Abstract
Full-thickness skin wounds occur in many different clinical cases and the use of biological acellular dermal matrices (ADMs) to reconstruct the damaged area is increasing in the field of plastic and reconstructive surgery. In particular, the ability of ADMs to maintain the structural properties of extracellular matrix as well as to provide a suitable environment for cell growth makes their use suitable for the improvement of wound healing and the reduction of side effects deriving from contracture and scar tissue formation. In this study, we describe the clinical use of a recently developed human dermal matrix (HDM) in combination with graft skin as an alternative reconstructive solution for the treatment of full-thickness skin wounds. The HDM was applied in combination with autologous graft skin on three different clinical cases in which full-thickness skin wounds occurred. The clinical outcomes were evaluated in the patients during their follow-up. Histological as well as ultra-structural analysis were also performed on skin biopsy of the clinical case 3 one year after the treatment with HDM. The use of HDM stimulates the wound healing process in all clinical cases of full-thickness skin wounds here described with a functional and aesthetic rescue of the damaged area. Histological and ultra-structural analysis show a regenerative healing of the wound area with well-organized/oriented connective tissue in which cellular infiltration as well as blood vessels are evident. Our results support the clinical use of HDM as a permanent dermal replacement for the treatment of full-thickness skin wounds.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Several clinical conditions are characterized by full-thickness skin wounds in which the dermal and epidermal layers result as totally destroyed. In these conditions, unlike the epidermis, the dermal layer is unable to regenerate and it is finally replaced by scar tissue lacking of properties required for functional and aesthetic rescue. The conventional surgical treatments with autograft or allograft skin are able to provide a life-saving skin replacement on the damaged area. On the other hand, a further injury at the donor site of the patient is required when auto-graft skin is applied while the use of allograft skin results in a final rejection of the homologous tissue applied for the presence of foreign cellular component (Ingham et al. 1993). In both these cases, a full recovery of dermal component is not achieved and scar tissue formation is produced as a consequence of their use (Cohen et al. 1992). To overcome limitations of these procedures, alternative approaches were taken into account and the use of ADMs is now increasing in the field of plastic and reconstructive surgery (Fosnot et al. 2011). ADMs are biological scaffolds able to maintain structural and biochemical properties of extracellular matrix (ECM) after the removal of cellular component. In fact, they provide a structural support and a biological environment suitable for the stimulation of cell growth as well as the attraction of underlying cells to the lesion area, improving and accelerating the healing process and, in turn, wound closure (Brown and Badylak 2014; Tabata 2008; Brigido et al. 2004). Thus the use of ADMs provides a biological replacement of the empty area in which dermis is missing reducing the possibility that it can be filled by scar tissue. In addition, the use of ADMs allows the application of a thinner auto-graft skin to cover the damaged area and the absence of cellular component avoids problems deriving from rejection. Unlike synthetic materials, ADMs are also biocompatible and well integrated with the wound receiving site. Moreover, biological scaffolds composed of ECM have been shown to be resistant to deliberate bacterial contamination in preclinical in vivo studies as well as to recruit and induce proliferation of tissue specific cell types (Badylak 2005; Brennan et al. 2006; Vorotnikova et al. 2010). All these ADMs properties have improved the surgical approaches to difficult clinical conditions such as head, neck or extremity surgery allowing their better management with excellent results (Fosnot et al. 2011). In this scenario, Emilia Romagna Regional (ERR) Skin Bank in collaboration with Rizzoli Orthopedic Institute realized a cell-free scaffold derived from human dermis (HDM) using a patented decellularization method (PTC/IB2008/002753) able to maintain a balance between the absence of viable cellular component and the ECM structure, required for its clinical use (Bondioli et al. 2014). In this study, we show the clinical outcomes of full-thickness skin wounds treatment in which HDM was used as a permanent dermal replacement in combination with overlying skin graft.
Materials and methods
Procurement, decellularization and assessment of HDM properties
Human dermis was taken from the trunk area of multi-organ and/or multi-tissue donors following National Rules on harvesting, processing and distributing tissues for transplantation (CNT 2016) and was then decellularized at the ERR Skin Bank of Bufalini Hospital, Cesena, Italy.
