]. This work demonstrates that the processes associated with vascularization during fracture repair are delayed or impaired by the aging process [135]. Further, bone volume and bone volume-to-total volume ratio in juvenile mice was higher than the middle-aged and elderly groups, suggesting an enhanced ability to form new bone in the youngest age group [166]. When assessed as a function of age and time, bone volume was higher in juvenile vs. middle-aged and elderly mice, indicating delayed healing in the older groups [166]. While many factors contribute to bone healing following fracture, the connection of vascular density with the appearance of new bone formation is noteworthy.
The Clinical Relevance of the Bone Vascular Network
This review has thus far highlighted the various physiological roles of the bone vascular network for bone and marrow function. While this review has focused on the age-related decrements in these three systems (i.e., vascular, bone, and marrow), we must recognize that advancing age is often associated with at least one morbidity and additional comorbidities. These morbidities and comorbidities often present with vascular challenges similar to those observed in advanced age. For example, declines in vasodilator (i.e., both endothelium-dependent and endothelium-independent) capacity of the rat femoral PNA were observed in a long-term (i.e., 20 weeks) type 2 diabetes mellitus model [167]. Type 2 diabetic rats also had elevated vasoconstriction to norepinephrine, enhanced myogenic vasoconstriction, and reduced mechanical distensibility vs. controls [167]. These vascular alterations corresponded with diminished bone and marrow blood flow [167] and decrements in various bone parameters (i.e., bone mineral density and strength) at several skeletal sites [168]. In addition to increasing the stiffness of the PNA, type 2 diabetes mellitus led to a more pro-vasoconstrictor phenotype of the vessel [167].
Loss of estrogen also alters the bone vascular network. While there is data demonstrating augmented bone blood flow, bone loss, and reduced bone mineral density and ash weight following ovariectomy and orchidectomy in young rats [169,170,171], more recent publications have documented the opposite. Estrogen-associated bone loss [7, 133] corresponded with diminished vasodilator capacity of bone arteries [7], enhanced vasoconstrictor capacity of bone arteries [172], reductions in erythropoietic marrow [133], vascular rarefaction [173], and declines in bone perfusion [133, 173]. Interestingly, strong, positive correlations existed between peak vasodilator capacity of bone arteries and bone volume, while poor associations existed between plasma estrogen and bone volume in animals with low circulating estrogen (i.e., old control and young and old ovariectomized) [7]. These data indicate that vascular function is a better predictor of bone loss than estrogen status [7].
The vascular alterations associated with hind limb unloading (i.e., a ground-based rodent model used to simulate bed rest, physical inactivity, and microgravity) drums home the clinical relevance associated with altered bone vascular function and structure in advanced age or morbidity. Following 2 weeks of hind limb unloading in 6-month-old male Fischer-344 rats, vasoconstriction to phenylephrine, an α1-receptor adrenergic agonist, was reduced in the femoral PNA [174]. Likewise, flow- and acetylcholine-mediated vasodilation (i.e., both endothelium-related mechanisms) were impaired in PNAs from hind limb unloaded animals [174]. In addition to the vasomotor changes, hind limb unloading structurally remodeled the PNA such that the intraluminal diameter (i.e., 146 ± 7 μm vs. 177 ± 10 μm, respectively) and medial wall thickness (i.e., 16 ± 2 μm vs. 22 ± 2 μm, respectively) were diminished in comparison to control rats [175]. Trabecular bone mineral density in the femur was also reduced [174]. The structural remodeling of the PNA resulted in an impaired ability to deliver blood [175]. Thus, 2 weeks of hind limb unloading remodeled the PNA and impaired its vasomotor function in such a way as to diminish its effectiveness at delivering blood to the femur upon re-initiation of weight-bearing activities [174, 175]. This set of experiments illustrates how alterations in vasomotor function or structure of bone arteries impact bone blood flow.
From a clinical perspective, the age- and/or disease-related alterations in the microcirculation of bone and marrow should not be overlooked, as they contribute to the declines in skeletal perfusion and presumably hinder the clinical treatment of bone and marrow. Singularly, one of these vascular dysfunctions (i.e., diminished vasodilator or enhanced vasoconstrictor activity, vascular rarefaction, arteriosclerosis, atherosclerosis, and ossification) could serve to diminish bone and marrow blood flow, initiating ischemia and affecting interstitial fluid flow and pressure, bone remodeling, fracture repair, and the egress of cell from the marrow. Suffering from more than one of these vascular pathologies, which is most likely the clinical scenario, could further exacerbate the ischemia and the decrements in bone and marrow blood flow. Clinically speaking, it may be prudent to consider the treatment of bone or marrow pathology as binary; i.e., the treatment of either of these organ systems should be coordinated with the treatment of an aged and/or diseased vascular system. Experimental and/or clinical data in regard to such a strategy, to date, is lacking. However, experimental evidence related to intermittent parathyroid hormone (PTH) administration may provide support.
Intermittent PTH administration is bone anabolic and augments bone mass. Thus, from a historical perspective, the efficacy of PTH treatment has been attributed to its effects on bone cellular activity. Recent data, however, illustrate its impact on bone vascular function and morphology. Intermittent PTH administration enhanced endothelium-dependent vasodilator function in young and old bone blood vessels [8, 176], augmented bone vascular density [173], redistributed the smallest bone blood vessels closer to bone-forming sites [177], and increased skeletal perfusion [103, 173]. All of these modifications serve to aid in the regulation of bone cellular activity and promote an environment supportive of bone formation. A note of caution, however, as recent evidence suggests a potential exacerbation of bone marrow blood vessel ossification with intermittent PTH administration [178]. Interestingly, 2 weeks of intermittent PTH administration improved the age-related decrements in aortic function in old rats [179]. While prescribed to augment bone mass, the totality of these findings demonstrate an unintended benefit of this treatment on bone blood vessels and overall vascular function.
