Abstract
Background
The etiology of non-healing ulcers depends on both systemic and local factors. The introduction of advanced dressing, negative wound therapy and compression therapy have undoubtedly improved clinical outcomes. The principal aim of study was to demonstrate the efficacy of dermal micrografts in the treatment of ulcers with different etiologies. The second aim was to investigate in vitro the action of micrografts in the regenerative process.
Methods
The dermal micro-grafts were obtained from mechanical disaggregation of small pieces of skin tissue through a medical device called Rigeneracons.
Results
We observed in vivo the ability of dermal autologous micrografts to improve the healing of venous, diabetic, pressure and post-traumatic ulcers after few week of treatment accomplished in general with a better quality of life for the patients. In vitro results showed that these micrografts express mesenchymal stem cells (MSCS) marker such as CD34, CD73, CD90 and CD105, and are able to form a viable and proliferative biocomplex with collagen sponge. Finally, the site of ulcers displayed a different expression of epidermal growth factors, insulin-like growth factors, platelet-derived growth factors and their receptors and tumor necrosis factor-β with respect to healthy skin samples.
Conclusion
We reported a good outcome for the treatment of chronic ulcers using dermal autologous micrografts. Finally, we suggest that the positivity to MSCs markers and the ability to interact with a scaffold can play a key role in their regenerative properties.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Wound healing is a complex process based on a coordinated phenomenon, which implies activation of several molecular and biochemical pathways regulating cell growth and differentiation [1]. The process of wound repair begins with platelet aggregation, formation of a provisional matrix of fibrin and release of growth factors from damaged cells, platelets and extracellular matrix (ECM) followed by the migration of inflammatory cells at the wound site [2]. Trophic ulcers of the lower limb are a classical example of chronic wounds affecting a large proportion of the population and are linked to various conditions, including venous hypertension (post-phlebitis), arterial diseases (arteriosclerosis, thromboembolism, arterial, vasculiti, Martorell ulcer and Raynaud’s diseases), neuropathic conditions, infectious or neoplastic diseases [3, 4]. Advanced wound care products such as dressings, negative pressure wound therapy (NPWT) devices and compression therapy have undoubtedly contributed to achievement of positive clinical outcomes but acute and chronic wounds remain still a major clinical issues [5, 6]. The treatment of these type of wounds is often multifaceted and multidisciplinary and involves correction, if possible, of the underlying disease. Many times, it is necessary supply of new skin to the wound, most commonly by autografting but often the implant procedures require a long waiting period, increasing the risk of ulcer infection besides complications arising from treatment of full-thickness lesions [7, 8]. In addition to these approaches, progress in cell culture and biomaterial technologies has developed commercially available autologous and allogeneic skin substitutes composed of keratinocytes and/or fibroblats, in part combined with allogeneic (fibrin) or xenogeneic (collagen, hyaluronan) matrix substances [9]. In this variety of therapeutic approaches, recent studies have found that autologous micrografts obtained by mechanical disaggregation of a small piece of dermal or connective tissue may improve the tissue repair of postoperative complex wounds [10–12], post-traumatic wounds [13] and hypertrophic scars [14] previously treated with other medications which failed to reach a complete wound healing.
On the basis of these considerations, the aim of our study was to demonstrate the efficacy of autologous dermal micrografts in the treatment of venous, diabetic, pressure and post-traumatic ulcers and to investigate the nature of these micrografts and the potential mechanism able to elucidate their regenerative role. The autologous dermal micrografts used in this study were obtained through Rigenera protocol which permits the disruption of human connective and skin tissues by the use of a disposable named Rigeneracons (Human Brain Wave srl, Italy). This medical device is a biological disruptor of human tissues able to select a population of progenitor cells commonly present in the healthy adult tissues [15].