The epidermis was first separated using an electric dermatome from the dermis trunk area and discarded in sterile conditions. Then dermis samples were dissected. The dermis grafts were rinsed with 0.9% NaCl solution and stored in this solution for transport (at 2–10 °C) to the treatment station (< 12 h), where they were aseptically submitted to a combined treatment of decellularization. The tissue was treated with 2.5% trypsin (Gibco, cat. no. 15090) and placed in an incubator overnight (5 ± 0.2% CO2 at 37 °C). Finally, the dermis was submitted to irradiation with γ-rays, cryo-frozen and stored in nitrogen vapor at − 180 °C to − 190 °C.
Microbiological and endotoxin analysis were performed to guarantee sterile conditions and, in turn, the clinical use of HDM. Histological analysis as well as 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay were also performed as tests of validation, in order to ensure respectively the maintenance of structural tissue properties and the loss of viable cellular component of HDM (data not shown).
Histology and ultrastructural analysis of biopsy
Histological and ultrastuctural analysis were performed on skin biopsy of the clinical case 3 one year after the treatment with HDM. Briefly, for histological analysis samples were fixed with 10% formalin solution and paraffin embedded. After processing, histological sections (5-μm in thickness) were stained with hematoxilin and eosin. Alternatively, Masson’s trichrome, Weigert or PAS staining were used in order to respectively identify the presence of collagen, elastic fibers or the basal membrane. A semiquantitative morphometric analysis was also performed on histological sections in order to evaluate cellular infiltration, the presence of multinucleated giant cells, vascularity and the organization of connective tissue according to scoring criteria previously established (Valentin et al. 2006) (Table 1). The mean value of four different fields analyzed by a pathologist at forty times magnification is reported in Table 2. Ultrastructural analysis has been performed on glutaraldehyde 2.5% in 0.1M phosphate buffer skin specimens. After post-fixation in OsO4 1% in Cacodylate buffer, specimens were dehydrated and embedded in Araldite epoxy resins. Thin sections were observed with a Philips 410 Philips Trasmissione Electron Microscope (TEM).
Surgical procedures
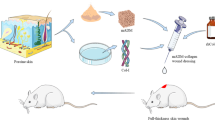
A schematic representation of surgical steps performed for each clinical case is reported in Table 3. After a surgical debridement to remove necrotic tissue, HDM previously fenestrated or not was applied on the exposed osteo-tendineous tissue of all wound areas (Fig. 1a, left panel, clinical case 1). In particular, the total exposition of Achilles tendon in the clinical case 2 was treated totally covering it with HDM (Fig. 1b, upper, right panel). A continuous negative pressure wound therapy (VAC therapy) was then immediately applied for 14 days on the wound area covered with HDM of the clinical case 1 and 2, in order to promote HDM engraftment on the wound bed maintaining its protection and perspiration. After this first treatment, auto-graft skin was also applied on the wound bed of both clinical cases. In particular, the extensive damaged area in the clinical case 2 required the use of auto-graft skin meshed in a ratio of 1:6 applied in combination with allograft skin, according to Alexander surgical technique. VAC therapy was finally performed for additional 14 days using a fat gauze as interface between HDM/skin autograft and the sponge of the VAC system. Differently from the clinical cases 1 and 2, the auto-graft skin was applied on HDM immediately before the application of VAC therapy and the fat gauze in the clinical case 3 (Fig. 1c, central panel). The combined treatment in a single surgical step was preferred in this clinical case to reduce risks of treatment failure since no comorbidity factors were evident. The VAC therapy was then accurately replaced after 3, 7 and 14 days. The study was exempt from approval from an ethics' board. In all cases, significant adverse reactions were not identified. The total healing time, the length of hospital stay, and the time between HDM application and healing of the wound for all clinical cases are reported in Table 4. The healing process as well as the functional and aesthetic rescue of the damaged area after HDM application in combination with graft skin was then evaluated after a follow-up of 4 months (Fig. 2a), 18 months (Fig. 2b) and 1 year (Fig. 2c) in the clinical case 1, 2 and 3, respectively. Histological and ultrastructural analysis were also performed during follow-up on skin biopsy of the clinical case 3 (Fig. 3).
