Abstract
Objectives
An immunotherapy was found to be effective in achieving long-term survival in some lung cancer patients. It has emerged to searching for new immune biomarkers for select the best candidates to this therapy. It is suggested that cancer stem cells (CSCs) are responsible for tumor initiation, maintenance and its metastatic potential. However, a role of CSCs in escape of cancer from immunosurveillance is unknown. The aim of the study was assess the phenotype of putative CSCs and to examine the expression of PD-L1 on CSCs in metastatic lymph nodes (LNs) in lung cancer patients.
Material and Methods
Flow cytometry was used for CSCs evaluation in peripheral blood and EBUS/TBNA aspirates from N1,N2 lymph nodes in lung cancer patients.
Results
Of 30 patients the LNs metastases were confirmed in 18 patients. We noticed presence of PD-L1 on putative lung CSCs- CD133 + EpCAM+ cells. A higher percentage of CD133 + EpCAM+PD-L1+ cells was observed in patients with metastatic in LNs- median value = 4.38% than in patients without LNs metastases– median value = 0,015% (p < 0.05). The highest proportion of PD-L1+ CSCs was found in adenocarcinoma patients and in those with oncogene addiction what indicate an particular biology of this type of lung cancer.
Conclusion
The presence of CSCs with expression of PD-L1 in the metastatic LNs might suggest their immunogenic potential. EBUS/TBNA is commonly used in diagnosis and staging of lung cancer, so the analysis of the cells in metastatic LNs may fit in “immunoscoring” before immunotherapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lung cancer is the most lethal cancer worldwide with 1.9 million estimated new cases each year and a poor prognosis with 5-year survival rates of approximately 15% [1]. An advanced stage of the disease at the time of diagnosis, observed in the majority of cases does not allow for introduction of radical treatment. Over the last 10 years an immunotherapy was a breakthrough in achieving long-term survival. The essence of modern immunotherapy is to improve the host antitumor immune defense. Nowadays, no specific clinical test exists to assess antitumor immunity evaluation in clinical settings.
The anticancer reaction is created in regional lymph nodes (LNs) and their samples may be useful in cancer immunological studies [2, 3]. Antigen presenting cells (APC) in lymph nodes are able to activate cytotoxic lymphocytes after recognition of cancer antigens. The outcome of a T cell-antigen encounter is modulated by T cell checkpoints such as cytotoxic T lymphocytes-associated protein 4 (CTLA-4) and programmed cell death protein (PD-1); both expressed on T-cells. PD-L1 (a ligand for PD-1)– is a molecule expressed in many solid tumors including lung cancer. As a consequence interaction between PD-1 and its ligands attenuate immune response [4]. It has been reported that PD-L1 is overexpressed in around 50% of non-small cell lung cancer (NSCLC) patients and is associated with poor prognosis [4, 5].
The cancer stem cell hypothesis states that cancers arise from genetic or epigenetic changes in tissue stem or progenitor cells [6]. Such aberration results in formation of cancer stem cells (CSCs). CSCs are the results of genetic and epigenetic changes in normal stem cells, progenitor cells or differentiated cells. It is believed that CSCs determine unrestricted growth of tumors and their different morphology.
Metastatic CSCs are released from primary tumor mass and responsible for developing metastases and invasion of distant places such as different organs and lymph nodes [7]. Isolation and characterization of CSCs represent a very attractive challenge, as they may comprise both the phenotypic and genetic composition on primary CSCs. The enumeration of metastatic CSCs and circulating CSCs might be relevant as a surrogate marker for tumor growth and aggressiveness [8,9,10,11]. CSCs are usually isolated according to presence of particular markers: CD133, CD44, CD90, EpCAM (epithelial cell adhesion molecule). These cells were able to form spheroid mass in vitro and initiate tumor growth in immunodeficient mice [12,13,14,15].
Endobronchial Ultrasound Trans-Bronchial Needle Aspiration (EBUS/TBNA) is becoming increasingly recognized as a useful, noninvasive method in the initial assessment of lung cancer staging with a high sensitivity (88–95%) as well as specificity (100%) [16, 17]. EBUS/TBNA, paratracheal lymph node stations (levels 2R, 2 L, 4R, 4 L), the subcarinal lymph node (level 7), the hilar (level 10), interlobar (level 11) and lobar (level 12), lymph nodes can be assessed.