To date, diagnosing bone vascular dysfunction in the clinical setting is difficult, if not impossible. Thus, the clinical treatment of bone vascular pathology without a clear diagnosis is not recommended. However, non-pharmacological remedies are available. Treadmill exercise training in young and old rodents resulted in increased vasodilator capacity of the femoral PNA [6], augmented blood flow to the femur [6], enhanced bone angiogenesis [180], and elicited exercise hyperemia to more bone regions [181] vs. sedentary controls. These vascular enhancements coincided with augmented bone volume [6, 180] and mineral density [180], except when rats were administered an anti-angiogenic agent which prevented bone angiogenesis and, thus, the changes in bone volume [180]. While clinical and experimental data may be lacking as to the effects of various medications on bone vascular function, a prescription of exercise training or physical activity to patients suffering from bone and marrow ailments may enhance or restore function in the bone and marrow microcirculation, particularly if the individuals are of advanced age.
Decreased Efficacy of Bone-Targeted Therapies in the Setting of a Compromised Vascular Network
To date, there are no studies examining the effects of bone vascular dysfunction on drug delivery to bone and marrow. Further, there is no experimental evidence indicating a decreased efficacy of bone-targeted therapy as a function of advance age and/or disease. However, “the absence of evidence is not evidence of absence.” Given the various physiological roles of the bone vascular network in supplying blood flow, removing waste products, regulating bone interstitial pressure and fluid flow, and mobilizing marrow cells for distribution into the peripheral circulation, it stands to reasons that compromises in this organ system would influence the ability to deliver drugs to bone and marrow. Perhaps it is time as a scientific and medical community to begin addressing these questions. At the extreme end of the clinical spectrum, steroid-induced osteonecrosis of the femoral head is associated with ischemia; i.e., diminish blood flow resulting from fat emboli or from hypertrophic adipocytes, which occlude or compress, respectively, the microcirculation [182,183,184]. To lesser extremes, circumstantial evidences provide associations between the various vascular pathologies and declines in bone health. For example, a vasoconstrictor phenotype of bone arteries in type 2 diabetes mellitus [7], age-, and/or disease-related diminished vasodilator capacity of bone blood vessels [4,5,6,7,8, 133], the presence of arteriosclerosis [12], atherosclerosis [11, 13] and ossification [9] in bone blood vessels, vascular rarefaction [9, 10, 41, 59, 128, 134], and reduced angiogenic capability [135] coincide with reduced skeletal blood flow [4, 6, 14,15,16, 110, 133, 167] and marrow ischemia [128]. Thus, in light of these connections, it may prove beneficial to begin clinical exploration as to whether an aging and/or diseased vascular system influences drug delivery or the efficacy of treating of bone and marrow pathology.
Conclusion
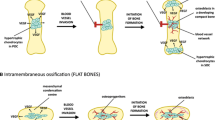
The vascular supply is essential for the maintenance of health and longevity of all tissues. In addition, the bone vascular network is vital for bone development (i.e., intramembranous and endochondral ossification), bone homeostasis (i.e., bone remodeling), and fracture repair. The regulation of bone blood flow by the nutrient arteries and arterioles allows for the fluidic nature of bone, which is essential for shear-mediated release of factors from vascular endothelial and bone cells, and for the transduction of mechanical stimuli into chemical signals. Finally, the mobilization and capture of mature blood cells depend upon the ability of the cells to penetrate the vascular wall, are reliant upon blood flow through the sinusoidal network, and are contingent upon pressure gradients between the sinusoids and the interstitial space. Due to these responsibilities, age- and/or disease-related vascular decline can have profound influences on bone and marrow homeostasis, as outlined in Fig. 2. The pathological consequences may include reduced bone and marrow blood flow, attenuated nutrient delivery and waste removal, impaired hematopoiesis, bone remodeling, and fracture healing, dysregulation of interstitial fluid flow and intramedullary pressure, impaired transference of mechanically-induced signals into bone cellular activity, and diminished egress of mature blood cells. The clinical manifestations of these abnormalities may present as bone loss, diminished fracture healing, increased fracture risk, senile anemia, and/or a compromised immune system. From a clinical standpoint, medications and treatments prescribed to alleviate these medical conditions may have reduced or no efficacy if relying upon a bone and marrow microcirculation with impaired or obstructed patency. These may be important clinical inquires to address to determine whether the efficacy of bone and marrow treatment is enhanced via the coinciding treatment of bone vascular dysfunction.
References
Kang L, Reyes RA, Muller-Delp JM. Aging impairs flow-induced dilation in coronary arterioles: role of NO and H(2)O(2). Am J Physiol Heart Circ Physiol. 2009;297:H1087–95.
Park Y, Prisby RD, Behnke BJ, Dominguez JM 2nd, Lesniewski LA, Donato AJ, et al. Effects of aging, TNF-α, and exercise training on angiotensin II-induced vasoconstriction of rat skeletal muscle arterioles. J Appl Physiol. 2012;113(7):1091–100.
Lesniewski L, Durrant JR, Connell ML, Henson GD, Black AD, Donato AJ, et al. Aerobic exercise reverses arterial inflammation with aging in mice. Am J Physiol Heart Circ Physiol. 2011;301(3):H1025–32.
Prisby RD, Ramsey MW, Behnke BJ, Dominguez JM, Donato AJ, Allen MR, et al. Aging reduces skeletal blood flow, endothelium-dependent vasodilation and nitric oxide bioavailability in rats. J Bone Miner Res. 2007;22:1280–8.
Prisby RD, Muller-Delp J, Delp MD, Nurkiewicz TR. Age, gender and hormonal status modulate the vascular toxicity of the diesel exhaust extract phenanthraquinone. J Toxicol Environ Health A. 2008;71:464–70.
Dominguez JM, Prisby RD, Muller-Delp JM, Allen MR, Delp MD. Increased nitric oxide-mediated vasodilation of bone resistance arteries is associated with increased trabecular bone volume after endurance training in rats. Bone. 2010;46:813–9. https://doi.org/10.1016/j.bone.2009.10.029.