Materials and Methods
Study Design
A total of 30 patients with a mean age of 64, 5 years were enrolled in the study and all signed written consent to participate according to the Declaration of Helsinki. Local Ethic Commitment approved the study. All patients were assessed for systemic and local diseases and clinical data were collected in the Table 1. Further, all patients reported persistent low back pain and walking difficulties. The wound duration, prior to applying the Rigenera protocol, was documented as either ‘less than 2 weeks’, ‘less than 6 weeks’, ‘less than 12 weeks’ or ‘12 weeks or more’. A total of 2 patients had wounds for less than 2 weeks, three patients had wounds for less than 6 weeks, five for less than 12 weeks, and five patients had wounds in excess of 12 weeks.
Rigenera Protocol
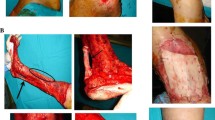
Ultrasonic debridement of damaged tissue was performed in all patients. For each patient we performed two 4 mm punch of skin sample from healthy skin (Fig. 1a–b) which are placed into the Rigeneracons medical device adding 1 ml of saline solution for each punch disaggregated (Fig. 1c). We obtained a total of 2 ml of micrografts: 1 ml was directly injected into the edges of the ulcer and 1 ml was used to soak a scaffold of equine collagen (Condress®, Smith and Nephew S.r.l. Italy), which was applied on the wound (Fig. 1e–d). A multi-composite wound dressing was applied and changed every week for a month.
Rigenera protocol to obtain dermal micrografts. a The surgeon performed a 4 mm punch of skin sample; b–c The punch skin sample was collected and the dermal sample without epidermal layer was placed into Rigeneracons medical device adding 1 ml of saline solution; d The suspension was collected, one part was injected into the edges of the ulcer and one part was injected on a collagen scaffold applied on the wound
Wound Size and Clinical Parameters Evaluation
For each ulcer, wound size was calculated at day 0, after 1 week of treatment and close to complete healing using a ruler and multiplying the greatest length for the perpendicular greatest width. Each ulcer was in average 5 cm of width and 2 cm of length. For an area of 10 cm2 we applied a total of 2 ml of micrografts obtained from disaggregation of 2 punches: 1 ml directly on the ulcer site and 1 ml on collagen sponge which was in turn placed on the ulcer.
Pain experience was measured before and during micrografts treatment using a numerical rating VAS scale, where −1 indicated absent pain and 10 severe pain. Digital photographs were also taken at these visits and were also scored subjectively to assess the amount of granulation tissue using an objective grading scale as previously reported (Grade 0, no granulation tissue; Grade 1, granulation tissue present but below skin edges at wound margin; Grade 2, granulation tissue present and level with wound edges; Grade 3, granulation tissue above, but not overlapping, skin edges at the wound margin; Grade 4, granulation tissue both above and overlapping the epithelial edges at the wound margin) [16]. A questionnaire was also administrated to the patients for the evaluation of quality of life such as sleep, mobility and daily living activities.
In Vitro Experiments
Cell Culture
Skin biopsies were obtained by 4 mm punch from fourteen volunteers and the procedure was carried out on an outpatient basis under local anesthesia. Samples were stored in transport media phosphate-buffered saline (PBS) containing penicillin and streptomycin for a maximum of 15–20 min from harvesting of the tissue. Skin biopsies were then rinsed three times in PBS containing antibiotics and antimycotics, disaggregated by Rigeneracons adding 1 ml of sterile saline solution, After disaggregation, a cell count was performed and 15,000 cells/ml were obtained. Afterwards, the cells were seeded into 96-well plates, cultured with Dulbecco’s modified Eagle’s medium (DMEM)/Ham’s F12 medium (DMEM/F12), 10 % fetal bovine serum (FBS), 2 ng/ml basic fibroblast growth factor (bFGF), 2 mM L-glutamine, 100 U/ml penicillin, and 100 mg/ml streptomycin (all purchased from Life Technologies, Italy) and incubated at 37 °C under 5 % CO2. The medium was changed twice a week and cells reached confluence in 5–7 days.