The aim of the study was:
-
1)
To develop the method to identify circulating CSCs in peripheral blood in lung cancer patients.
-
2)
To identify metastatic CSCs and examine the expression of PD-L1 on metastatic CSCs in LNs in lung cancer patients with assessment of differences between metastatic LNs and free of cancer LNs.
Patients
We investigated consecutive enrolled patients during lung cancer diagnosis, before treatment and next only patients with histologically confirmed lung cancers were included in the study groups. The actual TNM classification of disease stage was used [16]. The following exclusion criteria was applied: infection, chronic autoimmune diseases, prior anti-cancer therapy. The procedures were accepted by Ethic Committee of Medical University of Warsaw and patients signed their consent. Due to methodological approach of the study peripheral blood and LNs aspirates were collected from different lung cancer patients.
Peripheral Blood Analysis
The group consisted of 21 patients (5 women, 16 men, mean age 66.0 ± 9.3 years, all smokers with mean pack years- 40.0 ± 18.0). Histological types distribution was as follow: SCLC (small cell lung cancer)-7; SQCLC (squamous cell carcinoma)-2; ADC (adenocarcinoma)- 8; NOS (not otherwise specified subtype)-4 and stage distribution: IB/II/IIIA/IIIB/IVA/IVB- 0/1/3/6/4/7 respectively. Five healthy volunteers in the mean age 58 years old served as control group.
LNs Analysis
The group consisted of 30 patients and it was the main group of our investigation. The characteristic of this group is summarized in Table 1.
Methods
1 ml of peripheral blood was obtained and placed in tubes containing K2EDTA and processed for flow cytometry.
LNs group 7 or 10 or 12 aspirates were obtained during routine EBUS/TBNA procedure of lung cancer diagnosis. After diagnostic aspiration the additional sample was taken for flow cytometry analysis. About 1 ml of LNs aspirate was diluted in 0.9% NaCl, collected in tubes containing K2EDTA and processed for flow cytometry.
To identify lung CSCs flow cytometry and staining using monoclonal antibodies targeting the cell-surface expression of EpCAM and CD133 were used (BD, USA). Additionally anti PD-L1 antibody was applied to assess PD-L1 presence on putative lung CSCs in LNs apsirates (BD, USA).
Briefly, to each cytometric tube 100 μL of LNs aspirate or peripheral blood and 4 μl of specific monoclonal antibodies were added. After 15 min of incubation in the dark, at room temperature, erythrocytes were lysed with lysing solution for 10 min and washed with 2% newborn calf serum in physiological buffer solutions (PBS). The cells were subsequently fixed in 0.5% paraformaldehyde. The samples were processed by the FACS Canto II flow cytometer (BD, USA). Geometric mean fluorescence (GMF) intensity of PD-L1 on CSCs was measured.
Dot plots for the representative LNs immunocytochemical analysis are shown in the Fig. 1.
Analysis of PD-L1+ lung cancer stem cells (CSCs)in lymph nodes aspirates by flow cytometry, a/ CSCs among other cells- marked in red, b/CSCs in red vs. lymphocytes in green, c/confirmation of non-lymphoid origin of CSCs by CD45 marker- CSCsin blue, lymphocytes in green, d/CD133 + EpCAM+cells gate, e/CSCs PD-L1 +
Statistical Analysis
The Statistica 13.1 software (StatSoft) was used for a statistical analysis. For group comparison, the Mann–Whitney test was used. Relations between quantitative variables were analyzed by Spearman correlations. A p < 0.05 was considered as statistically significant.