Prisby RD, Dominguez JM 2nd, Muller-Delp J, Allen MR, Delp MD. Aging and estrogen status: a possible endothelium-dependent vascular coupling mechanism in bone remodeling. PLoS One. 2012;7:e48564.
Lee S, Bice A, Hood B, Ruiz J, Kim J, Prisby RD. Intermittent PTH 1-34 administration improves the marrow microenvironment and endothelium-dependent vasodilation in bone arteries of aged rats. J Appl Physiol. 2018;124(6):1426–37. https://doi.org/10.1152/japplphysiol.00847.2017.
Prisby R. Bone marrow blood vessel ossification and “microvascular dead space” in rat and human long bone. Bone. 2014;64:195–203. https://doi.org/10.1016/j.bone.2014.03.041.
Burkhardt R, Kettner G, Bohm W, Schmidmeier M, Schlag R, Frisch B, et al. Changes in trabecular bone, hematopoiesis and bone-marrow vessels in aplastic-anemia, primary osteoporosis, and old-age: a comparative histomorphometric study. Bone. 1987;8(3):157–64.
Bocchi L, Orso CA, Passarello F, Lio R, Petrelli L, Tanganelli P, et al. Atherosclerosis of the microcirculation in the femoral head: based on a study by optical and electron microscopy of femoral heads removed at operation. Ital J Orthop Traumatol. 1985;11(3):365–70.
Ramseier E. Untersuchungen uber arteriosklerotische veranderungen der knochenarterien. Virchows Archiv. A, Pathology. 1962;336(1):77–86.
Takasaki M, Tsurumi N, Harada M, Rokugo N, Ebihara Y, Wakasugi K. Changes of bone marrow arteries with aging. Jpn J Geriat. 1999;36:638–43.
Griffith JF, Yeung DK, Tsang PH, Choi KC, Kwok TC, Ahuja AT, et al. Compromised bone marrow perfusion in osteoporosis. J Bone Miner Res. 2008;23:1068–75.
Griffith JF, Yeung DKW, Antonia GE, Lee FKH, Hong AWL, Wong SYS, et al. Vertebral bone mineral density, marrow perfusion, and fat content in healthy men and men with osteoporosis: dynamic contrast-enhanced MR imaging and MR spectroscopy. Radiology. 2005;236:945–51.
Bloomfield S, Hogan HA, Delp MD. Decreases in bone blood flow and bone material properties in aging Fischer-344 rats. Clin Orthop Related Res. 2002;396:248–57.
Choi I, Chung CY, Cho TJ, Yoo WJ. Angiogenesis and mineralization during distraction osteogenesis. J Korean Med Sci. 2002;17:435–47.
Amir L, Becking AG, Jovanovic A, Perdijk FB, Everts V, Bronckers AL. Formation of new bone during vertical distraction osteogenesis of the human mandible is related to the presence of blood vessels. Clin Oral Implants Res. 2006;17:410–6.
Rowe N, Mehrara BJ, Luchs JS, Dudziak ME, Steinbrech DS, Illei PB, et al. Angiogenesis during mandibular distraction osteogenesis. Ann Plast Surg. 1999;42(5):470–5.
Kanczler JM, Oreffo RO. Osteogenesis and angiogenesis: the potential for engineering bone. Eur Cell Mater. 2008;15:100–14.
Hansen E. Mircovascularization, osteogenesis, and myelopoiesis in normal and pathological conditions. In: Schoutens A, Arlet J, Gardeniers JWM, Hughes SPF, editors. Bone circulation and vascularization in normal and pathological conditions, Nato Science Series: A. New York: Plenum Press; 1993. p. 29–41.
Thompson T, Owens PD, Wilson DJ. Intramembranous osteogenesis and angiogenesis in the chick embryo. J Anat. 1989;166:55–65.
Li G, Simpson AH, Kenwright J, Triffitt JT. Effect of lengthening rate on angiogenesis during distraction osteogenesis. J Orthop Res. 1999;17(3):362–7.
Reilly T, Seldes R, Luchetti W, Brighton CT. Similarities in the phenotypic expression of pericytes and bone cells. Clin Orthop Relat Res. 1998;346:95–103.
Kuznetsov S, Mankani MH, Gronthos S, Satomura K, Bianco P, Robey PG. Circulating skeletal stem cells. J Cell Biol. 2001;153:1133–40.
Lewinson D, Maor G, Rozen N, Rabinovich I, Stahl S, Rachmiel A. Expression of vascular antigens by bone cells during bone regeneration in a membranous bone distraction system. Histochem Cell Biol. 2001;116(5):381–8.
Bronckers A, Sasaguri K, Cavender AC, D'Souza RN, Engelse MA. Expression of Runx2/Cbfa1/Pebp2alphaA during angiogenesis in postnatal rodent and fetal human orofacial tissues. J Bone Miner Res. 2005;20:428–37.
Choi I, Ahn JH, Chung CY, Cho TJ. Vascular proliferation and blood supply during distraction osteogenesis: a scanning electron microscopic observation. J Orthop Res. 2000;18:698–705.
Aronson J, Harrison BH, Stewart CL, Harp JH Jr. The histology of distraction osteogenesis using different external fixators. Clin Orthop Relat Res. 1989;241:106–16.
Aldegheri R, Volino C, Ambito Z, Tessari G, Trivella G. Use of ultrasound to monitor limb lengthening by callotasis. J Pediatr Orthop. 1993;2(1):22–7.
Aronson L. The biology of distraction osteogenesis. Operative principles of Ilizarow. In: Fracture treatment, nonunion, osteomyelitis, lengthening, deformity correction. Baltimore: Williams and Wilkins; 1991.
Aronson J. Experimental assessment of bone regenerate quality during distraction osteogenesis. In: Brighton C, Friedlander GE, Lane JM, editors. Bone formation and repair. Illinois: The Amercian Academy of Orthopedic Surgeons; 1994. p. 441–63.