Application of HDM on the osteo-tendineous exposure on the marginal side of the right foot (clinical case 1) (a, left panel) and clinical results after 14 days of VAC therapy (a, right panel). Full-thickness skin wound due to necrotizing fasciitis with exposure of bone tissue and Achilles tendon (clinical case 2) (b, upper, left panel). Application of HDM on Achilles tendon (b, upper, right panel) and healing process after 14 days of VAC therapy (b, lower, left and right panels). Full-thickness skin wound with osteo-tendineous exposure on the malleolar region of the left lower limb due to car accident (clinical case 3) (c, upper panel). Application of HDM and autograft skin on the osteo-tendineous exposure (c, central panel). Engraftment of HDM/auto-graft skin after 14 days of VAC therapy (c, lower panel)
Histological analysis of the wound area after the clinical follow-up (clinical case 3) stained with hematoxylin and eosin (a, left panel) or PAS (a, right panel). Histological analysis of both un-implanted HDM (upper panels) and the replaced dermal layer (lower panels) stained respectively with hematoxylin and eosin (b, upper and lower left panels), Masson’s trichrome (b, upper and lower central panels) or Weigert (b, upper and lower right panels). Ultrastructural analysis performed on skin biopsy of the patient (c)
Results
The clinical cases here described are all characterized by full-thickness skin wounds in which osteo-tendineous exposure and lack of vascularization were identified. Thus, the use of conventional surgical treatments resulted unsuitable for their treatment and the application of HDM in combination with overlying graft skin appeared to be an alternative, efficient treatment to induce healing process.
In the clinical case 1, the full-thickness skin wound showed osteo-tendineous exposure on the marginal side of the right foot due to arterial vascular ulcer previously treated with conventional treatments without any improvement. In addition, the 91-year-old patient showed arteriosclerosis and diabetes, and a total amputation of the fifth toe was also required after the limb surgical revascularization and its aggressive debridement. In this case, HDM was applied on the wound bed in combination with auto-graft skin as described in “Materials and methods” section. A clear reduction of granulation tissue in the damaged area in which HDM was applied alone was already evident after the first 14 days of VAC therapy (Fig. 1a, right panel). Thus HDM was able to induce a modulating effect on the wound healing resulting in a rescue of physiological characteristics of the damaged tissue. Then the application of auto-graft skin on the wound bed followed by additional 14 days of VAC therapy was able to ensure skin engraftment on the wound area. The clinical follow-up was then performed after 4 months resulting in an excellent healing of the damaged area with little scar-like tissue (Fig. 2a). A functional and aesthetic rescue of the damaged area with minimal evidence of inflammatory response was also identified.
In the clinical case 2 a full-thickness skin wound due to necrotizing fasciitis in both legs was evident with exposure of bone tissue as well as Achilles tendon on the left leg (Fig. 1b, circle and arrow respectively, upper, left panel). A gap due to loss of tissue between the Achilles tendon and the remaining damaged area of the left leg was also identified (Fig. 1b, arrow, upper, right panel). In addition, the 55-year-old patient was affected by rheumatoid arthritis and she had been treated with systemic steroid therapy. An inflammatory response was also evident. Similar to the clinical case 1, HDM was immediately applied on bone tissue as well as on the Achilles tendon (Fig. 1b, upper, right panel). The healing process was then already evident on the wound area after 14 days of VAC therapy. In fact both bone tissue and Achilles tendon resulted vital, well vascularized and protected by HDM above them after this first treatment (Fig. 1b, lower, left and right panels). Surprisingly, the gap between Achilles tendon and the remaining damaged area of the left leg resulted totally covered by viable and well vascularized tissue, supporting a regenerative process of healing induced by HDM (Fig. 1b, lower, right panel). No significant adverse reactions were identified. After this first encouraging result, a meshed auto-graft skin (ratio 1:6) in combination with allograft skin was applied on both legs as described in “Materials and methods” section. Then a clinical follow-up of the wound areas was performed after 18 months. As shown in Fig. 2b, a rescue of the damaged area was identified in both legs with good wound healing and a complete engraftment of auto/allograft skin. In particular, the addition of HDM on the left leg was able to cover and protect the exposed bone tissue as well as the Achilles tendon so that similar clinical results were obtained in comparison with the other right, damaged leg (Fig. 2b, right and left panels). A reduction of inflammatory component and an improvement of functional and aesthetic properties of the damaged area was also identified, avoiding the limb’s amputation, previously considered as treatment of choice for the patient in other structures.