Flow Cytometry
After mechanical disaggregation, cells were incubated directly with fluorescent conjugated antibodies for 30 min at 4 °C, washed, and resuspended in 0.6 ml PBS. Samples were analyzed at cells confluence by flow cytometry using a FACS Aria II (Becton & Dickinson, Mountain View, CA,USA). The antibodies used are following: anti-CD45 Cy and PE (BD Pharmingen, Buccinasco, Milano, Italy); anti-CD105 FITC (AbCam, Cambridge, UK); anti-CD90 FITC (BD Pharmigen); anti-CD73 FITC and PE (AbCam) anti-CD34 PE (Miltenyi-Biotech); anti-CD31 FITC (Miltenyi-Biotech) and anti-CK15 FITC (Abcam).
Calcein and Haematoxylin & Eosin Staining
The micrografts cultured on collagen sponge were stained with calcein AM according to manufacturer’s instructions (Thermo Scientific, Italy) to determine the cell viability of the biocomplex. For Haematoxylin & Eosin staining, micrografts cultured on collagen sponge were fixed in 4 % paraformaldehyde for 24 h at 48 °C, washed in PBS, incubated in 30 % sucrose for 3 days, washed in PBS and then embedded in an optimal cutting temperature (OCT-purchased from Bio-Optica, Milan, Italy). Finally, they were stored at −80 °C for haematoxylin and eosin (H&E) staining. The frozen sections in the OCT were cut into 5mmthick slices, washed in distilled water for 10 min. For H&E staining, sections were placed in haematoxylin for 5 min. After three washes of distilled water, samples were placed in spring water for 20 min. Then, the samples were placed in eosin acidified with acetic acid for 30 s. Finally, they were placed in alcohol 95°, 75°, and 95° and mounted with DPX.
Semi-Quantitative PCR
Total RNA was extracted from a total of 7 venous and diabetic ulcers samples, and 7 healthy skin samples with TRI Reagent (Sigma, Milan, Italy). cDNA synthesis was carried out by SuperScript II reverse transcriptase (Invitrogen, San Giuliano Milanese, Milan, Italy). The human primers sequences used for gene expression analysis are listed in the Table 2 and were provided by Thermo Fischer Scientific (Milan, Italy). GAPDH was used as internal control (data no shown).
Statistical Analysis
All data on wound size and pain assessment were statistically analyzed using One-way ANOVA test. We considered significant P value ≤ 0.05. All analyses were performed using GraphPad 5.0 software.
Results
In Vivo Effects of Micrografts on Ulcers Healing
We reported some representative cases among of 30 patients treated with dermal autologous micrografts. In the Fig. 2 is represented a post-traumatic ulcer (Fig. 2a) in a patient affected by vascular deficit treated with micrografts plus collagen (Fig. 2b) and we observed an increase in exudation after the first week of therapy at first dressing change (Fig. 2c). At second dressing change, exudation had diminished and a fibrin layer in the ulcers was visible (Fig. 2d). Buttons of granulation tissue were also evident. A month later a distinct reduction in wound size and new tissue was visible around the wound (Fig. 2e). Similar results were also observed for diabetic ulcer of lower leg (Fig. 3) and sacred coccygeal ulcer (Fig. 4). We then measured a wound size of the ulcers observing a significant reduction already after 1 week from treatment and until the almost complete wound haeling (Fig. 5a). In addition, all patients reported a significant decrease of pain after micrografts application (Fig. 5b) and no infection signs, allergic reactions, maceration and inflammation around the lesion were reported. Furthermore, we reported a decreased level of exudation and increased level of granulation tissue after 15 days from treatment with a peak after 30 days, which from a clinical point of view was represented by the appearance of a fibrin layer (Fig. 5c). Lastly, 70 % of patients stated an improvement in physical symptoms, daily living activities, sleep, motility and social life (Fig. 5d).