Aronson J. Temporal and spatial increases in blood flow during distraction osteogenesis. Clin Orthop Relat Res. 1994;301:124–31.
Yasui N, Sato M, Ochi T, Kimura T, Kawahata H, Kitamura Y, et al. Three modes of ossification during distraction osteogenesis in the rat. J Bone Joint Surg Br. 1997;79(5):824–30.
Marks SC, Hermey DC. The structure and development of bone. In: Bilezikian JP, Raisz LG, Rodan GA, editors. Principles of bone biology. San Diego: Academic Press; 1996.
Ortega N, Wang K, Ferrara N, Werb Z, Vu TH. Complementary interplay between matrix metalloproteinase-9, vascular endothelial growth factor and osteoclast function drives endochondral bone formation. Dis Model Mech. 2010;3(3-4):224–35.
Gerber H-P, Vu TH, Ryan AM, Kowalski J, Werb Z, Ferrara N. VEGF couples hypertrophic cartilage remodeling, ossification and angiogenesis during endochondral bone formation. Medicine. 1999;5:623–8.
Ben Shoham A, Rot C, Stern T, Krief S, Akiva A, Dadosh T, et al. Deposition of collagen type I onto skeletal endothelium reveals a new role for blood vessels in regulating bone morphology. Development. 2016;143(21):3933–43.
Maes C, Kobayashi T, Selig MK, Torrekens S, Roth SI, Mackem S, et al. Osteoblast precursors, but not mature osteoblasts, move into developing and fractured bones along with invading blood vessels. Dev Cell. 2010;19:329–44.
Ramasamy S, Kusumbe AP, Wang L, Adams RH. Endothelial Notch activity promotes angiogenesis and osteogenesis in bone. Nature. 2014;507:376–80.
Kusumbe A, Ramasamy SK, Adams RH. Coupling of angiogenesis and osteogenesis by a specific vessel subtype in bone. Nature. 2014;507:323–8.
Doll B. Developmental biology of the skeletal system. In: Hollinger J, Einhorn TA, Doll BA, Sfeir C, editors. Bone tissue engineering. Boca Raton, FL: CRC Press; 2005. p. 3–26.
Frost H. The skeletal intermediary organization. Metab Bone Dis Relat Res. 1983;4(5):281–90.
Hauge EM, Qvesel D, Eriksen EF, Mosekilde L, Melsen F. Cancellous bone remodeling occurs in specialized compartments lined by cells expressing osteoblastic markers. J Bone Miner Res. 2001;16:1575–82.
Parfitt A. Osteonal and hemi-osteonal remodeling: the spatial and temporal framework for signal traffic in adult human bone. J Cell Biochem. 1994;55(3):273–86.
Parfitt AM. Mini-review: osteoclast precursors as leukocytes: importance of the area code. Bone. 1998;23:491–4.
Melsen F, Mosekilde L, Eriksen EF. Spatial distribution of sinusoids in relation to remodeling sites: evidence for specialized sinusoidal structures associated with formative sites. J Bone Miner Res. 1995;10:S209.
Ferguson C, Alpern E, Miclau T, Helms JA. Does adult fracture repair recapitulate embryonic skeletal formation? Mech Dev. 1999;87(1-2):57–66.
Paradis G, Kelly PJ. Blood flow and mineral deposition in canine tibial fractures. J Bone Joint Surg Am. 1975;57(2):220–6.
McCarthy ID, Hughes SPF. Extraction of 99mTC-methylene diphosphonate as a function of bone blood flow. In: Artlet J, Ficat RP, Hungerford DS, editors. Bone circulation. Baltimore: Williams and Wilkins; 1984. p. 167–70.
Santolini E, Goumenos SD, Giannoudi M, Sanguineti F, Stella M, Giannoudis PV. Femoral and tibial blood supply: a trigger for non-union? Injury. 2014;45:1665–73.
Tzioupis C, Giannoudis PV. Prevalence of long-bone non-unions. Injury. 2007;38:S3–9.
Brookes M, Revell WJ. Blood supply of bone: scientific aspects. London: Springer-Verlag; 1998.
McCarthy I. The physiology of bone blood flow: a review. J Bone Joint Surg Am. 2006;88:4–9.
Zamboni L, Pease DC. The vascular bed of red bone marrow. J Ultrastruct Res. 1961;5:65–85.
Cowin S, Cardoso L. Blood and interstitial flow in the hierarchical pore space architecture of bone tissue. J Biomech. 2015;48(5):842–54.
Montgomery R, Sutker BD, Bronk JT, Smith SR, Kelly PJ. Interstitial fluid flow in cortical bone. Microvasc Res. 1988;35:295–307.
Lam H, Brink P, Qin YX. Skeletal nutrient vascular adaptation induced by external oscillatory intramedullary fluid pressure intervention. J Orthop Surg Res. 2010;5. https://doi.org/10.1186/1749-1799X-1185-1118.
Grüneboom A, Hawwari I, Weidner D, Culemann S, Müller S, Henneberg S, et al. A network of trans-cortical capillaries as mainstay for blood circulation in long bones. Nat Metab. 2019. https://doi.org/10.1038/s42255-018-0016-5.
Delp MD, Evans MV, Duan C. Effects of aging on cardiac output, regional blood flow, and body composition in Fischer-344 rats. J Appl Physiol. 1998;85(5):1813–22.
Prisby R. Mechanical, hormonal and metabolic influences on blood vessels, blood flow and bone. J Endocrinol. 2017;235:R77–R100.
Kwon R, Meays DR, Tang WJ, Frangos JA. Microfluidic enhancement of intramedullary pressure increases interstitial fluid flow and inhibits bone loss in hindlimb suspended mice. J Bone Mineral Res. 2010;25:1798–807.
Thomas I, Gregg PJ, Walder DN. Intra-osseous phlebography and intramedullary pressure in the rabbit femur. J Bone and Joint Surg. 1982;64:239–42.