In the clinical case 3, the 41-year-old patient showed the full-thickness skin wound with osteo-tendineous exposure on the malleolar region of the left lower limb due to car accident (Fig. 1c upper panel). HDM was immediately applied in combination with auto-graft skin on the wound area (Fig. 1c, central panel). The damaged area was then evaluated after 3, 7 and 14 days of VAC therapy. An initial engraftment of HDM/autologous skin on the full thickness skin wound was already evident after 3 days of VAC therapy, although the two different layers were yet well recognized. Then a continuous improvement of their engraftment as well as an increase of skin thickness on the wound bed was carried out during the following days so that the damaged area appeared covered by a dry and well vascularized skin and the HDM scaffold below could no longer be identified at the end of VAC therapy (Fig. 1c, lower panel). The long term follow-up was then performed after 1 year showing a total healing of the full-thickness skin wound with low, soft and plain similar scar tissue (Fig. 2c). A functional and aesthetic rescue of the damaged area was also identified. In this clinical case, histological and ultrastructural analysis were also performed on skin biopsy of the patient after the clinical follow-up of 1 year. As shown in Fig. 3a (left panel), the histological sections showed a normal epidermis in which the basal layer appears full of keratinocytes that become less evident in the upper layers consistent with their commitment to differentiation. As expected in normal skin, the final loss of their nuclei in the outer corneal layer gave rise to the keratinized surface. The specific PAS staining also showed an intact and well defined basal membrane in the interface between the epidermal and dermal layers (Fig. 3a, right panel). Moreover, we also compared the un-implanted HDM (Fig. 3b upper, left panel) with dermal layer observed on the damaged area after 1 year (Fig. 3b, lower, left panel). The un-implanted HDM showed dense collagen fibers randomly organized (Fig. 3b upper, central panel) as well as few and disrupted elastic fibers (Fig. 3b upper, right panel) as expected (Ghetti et al. 2017a), while the replaced dermal layer showed the presence of well-organized/oriented collagen (Fig. 3b, lower, central panel) and elastic fibers (Fig. 3b lower, right panel) in which cellular infiltration as well as the presence of blood vessels was evident (Fig. 3b, lower left panel), supporting the progressive remodeling of the lesion area induced by HDM. In addition, the presence of multinucleated giant cells was not identified. The specific values of morphometric analysis reported in Table 2 were identified following scoring criteria of the semi-quantitative histological analysis (Valentin et al. 2006) (Table 1). Ultra-structural analysis (Fig. 3c) confirmed the presence of some monocytic infiltrating cells, as light inflammatory reactions (left panel), and a well-defined dermal structure in which not only collagen fibers, in a parallel array, are clearly visible, but also the elastic component (right panel).
