Abstract
Bone marrow-derived mesenchymal stem cells (MSC) can be differentiated into myocytes, as well as adipocytes, chondrocytes, and osteocytes in culture. Calcium channels mediate excitation-contraction coupling and are essential for the function of muscle. However, little is known about the expression of calcium channel subunits and calcium handling in stem cells. We examined whether the expression of calcium channel subunits in MSC is similar to that of skeletal muscle satellite cells and if their levels of expression are modified after treatment with bone morphogenetic protein-4 (BMP4). We found that during myogenic differentiation, MSC first express the α2δ1 subunit and the cardiac channel subunit Cav1.2. In contrast to the α2δ1 subunit levels, the Cav1.2 subunit decreases rapidly with time. The skeletal channel subunit Cav1.1 is detected at day 3 but its expression increases considerably, resembling more closely the expression of the subunits in satellite cells. Treatment of MSC with BMP4 caused a significant increase in expression of Cav1.2, a delay in expression of Cav1.1, and a reduction in the duration of calcium transients when extracellular calcium was removed. Calcium currents and transients followed a pattern related to the expression of the cardiac (Cav1.2) or skeletal (Cav1.1) α1subunits. These results indicate that differentiation of untreated MSC resembles differentiation of skeletal muscle and that BMP4 reduces skeletal muscle calcium channel expression and promotes the expression of cardiac calcium channels during myogenic differentiation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
It is well known that bone marrow mesenchymal stem cells (MSC) can be induced into myogenic differentiation [1–3]. However, little is known about their developmental pathway and whether or not it is similar to the one followed by early skeletal and cardiac cells. We have previously shown that during myogenic differentiation MSC co-express cardiac- and skeletal-specific proteins such as Nkx2.5, GATA4, cardiac troponin-T (cTnT), MyoD, and skeletal actin, among others [4]. In this study we evaluated the RNA and protein expression of the subunits of the skeletal and cardiac L-type calcium channels and the cardiac T-type calcium channel. We recently showed [4] that during myogenic differentiation a group of MSC seemed to follow the skeletal-like pathway by expressing proteins in a similar temporal pattern as satellite cells isolated from skeletal muscle. Specifically there was an early expression of cTnT (day 6), followed by a significant reduction in its expression by day 15, in addition to the formation of multinucleated myotubes with expression of skeletal actin distributed throughout the whole cell. In the same culture, there was another group of cells that followed a cardiac-like pathway characterized by remaining mostly mononucleated and expressing cTnT and MLC2V throughout the whole cell. In addition, in our previous studies, we showed that when MSC were treated with bone morphogenetic protein 4 (BMP4), there was a shift in the differentiation of MSC towards the cardiac-like pathway, with an increase in RNA expression of Nkx2.5 and cTnI, and an increase of the action potential duration compared to untreated MSC.
Supra-threshold depolarization of the plasma membrane in skeletal and cardiac muscle generates action potentials, an increase in myoplasmic calcium, and contraction. Key membrane proteins mediating the coupling of excitation and contraction are voltage-sensitive calcium channels in the plasma membrane. While the composition of skeletal muscle and cardiac L-type calcium channels is well established as being formed by α1, α2δ, β, and γ subunits, the cardiac T-type channel is formed by an α1 subunit and perhaps a γ subunit [5]. Interestingly, the subunits of skeletal muscle L-type calcium channel are not expressed simultaneously during differentiation of myotubes in vitro. We have shown that the α2δ1 subunit is the first one to be expressed in freshly dissociated satellite cells and is followed by expression of the α1, β, and γ subunits [6, 7]. Further, we have shown that more than 50 % of freshly isolated satellite cells express α2δ1 subunit and that these cells commit to the muscle lineage to form myotubes [7].
Although cardiac and skeletal muscle L-type calcium channels have the same type of subunits, the excitation-contraction coupling (ECC) mechanism is quite distinct. In cardiac muscle, entry of calcium through the L-type calcium channel activates the ryanodine receptor (RyR2), whose opening allows calcium release from the sarcoplasmic reticulum. This is termed calcium-induced calcium release (CICR). In contrast, calcium entry in adult skeletal muscle is not necessary for calcium release; instead, there is a direct mechanical coupling of the L-type calcium channel with the ryanodine receptor (RyR1) and activation of the L-type calcium channel causes opening of RyR1. During development, skeletal muscle has a mix of CICR and direct coupling of ECC mechanism until it matures and only the direct coupling mechanism remains. [8].