Results
Peripheral Blood Analysis
Analysis of peripheral blood revealed the presence of CD133+ cells in each sample, EpCAM+ cells in 19/21 samples and CD133 + EpCAM+ cells in 18/21 (86%) samples. In our opinion only the latter population is representative for CSCs. In healthy individuals we found only CD133+ cells in proportion up to 0.02%. The median (p25-p75) proportion of cells was as follow: CD133 + EpCAM = 3,6% (0,1-18,7%) CD133-EpCAM+ = 1,2% (0–5,9%), CD133 + EpCAM+ = 1,3% (0–8,2%). We observed a higher percentage of putative CSCs (CD133 + EpCAM+) in patients with than without metastases (median values (p25–75)- 1,3% (0–8,2%) versus 1,05% (0–3,4%), difference not significant). The highest proportion was observed in adenocarcinoma vs. NOS vs. squamous cell carcinoma (2,25% (0,1-3,8%) vs.1,65% (0,8-3,2%) vs.1,15% (0–1,85%), difference not significant).
Lymph Nodes Aspirates
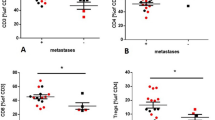
Analysis of LNs revealed the presence of EpCAM+ cells in each sample, CD133+ cells in 28/30 samples. CD133 + EpCAM+ cells were found in 25 patients (83%) whereas CD133 + EpCAM+PD-L1+ were found in 23 patients (77%). The proportion of each cell population is presented in the Table 2. Higher percentage of CD133 + EpCAM+ cells and CD133 + EpCAM+PD-L1+ were found in patients in metastatic LNs than in free LNs (p < 0.05), Table 2, Fig. 2. The mean GMF of PD-L1 on CSCs differed significantly between LNs with metastases when compared with free LNs (Table 2).
Among patients with confirmed metastases ADC subtype was recognized in 13/18 (73%), SQCLC in 1/18 (5%) and NOS in 4/17 (22%) patients. The fraction of CD133 + EpCAM+ and CD133 + EpCAM+PD-L1+ cells varied between the histopathological types of non-small cell lung cancer, and was increased in the LNs aspirates of patients with adenocarcinoma (because of disproportion in number of samples statistical analysis was not performed, Fig. 3). No significant difference in cell proportion between LNs stations was found. When group N2 was compared with group N1 we found higher proportion of CD133 + EpCAM+PD-L1+ in N1 group than in N2, difference not significant (4.09% (0.03–7.15) vs. 1.75% (0.01–4.54)). We did not find significant correlation between stage of the disease and cell proportions. However, the percentage of CD133 + EpCAM+PD-L1+ was higher in patients with IV stage than in lower stages of the disease (Fig. 4).
We observed significant correlation between proportion of CD133 + EpCAM+PD-L1+ cells and pack years smoked (r = 0.4, p < 0.05) and between GMF of PD-L1 and pack years smoked (r = 0.4, p < 0.05, Fig. 5).
Molecular analysis was performed in 10 patients with adenocarcinoma or NOS (the reason that it was done not in all patients has a clinical context). In four patients activated mutation of EGFR was confirmed, in two other patients- rearrangement of ALK/EML4 and in one patient KRAS mutation was found. These groups are too small for proper statistical analysis however, we observed significantly higher proportion of CD133 + EpCAM+ and CD133 + EpCAM+PD-L1+ in patients with confirmed oncogene addiction.
Discussion
We confirmed the presence of putative lung CSCs in peripheral blood and in LNs aspirates in lung cancer patients. Previously, expression of the stem cell markers CD133 and EpCAM was confirmed in tumor lung tissue and in the blood by flow cytometry [18]. CSCs might be prognostic factor in lung cancer, especially as a marker for tumor growth and aggressiveness. It has been reported, in other studies, that CSCs counts correlate with a poorer prognosis in lung cancer and in other tumor types [19, 20]. The findings of our study clearly show that the highest percentage of putative lung cancer stem cells is observed in patients with advanced-stage disease. However, we detected the presence of lung CSCs in LNs aspirates of patients with I and II stage of disease. The percentage of CSCs was lower than those observed in patients with IV stage of the disease. To assess their impact on disease progression and aggressiveness monitoring as well as response to the treatment the repeatable CSCs quantification could be applied. Retrospective study performed by Gwozdz et al. demonstrated that the presence of occult micrometastases in the mediastinal LNs was associated with reduced survival in stage I and II NSCLC patients due to tumor recurrence [21]. Their and our study demonstrated the usefulness of EpCAM marker for detection of early LNs cancer invasion.