Discussion
The continuous innovation in the field of plastic and reconstructive surgery has led in the last decade to the development of new and high-performance substrates presently available for different surgical applications (Shevchenko et al. 2010; Mulder et al. 2012; Hughes et al. 2016). The rescue of functional and aesthetic skin properties in clinical cases of skin wounds is affected by production of scar tissue during the healing process that, in turn, is inversely related to residual dermis on the wound site able to prevent wound contraction (Walden et al. 2000). Thus the presence of dermal layer plays an important role in the healing process and several methods based on skin replacement were developed for the treatment of full-thickness skin wounds (Wainwright and Bury 2011). Among them the use of acellular dermal matrices associated with autograft or allograft skin is increasing as a new reconstructive solution when conventional surgical treatments could not be effective. The strength in the use of acellular matrices is their ability to give a well maintained three-dimensional living place for cells in which the biological stimuli mediated by ECM induce tissue regeneration preventing or reducing contracture and scar tissue formation during wound healing (Aurora et al. 2007; Derwin et al. 2006; Reing et al. 2009). In our study we evaluated the effectiveness of the acellular dermal matrix (HDM) recently developed at Emilia Romagna Regional Skin Bank (Bondioli et al. 2014) for the treatment of three different clinical cases in which full-thickness skin wounds occurred. The clinical cases described were selected for this study since the invasiveness of other surgical techniques such as free flaps approach and their possible poor engraftment on the extensive wound area lacking of vascularization make their use unsuitable for the induction of healing process as well as for permanent wound closure. In particular, the treatment was based on HDM application on the osteo-tendineous exposure of the damaged area followed by skin graft. We identified a double action of the HDM/skin graft combined treatment: an HDM-induced protection of the exposed osteo-tendineous structures and a progressive wound healing, with evidence of tissue regeneration and rescue of functional and aesthetic skin properties of the damaged area during the clinical follow-up. In fact the application of an intact extracellular matrix on the wound area can act as a template, stimulating the production of a functional structure of dermis and avoiding its filling with scar tissue (Livesey et al. 1995). Consistent with the induction of regenerative process we identified cellular infiltration and the presence of new blood vessels on the replaced dermal layer as a sign of neovascularization. Moreover the fibroblast component recruited inside the wound site produced new and well oriented collagen and elastic fibers, as evidence of the progressive remodeling of the lesion area associated with the presence of well-defined elastic fiber as demonstrated also by ultrastructural analysis on skin biopsies. Thus, HDM could restore the lacking ECM structure and acts as a template giving a stimulus to induce its enrichment with host cells. According to these results, similar evidences after the application of HDM for the treatment of abdominal wall defects were recently published (Ghetti et al. 2017a). Moreover, HDM was also used for rotator cuff repair (Fini et al. 2012; Rotini et al. 2011), breast or pelvic reconstruction (Perrone et al. 2016), and abdominal wall reconstruction (Ghetti et al. 2017b) underling its high potential for different clinical applications. Similarly to us, other authors described the use of different biological matrices in a wide range of medical fields that were overviewed considering their general applications (Fosnot et al. 2011). In particular, the use of human dermal matrices in combination with graft skin was evaluated as suitable for the treatment of wounds (Wainwright and Bury 2011; Askari et al. 2011; Yim et al. 2010; Reyzelman et al. 2009; Wainwright 1995; Wainwright et al. 1996) resulting in tissue engraftment on the damaged area and revascularization of the wound bed without sign of necrosis. In our study we observed on the damaged area after the HDM/skin application both tissue engraftment and a rapid revascularization, probably related to the presence of necrotic vascular channels inside HDM (Bondioli et al. 2014) that can be used as preferred “route” by host cells for a rapid vascularization/repopulation of the wound site. Our histological analysis also showed the presence of all epidermal layers after the clinical follow-up of 1 year in the clinical case 3. Noteworthy, keratinocytes in the basal layer stretched over an intact and well defined basal membrane complex that is frequently lost after injury and whose integrity plays an important role during the wound healing process (Chetty et al. 1992) providing elasticity and strength against contractures to the damaged area. The HDM also resulted as well tolerated by the receiving patients without heavy signs of inflammatory response or giant cells in the analyzed histological sections. In other clinical conditions previously reported, different results were obtained after the use of xenograft scaffolds (Mulder et al. 2012; Browne et al. 2001; Zheng et al. 2005; Badylak and Gilbert 2008). On the other hand, the animal-derived matrices are often used for the treatment of skin wounds and their availability in large amount and size is independent from donation of skin tissue, required for human-derived matrices (Jiong et al. 2010). However, an equality to native dermal tissue of human origin is not naturally owned.
Taking into account all these considerations, we can conclude that HDM is able to provide a coverage of high quality, improving patient care when conventional surgical treatments were strongly limited by the local and general conditions of the patients. Thus the use of HDM can be considered as an innovative reconstructive solution for the treatment of full-thickness skin wounds and our previous and current clinical results prompt us to further enhance its use to different clinical fields as a permanent dressing able to induce a regenerative healing process.