Representative image of post-traumatic venous ulcer in patient with vascular deficit in the lower limbs. a Ulcer before micrografts application; b Ulcer treated with micrografts and collagen sponge; c Increase in exudation at first dressing change after the one week from micrografts application; d At second dressing change, after 15 days exudation is reduced with a visible fibrin layer and buttons of granulation tissue; e A month later a distinct reduction in wound size and new tissue was visible around the wound. The closure time was 31 days
Wound area and pain assessment before and after the micrografts treatment. a Wound area was evaluated using a ruler and multiplying the greatest length for the perpendicular greatest width and was expressed in cm2. b The pain was measured by VAS scale according to what reported by the patients before and after 1 week of treatment. (***P = 0.001 vs. before treatment condition). c Change in granulation and exudation tissue over treatment period calculated on digital photographs scored to assess the amount of granulation tissue using an objective grading scale. d Graph depicting exit survey responses provided by the patients to questionnaire of the impact of the treatment on various quality of life issues
In Vitro Results
Isolation and Characterization of Dermal Micro-Grafts
To elucidate the characteristics of micrografts, we carried out in vitro experiments on healthy skin biopsies disaggregated and cultured as described in the appropriate paragraph. In the Fig. 6, we showed that primary cells obtained by cultured skin biopsies displayed a typical fibroblast-like morphology after 7 and 14 days of culture (Fig. 6a–b). Then, flow cytometry analysis reported an high positivity for MSCs markers including CD34 (67 %), CD73 (83 %), CD105 (80 %) CD90 (72 %), while the cells are negative for CD31, CD45 (hematopoietic markers) and CK15 (keratinocyte marker) (Fig. 6c).
Representative image of cells derived from cultured micrografts. a–b The images were captured after 7 and 14 days of culture. c Representative image of flow cytometric analyses performed immediately after disaggregation with Rigeneracons medical device. A significant percentage of cells are clearly positive for mesenchymal stem cell markers, including CD90 (72 %), CD105 (80 %), CD34 (67 %), CD73 (83 %). In contrast, all cells were negative for hematopoietic markers such as CD31 and CD45 and negative for keratinocyte marker such as CK15
Interaction Between Dermal Micrografts and Collagen Sponge
To assess the interaction between dermal micrografts and collagen sponge to form a functional bio-complex, we firstly investigated a cell viability staining the biocomplex with calcein AM and we observed after 3, 7 and 14 days of culture a good green fluorescence suggesting an high cell viability (Fig. 7a–c). Then, we stained a biocomplex with H&E showing that cells appear homogeneously distributed throughout the whole sponge after 3 and 7 days of culture with an extensive spreading at Day 14 suggesting, in addition to cell viability, an increased time-dependent cell proliferation (Fig. 7d–l).
Cell viability and interaction of micrografts on collagen sponge. a–c Cell viability of biocomplex was evaluated by calcein staining after 3, 7 and 14 days of culture. d–f Histological staining with H&E after 3 days, g–i, 7 days, and j–l, 14 days at different magnification (X 10, 40, 100). The images showing that the cells were homogeneously distributed on scaffold with an increased cell proliferation time- dependent. Arrowhead shows the neo-formed matrix and the arrow shows the micro-grafts
Gene Expression in the Ulcer Site
Finally, we compared in injured and healthy skin the expression of growth factors such as EGF, IGF PDGF and TGF-Beta to understand the factors involved in the micro-grafts feeding and activation in both venous and diabetic ulcers. As indicated in the Fig. 8, we observed no expression for EGF (Epidermal Growth Factor), IGF (Insulin-Growth Factor) -I and -II in venous ulcers. The same results were also observed for diabetic ulcers, except to IGF-II that was instead expressed. We also observed a mild expression for PDGF (Platelet Derived Growth Factor)-AA and -BB as well as their receptors in both venous and diabetic ulcer samples. Lastly, in both venous and diabetic ulcers we revealed a strong expression of TGF-β (Transforming Growth Factor-β) (Fig. 8a–b). Skin biopsies from healthy subjects were used as control (Fig. 8c).
Discussion
Chronic wounds are considered a financial burden on the health care system in western countries and for this reason, it is important to consider the application of new clinical procedures to improve their management [17]. To date, one of clinical approaches is for example the application of skin substitutes but given the high cost of these products, future randomized large prospective studies are needed to guide their clinical applications [18]. Another approach is the skin graft through the application of a bilayer artificial skin in conjunction with compression bandaging. Although this approach increases venous ulcer healing compared with a simple dressing plus compression, more data are needed to assess whether other forms of skin grafts increase ulcer healing [19].