We found expression of PD-L1 on CD133 + EpCAM+ cells. EBUS TBNA may be a robust method for the evaluation of tumor PD-L1 expression in lung cancer [22]. The best to our knowledge our study is the first report in which PD-L1 presence on lung CSCs was examined. Schatton at al. reported evidence that melanoma CSCs downregulate activation of T cells [23]. Expression of PD-L1 was reported in head and neck carcinoma CSCs [24]. In concordance with these results, our study suggests the possibility that subsets of CSCs may be involved in the PD-1/PD-L1 interaction. We assume that CSCs can be largely responsible for the unfavorable modification of the immune system, which may be explained by the presence of PD-L1 on CSCs. This fits well with the supposed function attributed to CSCs i.e. their ability to survive in order to provide new, more differentiated neoplastic cells that will allow the growth and expansion of the tumor in the body [25]. The highest percentage of PD-L1 CSCs was observed in patients with IV stage of lung cancer. Consistent with our study Liu et al. reported higher PD-L1 expression in LNs in patients with advanced TNM stage and lymphatic metastases [26]. Unfortunately lower proportion of PD-L1 CSCs was observed in N2 than in N1 LNs. It only proves that biology of cancer is unpredictable, what is a nature of malignancy. The benefit of anti-PD-1/PD-L1 therapies in NSCLC was documented [5]. However, despite impressive outcomes in some patients, many others showed no response [27]. Our finding that CD133 + EpCAM+ CSCs specifically express PD-L1 may indicate the new markers able to identify patients for anti-PD-1/PD-L1 therapies.
The highest percentage of CD133 + EpCAM+ cells and CD133 + EpCAM+PD-L1+ cells was observed in lung ADC patients. PD-L1 expression in lung ADC tumor samples was reported by Kim et al. [28]. They demonstrated that PD-L1 expression is associated with epithelial-to-mesenchymal transition (EMT) phenotype in lung ADC. It is likely that EMT, immune cell recruitment and PD-L1 expression might be closely related to each other in EGFR-mutated ADC. Our findings on correlation of PD-L1+ cells proportion with molecular alterations may confirm this assumption. The new classification of ADC shows the heterogeneity of this type of NSCLC [29, 30]. ADC is the most common lung cancer subtype to show genomic changes and somatic mutations. It might have significance in case of CSCs. CSCs may suffer genetic and epigenetic changes, which can result in new properties such as generation new peptides, recruitment of immunosuppressive cells and creating poorly immunogenic stem cells population [31]. The expression of molecules involved in EMT was shown to correlate with advanced stage of lung cancer, PD-L1 expression, resistance to EGFR inhibitors and failure of targeted therapy in NSCLC [28, 32]. However, in our study we didn’t evaluate expression of EMT associated regulators in EBUS/TBNA samples; this needs further investigations.
Our study is probably the first one in which lung metastatic CSCs presence in LNs aspirates were analyzed. Presence of metastatic CSCs in LNs (in mouse model) was reported in neck cancer and in LNs resected from patients with pancreatic carcinoma [33, 34]. Unfortunately aspirates obtained during EBUS/TBNA procedure is insufficient for complete evaluation of cellular infiltration, whole CSCs metastatic niche and complex communication network known as “cross talk” with the CSCs at the center stage. However, it is a noninvasive procedure potentially eligible for flow cytometry. We confirmed that EBUS/TBNA is an easy and repeatable method. The median percentage of lung CSCs and PD-L1+ lung CSCs in LNs aspirates relatively higher than number of circulating CSCs detected in peripheral blood [18, 19]. Such changes may result from fact that CSCs from LNs aspirates are obtained directly from metastatic site. Similar conclusion were made in Hashimoto et al. and Murlidhar et al. studies in which the higher number of circulating CSCs was found in pulmonary vein, which is first tumor draining vein than peripheral vein [35, 36].
Conclusion
We confirmed, for the first time PD-L1 presence on metastatic CSCs in LNs aspirates. Our results reveal CSCs as a potential strategy to enhance cancer immunotherapy efficacy. The development of predictive biomarkers is needed to optimize patient benefit and guide combination approaches.
References
http://gco.iarc.fr. Accessed 6 Oct 2018.