References
Askari M, Cohen MJ, Grossman PH, Kulber DA (2011) The use of acellular dermal matrix in release of burn contracture scars in the hand. Plast Reconstr Surg 127:1593–1599. https://doi.org/10.1097/PRS.0b013e31820a6511
Aurora A, McCarron J, Iannotti JP, Derwin K (2007) Commercially available extracellular matrix materials for rotator cuff repairs: state of the art and future trends. J Shoulder Elbow Surg 18:982. https://doi.org/10.1016/j.jse.2007.03.008
Badylak SF (2005) Regenerative medicine and development biology: the role of the extracellular matrix. Anat Rec New Anat 287:36–41. https://doi.org/10.1002/ar.b.20081
Badylak SF, Gilbert TW (2008) Immune response to biologic scaffold materials. Semin Immunol 20:109–116. https://doi.org/10.1016/j.smim.2007.11.003
Bondioli E, Fini M, Veronesi F et al (2014) Development and evaluation of a decellularized membrane from human dermis. J Tissue Eng Regen Med 8:325–336. https://doi.org/10.1002/term.1530
Brennan EP, Reing J, Chew D, Myers-Irvin JM, Young EJ, Badylak SF (2006) Antibacterial activity within degradation products of biological scaffolds composed of extracellular matrix. Tissue Eng 12:2945–2949. https://doi.org/10.1089/ten.2006.12.2949
Brigido SA, Boc SF, Lopez RC (2004) Effective management of major lower extremity wounds using an acellular regenerative tissue matrix: a pilot study. Orthopedics 27(1 Suppl):S145–S149
Brown BN, Badylak SF (2014) Extracellular matrix as an inductive scaffold for functional tissue reconstruction. Transl Res 163:268–285. https://doi.org/10.1016/j.trsl.2013.11.0030
Browne AC, Vearncombe M, Sibbald RG (2001) High bacterial load in asymptomatic diabetic patients with neurotrophic ulcers retards wound healing after application of Dermagraft. Ostomy Wound Manag 47:44–49
Chetty BV, Boissy RE, Warden GD, Nordlund JJ (1992) Basement membrane and fibroblast aberration in blisters at the donor, graft, and spontaneously healed sites in patients with burns. Arch Dermatol 128:181–186
Cohen IK, Diegelmann RF, Lindblad WJ (1992) Wounding healing: biochemical and clinical aspects. WB Saunders Company, Philadelphia
Derwin KA, Baker AR, Spragg RK, Leigh DR, Iannotti JP (2006) Commercial extracellular matrix scaffolds for rotator cuff tendon repair. Biomechanical, biochemical, and cellular properties. J Bone Jt Surg Am 88:2665–2672. https://doi.org/10.2106/JBJS.E.01307
Fini M, Bondioli E, Castagna A et al (2012) Decellularized human dermis to treat massive rotator cuff tears: in vitro evaluations. Connect Tissue Res 53:298–306. https://doi.org/10.3109/03008207.2011.649929
Fosnot J, Kovach SJ 3rd, Serletti JM (2011) Acellular dermal matrix: general principles for the plastic surgeon. Aesthet Surg J 1(7 Suppl):5S–12S. https://doi.org/10.1177/1090820X11417576
Ghetti M, Papa V, Deluca G et al (2017a) Histological and ultrastructural evaluation of human decellularized matrix as a hernia repair device. Ultrastruct Pathol 1:1–7. https://doi.org/10.1080/01913123.2017.1365788
Ghetti M, Bondioli E, Purpura V, Cenacchi G, Ruscelli P, Melandri D (2017b) Decellularized human dermal matrix produced by a skin bank: a new treatment for abdominal wall defects. Ann Ital Chir 5:443–448
Hughes OB, Rakosi A, Macquhae F (2016) A review of cellular and acellular matrix products: indications, techniques and outcomes. Plast Reconstr Surg 138(3 Suppl):138S–147S. https://doi.org/10.1097/PRS.0000000000002643
Ingham E, Matthews J-B, Kearney JN, Gowland G (1993) The effects of variation of cryopreservation protocols on the immunogenicity of allogeneic skin graft. Cryobiology 30:443–458
Jiong C, Jiake C, Chunmao H et al (2010) Clinical application and long-term follow-up study of porcine acellular dermal matrix combined with autoskin grafting. J Burn Care Res 31:280–285. https://doi.org/10.1097/BCR.0b013e3181d0f42d