In this study, we showed that autologous dermal micrografts obtained by Rigenera protocol improve the process of tissue repair in venous, diabetic, pressure and post traumatic ulcers, moreover we observed a better healing of ulcer site after micrografts application with a reduction of wound size, an increased granulation and reduced exudation. These results are according to an independent study where was demonstrated the ability of autologous micrografts to trigger the wound healing of multifactorial ulcers [20]. The successful outcomes of this novel approach are also evident in other clinical fields including dentistry where micro-grafts derived from dental pulp were used for periodontal regeneration and alveolar socket preservation [21–23] and in dermatology where an engraftment of transplanted hair through micro-grafts application was observed [24].
Chronic wounds have a significant impact on quality of life of the patients who are often affected by immobility and pain and need of continuous topical treatment [17, 25]. Regarding this aspect, we reported in this study a significant amelioration of patient’s quality of life in terms of sleep, motility and implementation of daily activities. Interestingly, patients reported abatement of pain within a few hours of micrografts application without previous administrations of analgesics.
On the basis of clinical evidences of ulcers site improvement, we tried to understand the potential mechanism implicated in this effect. For this reason, after the disaggregation of healthy skin biopsies, we cultured the dermal micrografts observing that these after 7 and 14 days of culture exhibited a fibroblasts-like morphology and mainly were positive to mesenchymal stem cell markers. These results are according to other studies where we showed that this type of tissue disaggregation yields viable micro-grafts positive for mesenchymal stem cells (MSCs) markers such as CD73, CD90, CD105 [15, 23]. This data is very interesting because of MSCs are object of intensive research for their regenerative properties [26]. Moreover, preclinical and clinical trials show that MSC therapy accelerates wound closure suggesting that this therapy can be promising for treating wounds with delayed healing [27]. MSCs promote different stages of the wound repair process and, although these may differentiate in the wound, it has been shown that MSCs enhance wound healing through modulation of inflammation, promotion of angiogenesis, and stimulation of cell movement during epithelial remodeling [28]. On the basis of these evidences, we can suppose that regenerative properties of micrografts can be in part determined by MSCs positivity reported in this and other above cited studies [10, 13, 15].
In this study we used at the same time the micrografts both alone and in combination with a collagen sponge assuming that topical delivery of micrografts can improve wound healing of treated ulcers. Our in vitro results on interaction between micrografts and collagen showed that when combined, these form a biocomplex which displays both cell viability and proliferation, therefore confirming a their regenerative potential.
Finally, in this study we evaluated the gene expression of any growth factors in the ulcer site to investigate the reason for delayed healing of the ulcers. Among these, epidermal growth factor plays a central role in wound healing acting on epithelial cells and fibroblasts and promoting restoration of damaged epithelium. Our results reported no gene expression for EGF in both venous and diabetic ulcers site according to an impaired bioavailability in chronic diabetic foot ulcers [29]. Chronic wounds are also characterized by a lower expression of PDGF-AA and -BB which promote mitogenesis, angiogenesis and macrophage activation [30]. According to this, we observed a mild expression of PDGF-AA and –BB and their receptors in the ulcer site when compared with control samples. We will evaluate the possibility to analyze the gene expression of these factors in micrografts-treated patients but for now no data are available.
The use of autologous micrografts obtained by Rigenera protocol represents an innovative approach in the clinical practice and in literature are already reported several studies on their efficacy both when applied on a scaffold and when applied alone. For example, it was reported the improvement of appearance and texture of exaggerated scars when applied directly around the lesions without the use of any scaffold [14]. Other studies reported the combined use of these micrografts injected both around the lesion and applied on collagen sponge [12, 20] or their application only on collagen sponge [11, 13]. In summary, our results indicate that dermal micro-grafts could endorse healing of different type of ulcers reducing wound size and promoting the formation of granulation tissue accompanied by an abatement of pain probably due a paracrine effect in the surrounding cells and in general by a better quality of life for patients. We also tried in vitro to elucidate the potential mechanism involved in this effect and we showed that micrografts contain cells expressing MSCs suggesting a their role in the micrografts-induced tissue repair even if further investigations are required to better elucidate both their role and cellular processes involved in the development and healing of ulcers.