Piekarski K, Munro M. Transport mechanism operating between blood supply and osteocytes in long bones. Nature. 1977;269:80–2.
Wolff J. The law of bone remodeling. Berlin: Springer; 1986.
Qin Y, Kaplan T, Saldanha A, Rubin C. Fluid pressure gradients, arising from oscillations in intramedullary pressure, is correlated with the formation of bone and inhibition of intracortical porosity. J Biomech. 2003;36(10):1427–37.
Revell WJ, Brookes M. Haemodynamic changes in the rat femur and tibia following femoral vein ligation. J Anat. 1994;184:625–33.
Kelly P, Bronk JT. Venous pressure and bone formation. Microvasc Res. 1990;39:364–75.
Hu M, Cheng J, Bethel N, Serra-Hsu F, Ferreri S, Lin L, et al. Interrelation between external oscillatory muscle coupling amplitude and in vivo intramedullary pressure related bone adaptation. Bone. 2014;66:178–81.
Johnson DL, McAllister TN, Frangos JA. Fluid flow stimulates rapid and continuous release of nitric oxide in osteoblasts. Am J Phys. 1996;271:E205–8.
McAllister TN, Du T, Frangos JA. Fluid shear stress stimulates prostaglandin and nitric oxide release in bone marrow-derived preosteoclast-like cells. Biochem Biophys Res Commun. 2000;270:643–8.
McAllister TN, Frangos JA. Steady and transient fluid shear stress stimulate NO release in osteoblast through distinct biochemical pathways. J Bone Miner Res. 1999;14:930–6.
Zaman G, Pitsillides AA, Rawlinson SC, Suswillo RF, Mosley JR, Cheng MZ, et al. Mechanical strain stimulates nitric oxide production by rapid activation of endothelial nitric oxide synthase in osteocytes. J Bone Miner Res. 1999;14(7):1123–31.
Hikiji H, Shin WS, Oida S, Takato T, Koizumi T, Toyo-Oka T. Direct action of nitric oxide on osteoblastic differentiation. FEBS Lett. 1997;410:238–42.
Riancho JA, Salas E, Zarrabeitia MT, Olmos JM, Amado JA, Fernandez-Luna JL, et al. Expression and functional role of nitric oxide synthase in osteoblast-like cells. J Bone Miner Res. 1995;10:439–46.
Kasten TP, Collin-Osdoby P, Patel N, Osdoby P, Krukowski M, Misko TP, et al. Potentiation of osteoclast bone-resorption activity by inhibition of nitric oxide synthase. Proc Natl Acad Sci U S A. 1994;91:3569–73.
Akamine T, Jee WS, Ke HZ, Li XJ, Lin BY. Prostaglandin E2 prevents bone loss and adds extra bone to immobilized distal femoral metaphysis in female rats. Bone. 1991;13:11–22.
Alam A, Gallagher A, Shankar V, Ghate MA, Datta HK, Huang CL, et al. Endothelin inhibits osteoclastic bone resorption by a direct effect on cell motility: implications for the vascular control of bone resorption. Endocrinology. 1992;130:3617–24.
Fiorelli G, Orlando C, Benvenuti S, Franceschelli F, Bianchi S, Pioli P, et al. Characterization, regulation, and function of specific cell membrane receptors for insulin-like growth factor I on bone endothelial cells. J Bone Miner Res. 1994;9:329–37.
Shinozuka K, Hashimoto M, Masumura S, Bjur RA, Westfall DP, Hattori K. In vitro studies of release of adenine nucleotides and adenosine from rat vascular endothelium in response to alpha 1-adrenoceptor stimulation. Br J Pharmacol. 1994;113:1203–8.
Zhang Y, Fujita N, Oh-hara T, Morinaga Y, Nakagawa T, Yamada M, et al. Production of interleukin-11 in bone-derived endothelial cells and its role in the formation of osteolytic bone metastasis. Oncogene. 1998;16(6):693–703.
Kage K, Fujita N, Oh-hara T, Ogata E, Fujita T, Tsuruo T. Basic fibroblast growth factor induces cyclooxygenase-2 expression in endothelial cells derived from bone. Biochem Biophys Res Commun. 1999;254:259–63.
Ishida A, Fujita N, Kitazawa R, Tsuruo T. Transforming growth factor-beta induces expression of receptor activator of NF-kappa B ligand in vascular endothelial cells derived from bone. J Biol Chem. 2002;277:26217–24.
Brandi ML, Collin-Osdoby P. Vascular biology and the skeleton. J Bone Miner Res. 2006;21(2):183–92.
Gonzalez C, Rosas-Hernandez H, Jurado-Manzano B, Ramirez-Lee MA, Salazar-Garcia S, Martinez-Cuevas PP, et al. The prolactin family hormones regulate vascular tone through NO and prostacyclin production in isolated rat aortic rings. Acta Pharmacol Sin. 2015;36:572–86.
Wen Q, Lee KO, Sim SZ, Xu XG, Sim MK. Des-aspartate-angiotensin I causes specific release of PGE2 and PGI2 in HUVEC via the angiotensin AT1 receptor and biased agonism. Eur J Pharmacol. 2015;768:173–81.
Gutterman D, Chabowski DS, Kadlec AO, Durand MJ, Freed JK, Ait-Aissa K, et al. The human microcirculation: regulation of flow and beyond. Circ Res. 2016;118(1):157–72.
Kiel MJ, Morrison SJ. Uncertainty in the niches that maintain haematopoietic stem cells. Nat Rev Immunol. 2008;8:290–301.
Goldsby RA, Kindt TJ, Osborne BA. Immunology. 4th ed. New York: W.H. Freeman and Company; 2000.
Zhu L, Emerson SG. A new bone to pick: osteoblasts and the haematopoietic stem-cell niche. BioEssays. 2004;26:595–9.
Fliedner T, Graessle D, Paulsen C, Reimers K. Structure and function of bone marrow hemopoiesis: mechanisms of response to ionizing radiation exposure. Cancer Biother Radiopharm. 2002;17(4):405–26.