Livesey S-A, Herndon DN, Hollyoak MA, Atkinson YH, Nag A (1995) Transplanted acellular allograft dermal matrix. Potential as a template for the reconstruction of viable dermis. Transplantation 60:1–9
Mulder G, Wallin K, Tenenhaus M (2012) Regenerative materials that facilitate wound healing. Clin Plast Surg 39:249–267. https://doi.org/10.1016/j.cps.2012.05.006
Perrone AM, Livi A, Fini M et al (2016) A surgical multi-layer technique for pelvic reconstruction after total exenteration using a combination of pedicled omental flap, human acellular dermal matrix and autologous adipose derived cells. Gynecol Oncol Rep 18:36–39. https://doi.org/10.1016/j.gore.2016.10.006
Reing JE, Zhang L, Myers-Irvin J et al (2009) Degradation products of extracellular matrix affect cell migration and proliferation. Tissue Eng Part A 15:605–614. https://doi.org/10.1089/ten.tea.2007.0425
Reyzelman A, Crews RT, Moore JC et al (2009) Clinical effectiveness of an acellular dermal regenerative tissue matrix compared to standard wound management in healing diabetic foot ulcers: a prospective, randomised, multicentre study. Int Wound J 6:196–208. https://doi.org/10.1111/j.1742-481X.2009.00585.x
Rotini R, Marinelli A, Guerra E et al (2011) Human dermal matrix scaffold augmentation for large and massive rotator cuff repairs: preliminary clinical and MRI results at 1-year follow-up. Musculoskelet Surg 95(Suppl 1):S13–S23. https://doi.org/10.1007/s12306-011-0141-8
Shevchenko RV, James SL, James SE (2010) A review of tissue-engineered skin bioconstructs available for skin reconstruction. J R Soc Interface 7:229–258. https://doi.org/10.1098/rsif.2009.0403
Tabata Y (2008) Current status of regenerative medical therapy based on drug delivery technology. Reprod Biomed Online 16:70–80
Valentin JE, Badylak JS, McCabe GP (2006) Extracellular matrix bioscaffolds for orthopaedic applications. A comparative histologic study. J Bone Jt Surg Am. 88:2673–2686. https://doi.org/10.2106/JBJS.E.01008
Vorotnikova E, McIntosh D, Dewilde A et al (2010) Extracellular matrix-derived products modulate endothelial and progenitor cell migration and proliferation in vitro and stimulate regenerative healing in vivo. Matrix Biol 29:690–700. https://doi.org/10.1016/j.matbio.2010.08.007
Wainwright DJ (1995) Use of an acellular allograft dermal matrix (AlloDerm) in the management of full-thickness burns. Burns 21:243–248
Wainwright DJ, Bury SB (2011) Acellular dermal matrix in the management of the burn patient. Aesthet Surg J 31(7 Suppl):13S–23S. https://doi.org/10.1177/1090820X11418202
Wainwright D, Madden M, Luterman A et al (1996) Clinical evaluation of an acellular allograft dermal matrix in full-thickness burns. J Burn Care Rehabil 17:124–136
Walden JL, Garcia H, Hawkins H et al (2000) Both dermal matrix and epidermis contribute to an inhibition of wound contraction. Ann Plast Surg 45:162–166
Yim H, Cho YS, Seo CH et al (2010) The use of AlloDerm on major burn patients: AlloDerm prevents post-burn joint contracture. Burns 36:322–328. https://doi.org/10.1016/j.burns.2009.10.018
Zheng MH, Chen J, Kirilak Y, Willers C, Xu J, Wood D (2005) Porcine small intestine submucosa (SIS) is not an acellular collagenous matrix and contains porcine DNA: possible implications in human implantation. J Biomed Mater Res B Appl Biomater 73:61–67. https://doi.org/10.1002/jbm.b.30170
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interests.
Ethical approval
This article does not contain any studies with human participants performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bondioli, E., Purpura, V., Orlandi, C. et al. The use of an acellular matrix derived from human dermis for the treatment of full-thickness skin wounds. Cell Tissue Bank 20, 183–192 (2019). https://doi.org/10.1007/s10561-019-09755-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10561-019-09755-w