Ogino, S., Galon, J., Fuchs, C. S., & Dranoff, G. (2011). Cancer immunology-analysis of host and tumor factors for personalized medicine. Nature Reviews. Clinical Oncology, 8(12), 711–719.
Domagala-Kulawik, J., Kwiecien, I., Pankowski, J., et al. (2017). Elevated Foxp3/CD8 ratio in lung adenocarcinoma metastatic lymph nodes resected by transcervical extended mediastinal lymphadenectomy. BioMed Research International, 5185034. https://doi.org/10.1155/2017/5185034.
Rizvi, N. A., Hellmann, M. D., Snyder, A., Kvistborg, P., Makarov, V., Havel, J. J., Lee, W., Yuan, J., Wong, P., Ho, T. S., Miller, M. L., Rekhtman, N., Moreira, A. L., Ibrahim, F., Bruggeman, C., Gasmi, B., Zappasodi, R., Maeda, Y., Sander, C., Garon, E. B., Merghoub, T., Wolchok, J. D., Schumacher, T. N., & Chan, T. A. (2015). Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non–small cell lung cancer. Science, 348, 124–128.
Sun, J. M., Zhou, W., Choi, Y. L., Choi, S. J., Kim, S. E., Wang, Z., Dolled-Filhart, M., Emancipator, K., Wu, D., Weiner, R., Frisman, D., Kim, H. K., Choi, Y. S., Shim, Y. M., & Kim, J. (2016). Prognostic significance of PD-L1 in patients with non-small cell lung cancer: a large cohort study of surgically resected cases. Journal of Thoracic Oncology, 11, 1003–1011.
Regenbrecht, C. R. A., Lehrach, H., & Adjaye, J. (2008). Stemming cancer: functional genomics of cancer stem cells in solid tumors. Stem Cell Reviews and Reports. https://doi.org/10.1007/s12015-008-9034-0.
Vermeulen, L., de Sousa e Melo, F., Richel, D. J., & Medema, J. P. (2012). The developing cancer stem-cell model: clinical challenges and opportunities. Lancet Oncology, 13, 83–89.
Sales, K. M., Winslet, M. C., & Seifalian, A. M. (2007). Stem cells and cancer: an overview. Stem Cell Reviews and Reports. https://doi.org/10.1007/s12015-007-9002-0.
Yu, S., & Bian, X. (2009). Enrichment of Cancer stem cells based on heterogeneity of invasiveness. Stem Cell Reviews and Reports, 5, 66–71. https://doi.org/10.1007/s12015-008-9047-8.
Maheswaran, S., Sequist, L. V., Nagrath, S., Ulkus, L., Brannigan, B., Collura, C. V., Inserra, E., Diederichs, S., Iafrate, A. J., Bell, D. W., Digumarthy, S., Muzikansky, A., Irimia, D., Settleman, J., Tompkins, R. G., Lynch, T. J., Toner, M., & Haber, D. A. (2008). Detection of mutations in EGFR in circulating lung-cancer cells. New England Journal of Medicine, 359, 366–377.
Krebs, M. G., Sloane, R., Priest, L., Lancashire, L., Hou, J. M., Greystoke, A., Ward, T. H., Ferraldeschi, R., Hughes, A., Clack, G., Ranson, M., Dive, C., & Blackhall, F. H. (2011). Evaluation and prognostic significance of circulating tumor cells in patients with non-small cell lung cancer. Journal of Clinical Oncology, 29, 1556–1563.
Morrison, R., Schleicher, S. M., Sun, Y., Niermann, K. J., Kim, S., & Spratt, D. E. (2011). Targeting the mechanisms of resistance to chemotherapy and radiotherapy with the cancer stem cell hypothesis. Journal of Oncology, 2011, 1–13. https://doi.org/10.1155/2011/941876.
Dragu, D. L., Necula, L. G., Bleotu, C., Diaconu, C. C., & Chivu-Economescu, M. (2015). Therapies targeting cancer stem cells: Current trends and future challenges. World Journal of Stem Cells., 7, 1185–1201.
Roudi, R., Madjd, Z., Korourian, A., Mehrazma, M., Molanae, S., Sabet, M. N., & Shariftabrizi, A. (2014). Clinical significance of putative cancer stem cell marker CD44 in different histological subtypes of lung cancer. Cancer Biomarkers, 14, 457–467.