References
Xue, M., & Jackson, C. J. (2015). Extracellular matrix reorganization during wound healing and its impact on abnormal scarring. Advances in Wound Care (New Rochelle), 4, 119–136.
Schultz, G. S., & Wysocki, A. (2009). Interactions between extracellular matrix and growth factors in wound healing. Wound Repair and Regeneration, 17, 153–162.
Ahmed, I., & Mistry, J. (2015). The management of acute and chronic elbow instability. The Orthopedic Clinics of North America, 46, 271–280.
Asamova, N. R., & Karimov, Z. Z. (2009). New prospects in treatment of lower-limb venous trophic ulcers. Angiol Sosud Khir, 15, 153–156.
Kasai, Y., Nemoto, H., Kimura, N., Ito, Y., & Sumiya, N. (2012). Application of low-pressure negative pressure wound therapy to ischaemic wounds. Journal of Plastic, Reconstructive & Aesthetic Surgery, 65, 395–398.
Frykberg, R. G., & Banks, J. (2015). Challenges in the treatment of chronic wounds. Advances in Wound Care (New Rochelle), 4, 560–582.
Vapniarsky, N., Arzi, B., Hu, J. C., Nolta, J. A., & Athanasiou, K. A. (2015). Concise review: human dermis as an autologous source of stem cells for tissue engineering and regenerative medicine. Stem Cells Translational Medicine, 4, 1187–1198.
Hamnerius, N., Wallin, E., Svensson, Å., Stenström, P., Svensjö, T. (2016). Fast and standardized skin grafting of leg wounds with a new technique: report of 2 cases and review of previous methods. Eplasty, 16, e14. eCollection 2016
Hunziker, T. (2004). Autologous cultured skin substitutes. Hautarzt, 55(11), 1077–1084. quiz 1085.
Giaccone, M., Brunetti, M., Camandona, M., Trovato, L., & Graziano, A. (2014). A new medical device, based on rigenera protocol, in the management of complex wounds. Journal of Stem Cells Research, Reviews & Reports, 1, 3.
Marcarelli, M., Trovato, L., Novarese, E., Riccio, M., & Graziano, A. (2016). Rigenera protocol in the treatment of surgical wound dehiscence. International Wound Journal. doi:10.1111/iwj.12601.
Baglioni, E., Trovato, L., Marcarelli, M., Frenello, A., & Bocchiotti, M. A. (2016). Treatment of oncological post-surgical wound dehiscence with autologous skin micrografts. Anticancer Research, 36(3), 975–980.
Purpura, V., Bondioli, E., Graziano, A., et al. (2016). Tissue characterization after a new disaggregation method for skin micro-grafts generation. Journal of Visualized Experiments, (109), doi:10.3791/53579.
Svolacchia, F., De Francesco, F., Trovato, L., Graziano, A., & Ferraro, G. A. (2016). An innovative regenerative treatment of scars with dermal micrografts. Journal of Cosmetic Dermatology. doi:10.1111/jocd.12212.
Trovato, L., Monti, M., Del Fante, C., et al. (2015). A new medical device rigeneracons allows to obtain viable micro-grafts from mechanical disaggregation of human tissues. Journal of Cellular Physiology, 230, 2299–2303.
Wollina, U., Liebold, K., Schmidt, W. D., Hartmann, M., & Fassler, D. (2002). Biosurgery supports granulation and debridement in chronic wounds—clinical data and remittance spectroscopy measurement. International Journal of Dermatology, 41(10), 635–639.
Green, J., Jester, R., McKinley, R., & Pooler, A. (2014). The impact of chronic venous leg ulcers: a systematic review. Journal of Wound Care, 23, 601–612.