Schofield R. The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells. 1978;4:7–25.
Yin T, Li L. The stem cell niches in bone. J Clin Invest. 2006;116:1195–201.
Arai F, Hirao A, Ohmura M, Sato H, Matsuoka S, Takubo K, et al. Tie2/angiopoietin-1 signaling regulates hematopoietic stem cell quiescence in the bone marrow niche. Cell. 2004;118(2):149–61.
Nilsson SK, Johnston HM, Coverdale JA. Spatial localization of transplanted hemopoietic stem cells: inferences for the localization of stem cell niches. Blood. 2001;97:2293–9.
Gong JK. Endosteal marrow: a rich source of hematopoietic stem cells. Science. 1978;199(4336):1443–5.
Heissig B, Hattori K, Dias S, Friedrich M, Ferris B, Hackett NR, et al. Recruitment of stem and progenitor cells from the bone marrow niche requires MMP-9 mediated release of kit-ligand. Cell. 2002;109(5):625–37.
Arai F, Suda T. Maintenance of quiescent hematopoietic stem cells in the osteoblastic niche. Ann N Y Acad Sci. 2007;1106:41–53.
Calvi L, Link DC. The hematopoietic stem cell niche in homeostasis and disease. Blood. 2015;126(22):2443–51.
Schiff L, Poncz M, Bergman A, Frenette PS. Megakaryocytes regulate hematopoietic stem cell quiescence through CXCL4 secretion. Nat Med. 2014;20:1315–20.
Acar M, Kocherlakota KS, Murphy MM, Peyer JG, Oguro H, Inra CN, et al. Deep imaging of bone marrow shows non-dividing stem cells are mainly perisinusoidal. Nature. 2015;526:126–30.
Dennis JE, Charbord P. Origin and differentiation of human and murine stroma. Stem Cells. 2002;20(3):205–14.
Moore A, Blake GM, Taylor KA, Rana AE, Wong M, Chen P, et al. Assessment of regional changes in skeletal metabolism following 3 and 18 months of teriparatide treatment. J Bone Miner Res. 2010;25:960–7.
Taichman R. Blood and bone: two tissues whose fates are intertwined to create the hematopoietic stem-cell niche. Blood. 2005;105(7):2631–9.
Gurkan U, Akkus O. The mechanical environment of bone marrow: a review. Ann Biomed Eng. 2008;36:1978–91.
Brookes M, Lloyd EG. Marrow vascularization and oestrogen-induced endosteal bone formation in mice. J Anat. 1961;95:220–8.
Bianco P, Riminucci M, Kuznetsov S, Robey PG. Multipotential cells in the bone marrow stroma: regulation in the context of organ physiology. Crit Rev Eukaryot Gene Expr. 1999;9(2):159–73.
Kiel M, Yilmaz OH, Iwashita T, Yilmaz OH, Terhorst C, Morrison SJ. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121:1109–21.
Nombela-Arrieta C, Pivarnik G, Winkel B, Canty KJ, Harley B, Mahoney JE, et al. Quantitative imaging of haematopoietic stem and progenitor cell localization and hypoxic status in the bone marrow microenvironment. Nat Cell Biol. 2013;15(5):533–43.
Ramasamy S, Kusumbe AP, Schiller M, Zeuschner D, Bixel MG, Milia C, et al. Blood flow controls bone vascular function and osteogenesis. Nat Commun. 2016;7. https://doi.org/10.1038/ncomms13601.
Wickramasinghe S. Observations on the ultrastructure of sinusoids and reticular cells in human bone marrow. Clin Lab Haematol. 1991;13:263–78.
Hudson G, Yoffey JM. The passage of lymphocytes through the sinusoidal endothelium of guinea-pig bone marrow. Proc R Soc Lond B Biol Sci. 1966;165:486–96.
Weiss L. The structure of bone marrow. Functional interrelationships of vascular and hematopoietic compartments in experimental hemolytic anemia: an electron microscopic study. J Morphol. 1965;117:467–537.
De Bruyn P, Michelson S, Thomas TB. The migration of blood cells of the bone marrow through the sinusoidal wall. J Morphol. 1971;133(4):417–37.
Campbell F. Ultrastructural studies of transmural migration of blood cells in the bone marrow of rats, mice and guinea pigs. Am J Anat. 1972;135(4):521–35.
Tavassoli M, Crosby WH. Fate of the nucleus of the marrow erythroblast. Science. 1973;179(4076):912–3.
Giordano G, Lichtman MA. Marrow cell egress. The central interaction of barrier pore size and cell maturation. J Clin Invest. 1973;52(5):1154–64.
Itkin T, Gur-Cohen S, Spencer JA, Schajnovitz A, Ramasamy SK, Kusumbe AP, et al. Distinct bone marrow blood vessels differentially regulate haematopoiesis. Nature. 2016;532:323–8.
Ellis S, Grassinger J, Jones A, Borg J, Camenisch T, Haylock D, et al. The relationship between bone, hemopoietic stem cells, and vasculature. Blood. 2011;118:1516–24.
Haylock D, Nilsson SK. The role of hyaluronic acid in hemopoietic stem cell biology. Regen Med. 2006;1:437–45.
Füreder W, Krauth MT, Sperr WR, Sonneck K, Simonitsch-Klupp I, Müllauer L, et al. Evaluation of angiogenesis and vascular endothelial growth factor expression in the bone marrow of patients with aplastic anemia. Am J Pathol. 2006;168(1):123–30.
Lapidot T, Dar A, Kollet O. How do stem cells find their way home? Blood. 2005;106:1901–10.
Dabrowski Z, Szyguła Z, Miszta H. Do changes in bone marrow pressure contribute to the egress of cell from bone marrow? Acta Phys Pol A. 1981;32:729–36.
Martin C, Burdon PC, Bridger G, Gutierrez-Ramos JC, Williams TJ, Rankin SM. Chemokines acting via CXCR2 and CXCR4 control the release of neutrophils from the bone marrow and their return following senescence. Immunity. 2003;19(4):583–93.