Miranda-Lorenzo, I., Dorado, J., & Lonardo, E. (2014). Intracellular autofluorescence: a biomarker for epithelial cancer stem cells. Nature Methods, 11, 1161–1169.
Detterbeck, F. C., Boffa, D. J., Kim, A. W., & Tanoue, L. T. (2017). The eight edition lung cancer classification. Chest Journal, 151, 193–203.
Dziedzic, D., Peryt, A., Szolkowska, M., Langfort, R., & Orlowski, T. (2016). Evaluation of the diagnostic utility of endobronchial ultrasoundguided transbronchial needle aspiration for metastatic mediastinal tumors. Endoscopic Ultrasound, 5(3), 173–177.
Skirecki, T., Hoser, G., Kawiak, J., Dziedzic, D., & Domagała-Kulawik, J. (2014). Flow cytometric analysis of CD133- and EpCAM- positive cells in the peripheral blood of patients with lung cancer. Archivum Immunologiae et Therapiae Experimentalis, 62, 67–75.
Hou, J.-M. M., Krebs, M., & Ward, T. (2011). Circulating tumor cells as a window on metastasis biology in lung Cancer. Americal Journal of Pathology, 178, 989–996.
Funaki, S., Sawabata, N., Abulaiti, A., Nakagiri, T., Shintani, Y., Inoue, M., Minami, M., & Okumura, M. (2013). Significance of tumour vessel invasion in determining the morphology of isolated tumour cells in the pulmonary vein in non-small-cell lung cancer. European Journal of Cardiothoracic Surgery, 43, 1126–1130.
Gwóźdź, P., Pasieka-Lis, M., & Kołodziej, K. (2018). Prognosis of patients with stages I and II non-small cell lung Cancer with nodal micrometastases. Annals of Thoracic Surgery, 105(5), 1551–1557.
Sakakibara, R., Inamura, K., Tambo, Y., Ninomiya, H., Kitazono, S., Yanagitani, N., Horiike, A., Ohyanagi, F., Matsuura, Y., Nakao, M., Mun, M., Okumura, S., Inase, N., Nishio, M., Motoi, N., & Ishikawa, Y. (2017). EBUS-TBNA as a promising method for the evaluation of tumor PD-L1 expression in lung Cancer. Clinical Lung Cancer, 18(5), 527–534.
Schatton, T., Schutte, U., Frank, N. Y., Zhan, Q., Hoerning, A., Robles, S. C., Zhou, J., Hodi, F. S., Spagnoli, G. C., Murphy, G. F., & Frank, M. H. (2010). Modulation of T-cell activation by malignant melanoma initiating cells. Cancer Research, 70, 697–708.
Lee, Y., & Sunwoo, J. (2014). PD-L1 is preferentially expressed on CD44+ tumor-initiating cells in head and neck squamous cell carcinoma. Journal for Immunotherapy of Cancer, 2, 270.
Abetov, D., Mustapova, Z., Saliev, T., Bulanin, D., Batyrbekov, K., & Gilman, C. P. (2015). Novel small molecule inhibitors of Cancer stem cell signaling pathways. Stem Cell Reviews and Reports, 11, 909–918.
Liu, Y., Dong, Z., Jiang, T., Hou, L., Wu, F., Gao, G., He, Y., Zhao, J., Li, X., Zhao, C., Zhang, W., Tian, Q., Pan, Y., Wang, Y., Yang, S., Wu, C., Ren, S., Zhou, C., Zhang, J., & Hirsch, F. R. (2018). Heterogeneity of PD-L1 expression among the different histological components and metastatic lymph nodes in patients with resected lung Adenosquamous carcinoma. Clinical Lung Cancer, 19, e421–e430. https://doi.org/10.1016/j.cllc.2018.02.008.
Grigg, C., & Rizvi, N. A. (2016). PD-L1 biomarker testing for non-small cell lung cancer: truth or fiction? Journal for Immunotherapy of Cancer, 4, 48.