Nyame, T. T., Chiang, H. A., & Orgill, D. P. (2014). Clinical applications of skin substitutes. The Surgical Clinics of North America, 94(4), 839–850. doi:10.1016/j.suc.2014.05.013.
Jones, J.E., Nelson, E.A., Al-Hity, A. (2013). Skin grafting for venous leg ulcers. Cochrane Database of Systematic Reviews, (1), CD001737. doi:10.1002/14651858.CD001737.pub4.
Trovato, L., Failla, G., Serantoni, S., Palumbo, F.P. (2016). Regenerative surgery in the management of the leg ulcers. Journal of Cell Science & Therapy, 7, 238. doi:10.4172/2157-7013.1000238.
Brunelli, G., Motroni, A., Graziano, A., D’Aquino, R., Zollino, I., & Carinci, F. (2013). Sinus lift tissue engineering using autologous pulp micro-grafts: a case report of bone density evaluation. Journal of Indian Society of Periodontology , 17, 644–647.
Graziano, A., Carinci, F., Scolaro, S., & D’Aquino, R. (2013). Periodontal tissue generation using autologous dental ligament micro-grafts: case report with 6 months follow-up. Annals of Oral & Maxillofacial Surgery, 1, 20.
d’Aquino, R., Trovato, L., Graziano, A., et al. (2016). Periosteum-derived micro-grafts for tissue regeneration of human maxillary bone. Journal of Translational Science, 2(2), 125–129. doi:10.15761/JTS.1000128.
Zanzottera, F., Lavezzari, E., Trovato, L., Icardi, A., & Graziano, A. (2014). Adipose derived stem cells and growth factors applied on hair transplantation. Follow-up of clinical outcome. Journal of Cosmetics Dermatological Sciences and Applications, 24, 268–274. doi:10.4236/jcdsa.2014.44036.
Mousa, A. Y., Richmond, B. K., & AbuRahma, A. F. (2014). Review and update on new horizon in the management of venous ulcers. Vascular and Endovascular Surgery, 48(2), 93–98. doi:10.1177/1538574413510625.
Garcia-Gomez, I., Elvira, G., Zapata, A. G., et al. (2010). Mesenchymal stem cells: biological properties and clinical applications. Expert Opinion on Biological Therapy, 10, 1453–1468.
Hocking, A. M. (2012). Mesenchymal stem cell therapy for cutaneous wounds. Advances in Wound Care, 1, 166–171.
Nuschke, A. (2014). Activity of mesenchymal stem cells in therapies for chronic skin wound healing. Organogenesis, 10, 29–37.
Tiaka, E. K., Papanas, N., Manolakis, A. C., & Georgiadis, G. S. (2012). Epidermal growth factor in the treatment of diabetic foot ulcers: an update. Perspectives in Vascular Surgery and Endovascular Therapy, 24(1), 37–44. doi:10.1177/1531003512442093.
Patel, S., Maheshwari, A., & Chandra, A. (2016). Biomarkers for wound healing and their evaluation. Journal of Wound Care, 25, 46–55.
Acknowledgments
We thank Prof. Giuseppina Caraglia B.A., English language expert and assistant for Department of Sciences and Environmental, Biological, Pharmaceutical Technologies, Caserta, Second University of Naples for providing excellent technical revision and support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors A.G. and L.T. are members of Scientific Division of Human Brain Wave srl, the manufacturer of the Rigeneracons medical device used in this study. No funding. Other authors declare that they have no conflict of interest.
Additional information
The original version of this article was revised: The name of the seventh author was incorrectly listed as Gabriella Maria Casella De Angelis, when it is actually Gabriella Maria Cusella De Angelis.
M. Riccio and G. A. Ferraro contributed equally to this work.
An erratum to this article is available at http://dx.doi.org/10.1007/s12015-016-9698-9.
Rights and permissions
About this article
Cite this article
De Francesco, F., Graziano, A., Trovato, L. et al. A Regenerative Approach with Dermal Micrografts in the Treatment of Chronic Ulcers. Stem Cell Rev and Rep 13, 139–148 (2017). https://doi.org/10.1007/s12015-016-9692-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-016-9692-2