Penninx B, Guralnik JM, Onder G, Ferrucci L, Wallace RB, Pahor M. Anemia and decline in physical performance among older persons. Am J Med. 2003;115(2):104–10.
Makovey J, Macara M, Chen JS, Hayward CS, March L, Sambrook PN. High osteoporotic fracture risk and CVD risk co-exist in postmenopausal women. Bone. 2013;52:120–5.
Gaudio A, Xourafa A, Rapisarda R, Castellino P, Signorelli SS. Peripheral artery disease and osteoporosis: not only age-related (review). Mol Med Rep. 2018. https://doi.org/10.3892/mmr.2018.9512.
Bridgeman G, Brookes M. Blood supply to the human femoral diaphysis in youth and senescence. J Anat. 1996;188:611–21.
Spencer H, Hausinger A, Laszlo D. The calcium tolerance test in senile osteoporosis. J Am Geriatr Soc. 1954;2(1):19–25.
Vogt M, Cauley JA, Kuller LH, Nevitt MC. Bone mineral density and blood flow to the lower extremities: the study of osteoporotic fractures. J Bone Miner Res. 1997;12(2):283–9.
Trueta J, Little K. The vascular contribution to osteogenesis. II. Studies with the electron microscope. J Bone Joint Surg Br. 1960;42-B:367–76.
Trueta J, Morgan JD. The vascular contribution to osteogenesis. I. Studies by the injection method. J Bone Joint Surg Br. 1960;42-B(1):97–109.
Griffith J, Wang YX, Zhou H, Kwong WH, Wong WT, Sun YL, et al. Reduced bone perfusion in osteoporosis: likely causes in an ovariectomy rat model. Radiology. 2010;254:739–46.
Bick E, Copel JW. The senescent human vertebra; contribution to human osteogeny. III J Bone Joint Surg Am. 1952;34-A(1):110–4.
Lu C, Hansen E, Sapozhnikova A, Hu D, Miclau T, Marcucio RS. Effect of age on vascularization during fracture repair. J Orthop Res. 2008;26:1384–9.
Brenneise CV, Squier CA. Blood flow in maxilla and mandible of normal and atherosclerotic rhesus monkeys. J Oral Pathol. 1985;14(10):800–8.
Mahlknecht U, Kaiser S. Age-related changes in peripheral blood counts in humans. Exp Ther Med. 2010;1(6):1019–25.
den Elzen W, Willems JM, Westendorp RC, de Craen AJ, Assendelft WJ, Gussekloo J. Effect of anemia and comorbidity on functional status and mortality in old age: results from the Leiden 85-plus study. CMAJ. 2009;181(3-4):151–7.
Chaves P. Functional outcomes of anemia in older adults. Semin Hematol. 2008;45(4):255–60.
Balducci L. Epidemiology of anemia in the elderly: information on diagnostic evaluation. J Am Geriatr Soc. 2003;51:S2–9.
Gabrilove J. Anemia and the elderly: clinical considerations. Best Pract Res Clin Haematol. 2005;3:417–22.
Ohta M. Management of anemia in the elderly. JMAJ. 2009;52:219–23.
Hayflick L. The limited in vitro lifetime of human diploid cell strains. Exp Cell Res. 1965;37:614–36.
Lipschitz D, Udupa KB, Milton KY, Thompson CO. Effect of age on hematopoiesis in man. Blood. 1984;63(3):502–9.
Nilsson-Ehle H, Jagenburg R, Landahl S, Svanborg A. Blood haemoglobin declines in the elderly: implications for reference intervals from age 70 to 88. Eur J Haematol. 2000;65:297–305.
Young D. Pre-analytical variability in the elderly. Geriatr Clin Chem. 1994:19–39.
Carpenter M, Kendall RG, O'Brien AE, Chapman C, Sebastian JP, Belfield PW, et al. Reduced erythropoietin response to anaemia in elderly patients with normocytic anaemia. Eur J Haematol. 1992;49(3):119–21.
Kario K, Matsuo T, Kodama K, Nakao K, Asada R. Reduced erythropoietin secretion in senile anemia. Am J Hematol. 1992;41(4):252–7.
Nafziger J, Pailla K, Luciani L, Andreux JP, Saint-Jean O, Casadevall N. Decreased erythropoietin responsiveness to iron deficiency anemia in the elderly. Am J Hematol. 1993;43(3):172–6.
Timaffy M. A comparative study of bone marrow function in young and old individuals. Gerontol Clin (Basel). 1962;4:13–8.
Compston JE. Bone marrow and bone: a functional unit. J Endocrinol. 2002;173(3):387–94.
Kita K, Kawai K, Hirohata K. Changes in bone marrow blood flow with aging. J Orthop Res. 1987;5(4):569–75.
Vogel J. Hematologic problems of the aged. Mt Sinai J Med. 1980;47:150–65.
Kusumbe A, Ramasamy SK, Itkin T, Mäe MA, Langen UH, Betsholtz C, et al. Age-dependent modulation of vascular niches for haematopoietic stem cells. Nature. 2016;532:380–4.
Méndez-Ferrer S, Michurina TV, Ferraro F, Mazloom AR, Macarthur BD, Lira SA, et al. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010;466(7308):829–34.
Ding L, Saunders TL, Enikolopov G, Morrison SJ. Endothelial and perivascular cells maintain haematopoietic stem cells. Nature. 2012;481(7382):457–62.
Zsebo K, Williams DA, Geissler EN, Broudy VC, Martin FH, Atkins HL, et al. Stem cell factor is encoded at the Sl locus of the mouse and is the ligand for the c-kit tyrosine kinase receptor. Cell. 1990;63:213–24.
Chambers S, Shaw CA, Gatza C, Fisk CJ, Donehower LA, Goodell MA. Aging hematopoietic stem cells decline in function and exhibit epigenetic dysregulation. PLoS Biol. 2007;5(8):e201.