Kim, S., Koh, J., Kim, M., et al. (2016). PD-L1 expression is associated with epithelial-to-mesenchymal transition in adenocarcinoma of the lung. Human Pathology, 58, 7–1.
Travis, W. D., Brambilla, E., Nicholson, A. G., Yatabe, Y., Austin, J. H. M., Beasley, M. B., Chirieac, L. R., Dacic, S., Duhig, E., Flieder, D. B., Geisinger, K., Hirsch, F. R., Ishikawa, Y., Kerr, K. M., Noguchi, M., Pelosi, G., Powell, C. A., Tsao, M. S., Wistuba, I., & WHO Panel. (2015). The 2015 World Health Organization classification of lung tumors: impact of genetic, clinical and radiologic advances since the 2004 classification. Journal of Thoracic Oncology, 10, 1243–1260.
Langfort, R., & Szołkowska, M. (2012). Recommendation concerning the microscopic classification of lung adenocarcinoma presented by International Association for the Study of Lung Cancer, American Thoracic Society, and European Respiratory Society. Pneumonologia i Alergologia Polska, 80(2), 99–100.
Dunn, G. P., Old, L. J., & Schreiber, R. D. (2004). The three Es of cancer immunoediting. Annual Review of Immunology, 22, 329–360.
Lou, Y., Diao, L., Parra Cuentas, E. R., et al. (2016). Epithelial-mesenchymal transition is associated with a distinct tumor microenvironment including elevation of inflammatory signals and multiple immune checkpoints in lung adenocarcinoma. Clinical Cancer Research, 22(14), 3630–3642.
Wu, T. F., Chen, L., Bu, L. L., Gao, J., Zhang, W. F., & Jia, J. (2017). CD44+ cancer cell-induced metastasis: a feasible neck metastasis model. European Journal of Pharmaceutical Sciences, 101, 243–250. https://doi.org/10.1016/j.ejps.2017.02.020.
Zhang, L., Wang, D., Li, Y., Liu, Y., Xie, X., Wu, Y., Zhou, Y., Ren, J., Zhang, J., Zhu, H., & Su, Z. (2016). CCL21/CCR7 axis contributed to CD133(+) pancreatic cancer stem-like cell metastasis via EMT and Erk/NF-κB pathway. PLoS One, 11, e0158529.
Hashimoto, M., Tanaka, F., Yoneda, K., Takuwa, T., Matsumoto, S., Okumura, Y., Kondo, N., Tsubota, N., Tsujimura, T., Tabata, C., Nakano, T., & Hasegawa, S. (2014). Significant increase in circulating tumour cells in pulmonary venous blood during surgical manipulation in patients with primary lung cancer. Interactive Cardiovascular and Thoracic Surgery, 18(6), 775–783.
Murlidhar, V., Reddy, R. M., Fouladdel, S., Zhao, L., Ishikawa, M. K., Grabauskiene, S., Zhang, Z., Lin, J., Chang, A. C., Carrott, P., Lynch, W. R., Orringer, M. B., Kumar-Sinha, C., Palanisamy, N., Beer, D. G., Wicha, M. S., Ramnath, N., Azizi, E., & Nagrath, S. (2017). Poor prognosis indicated by venous circulating tumor cell clusters in early stage lung cancers. Cancer Research, 77, 5194–5206.
Acknowledgments
Marta Dabrowska MD, PhD, and Rafał Sokołowski MD for the help in EBUS/TBNA performing.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The Authors declare no conflict of interest.
Additional information
Micro Abstract
• Lung cancer stem cells- CSCs could be responsible for escape from immunosurveillance.
• CSCs are identified by expression of CD133 and EpCAM.
• CD133+EpCAM+PD-L1+ cells are found in EBUS/TBNA samples of lymph nodes.
• Proportion of CSCs PD-L1+ is elevated in metastatic lymph nodes and in adenocarcinoma.
Rights and permissions
About this article
Cite this article
Raniszewska, A., Polubiec-Kownacka, M., Rutkowska, E. et al. PD-L1 Expression on Lung Cancer Stem Cells in Metastatic Lymph Nodes Aspirates. Stem Cell Rev and Rep 15, 324–330 (2019). https://doi.org/10.1007/s12015-018-9860-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-018-9860-7