Dykstra B, Olthof S, Schreuder J, Ritsema M, de Haan G. Clonal analysis reveals multiple functional defects of aged murine hematopoietic stem cells. J Exp Med. 2011;208:2691–703.
Davis TRC, Wood MB. Bone blood flow. In: Wood MB, Gilber A, editors. Microvascular bone reconstruction. London: The Livery House; 1997. p. 13–7.
Mazo I, von Andrian UA. Adhesion and homing of blood-borne cells in bone marrow microvessels. J Leukoc Biol. 1999;66:25–32.
Gruver AL, Hudson LL, Sempowski GD. Immunosenescence of ageing. J Pathol. 2007;211(2):144–56.
Cauley J, Thompson DE, Ensrud KC, Scott JC, Black D. Risk of mortality following clinical fractures. Osteoporos Int. 2000;11:556–61.
Bak B, Andreassen TT. The effect of aging on fracture healing in the rat. Calcif Tissue Int. 1989;45:292–7.
Meyer RJ, Meyer MH, Tenholder M, Wondracek S, Wasserman R, Garges P. Gene expression in older rats with delayed union of femoral fractures. J Bone Joint Surg Am. 2003;85-A(7):1243–54.
Lu C, Miclau T, Hu D, Hansen E, Tsui K, Puttlitz C, et al. Cellular basis for age-related changes in fracture repair. J Orthop Res. 2005;23:1300–7.
Stabley J, Prisby RD, Behnke BJ, Delp MD. Type 2 diabetes alters bone and marrow blood flow and vascular control mechanisms in the ZDF rat. J Endocrinol. 2015;225(1):47–58.
Prisby R, Swift JM, Bloomfield SA, Hogan HA, Delp MD. Altered bone mass, geometry and mechanical properties during the development and progression of type 2 diabetes in the Zucker diabetic fatty rat. J Endocrinol. 2008;199:379–88.
Kapitola J, Kubícková J. Estradiol benzoate decreases the blood flow through the tibia of female rats. Exp Clin Endocrinol. 1990;96(1):117–20.
Kapitola J, Andrle J, Kubícková J. Possible participation of prostaglandins in the increase in the bone blood flow in oophorectomized female rats. Exp Clin Endocrinol. 1994;102:414–6.
Egrise D, Martin D, Neve P, Vienne A, Verhas M, Schoutens A. Bone blood flow and in vitro proliferation of bone marrow and trabecular bone osteoblast-like cells in ovariectomized rats. Calc Tissue Int. 1992;50(4):336–41.
Hansen VB, Forman A, Lundgaard A, Aalkjær C, Skajaa K, Hansen ES. Effects of oophorectomy on functional properties of resistance arteries isolated from the cancellous bone of the rabbit femur. J Orthop Res. 2001;19(3):391–7.
Roche B, Vanden-Bossche A, Malaval L, Normand M, Jannot M, Chaux R, et al. Parathyroid hormone 1-84 targets bone vascular structure and perfusion in mice: impacts of its administration regimen and of ovariectomy. J Bone Miner Res. 2014;29(7):1608–18.
Prisby R, Behnke BJ, Allen MR, Delp MD. Effects of skeletal unloading on the vasomotor properties of the rat femur principal nutrient artery. J Appl Physiol. 2015;118(8):980–8.
Stabley J, Prisby RD, Behnke BJ, Delp MD. Chronic skeletal unloading of the rat femur: mechanisms and functional consequences of vascular remodeling. Bone. 2013;57(2):355–60.
Prisby R, Menezes T, Campbell J. Vasodilation to PTH (1-84) in bone arteries is dependent upon the vascular endothelium and is mediated partially via VEGF signaling. Bone. 2013;54:68–75.
Prisby R, Guignandon A, Vanden-Bossche A, Mac-Way F, Linossier MT, Thomas M, et al. Intermittent PTH(1-84) is osteoanabolic but not osteoangiogenic and relocates bone marrow blood vessels closer to bone-forming sites. J Bone Miner Res. 2011;26(11):2583–96.
Lee S, Prisby RD. Short-term intermittent PTH 1-34 administration and bone marrow blood vessel ossification in mature and middle-aged C57BL/6 mice. Bone Rep. 2019;10. https://doi.org/10.1016/j.bonr.2018.100193.
Guers J, Prisby RD, Edwards DG, Lennon-Edwards S. Intermittent parathyroid hormone administration attenuates endothelial dysfunction in old rats. J Appl Physiol. 2017;122(1):76–81.
Yao Z, Lafage-Proust MH, Plouët J, Bloomfield S, Alexandre C, Vico L. Increase of both angiogenesis and bone mass in response to exercise depends on VEGF. J Bone Miner Res. 2004;19(9):1471–80.
Stabley J, Moningka NC, Behnke BJ, Delp MD. Exercise training augments regional bone and marrow blood flow during exercise. Med Sci Sports Exerc. 2014;46(11):2107–12.
Boss J, Misselevich I. Osteonecrosis of the femoral head of laboratory animals: the lessons learned from a comparative study of osteonecrosis in man and experimental animals. Vet Pathol. 2003;40(4):345–54.
Yamamoto T, Irisa T, Sugioka Y, Sueishi K. Effects of pulse methylprednisolone on bone and marrow tissues: corticosteroid-induced osteonecrosis in rabbits. Arthritis Rheum. 1997;40:2055–64.
Wang G, Sweet DE, Reger SI, Thompson RC. Fat-cell changes as a mechanism of avascular necrosis of the femoral head in cortisone-treated rabbits. J Bone Joint Surg Am. 1977;59(6):729–35.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The author declares that she has no conflicts of interest.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Prisby, R.D. The Clinical Relevance of the Bone Vascular System: Age-Related Implications. Clinic Rev Bone Miner Metab 17, 48–62 (2019). https://doi.org/10.1007/s12018-019-09259-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12018-019-09259-x