In this study, we examined whether the expression of calcium channel subunits in MSC is similar to those of satellite cells and whether their expression is modified after treatment with BMP4. Our results show that α2δ1 is the first subunit to be expressed in MSC. In addition, MSC express very little of the cardiac L-type calcium channel α1 subunit (Cav1.2) and significantly increase the expression of the skeletal muscle calcium channel α1 subunit (Cav1.1), resembling more closely the calcium channel expression of satellite cells. However, after treatment of MSC with BMP4, there was a significant increase in the expression of Cav1.2 and a delayed expression of Cav1.1. We also measured calcium currents from each of the cell types and our results show a shift in the membrane voltage at which the maximum calcium current occurs. In addition, recordings of calcium transients show that BMP4-treated MSC are more sensitive to the presence of extracellular calcium, producing a significant reduction in the calcium transient signal amplitude and duration in the absence of extracellular calcium compared to untreated MSC and satellite cells. The changes in calcium transients in the absence of calcium are reminiscent of the calcium-induced calcium release mechanism of cardiac cells. These data improve our understanding of MSC and satellite cell myogenic differentiation as it relates to calcium channels and demonstrate that the early expression of the α2δ1 subunit is a common phenomenon in MSC and satellite cells. Furthermore, it shows that BMP4 reduces skeletal muscle calcium channel expression and promotes the expression of cardiac calcium channels during myogenic differentiation.
Materials and Methods
Animals
Mice were maintained in accordance with the Guide for the Care and Use of Laboratory Animals (National Research Council, revised 2011). Experimental protocols were approved by the Institutional Animal Care and Use Committee.
Harvesting of Bone Marrow Mesenchymal Stem Cell (MSC)
Bone marrow was isolated from 3 to 6 month old FVB.Cg-Tg(GFPu)5Nagy/J mice (Jackson Laboratory) as previously described [9, 10]. Briefly, tibia and femur bones were stripped of muscle and placed in ice cold PBS + 2 % FBS. The epiphyseal ends were removed and the bones were centrifuged at 4000 g for 1 min in a microfuge tube. The bone marrow cells were suspended in ice cold PBS + 2 % FBS, passed through a 70 μm filter and counted. Filtered bone marrow cells were enriched for lineage negative (Lin-) cells using the SpinSep system (Stem Cell Technologies). Cells were incubated with murine progenitor enrichment cocktail (anti-CD5, anti-CD45R, anti-CD11b, anti-Gr-1, anti-TER119, and anti-7/4; Stem Cell Technologies) on ice for 30 min and, after washing, incubated with dense particles on ice for 20 min. Cells were layered on density medium, centrifuged at 1200 g for 10 min, and the layer of cells at the density medium/PBS interface was collected, washed, and counted. Enriched bone marrow cells were placed on tissue culture-treated plates at a density of 0.1 × 106 cells/cm2 in murine Mesencult media (Stem Cell Technologies) with 100 units/ml penicillin, 100 μg/ml streptomycin, and 0.25 μg/ml amphotericin B. The media was changed after 48 h. Adherent cells [11] were maintained in culture with media changes twice a week. After 4 weeks the confluent cells were detached with trypsin and split 3:1. Lin− MSC were characterized for surface antigens using flow cytometry as described in Boomsma et al. [9].
MSC Differentiation Treatment with 2 % or 5 % BCS and Treatment with BMP4
Cells passages P11-P13 were allowed to attach to the tissue culture plates (BD Falcon) by culturing them for 24 h in Eagle’s MEM with 15 % BCS. The next day corresponds to the beginning of differentiation (D0), when cells were treated with low serum media (2 % BCS) for 72 h, after which the media was replaced every three to four days using either MEM with 2 % or 5 % BCS and 5 μg/ml of insulin and transferrin, 5 ng/ml of sodium selenite (Sigma: ITS, I1884 prepared as recommended) for each media group. For RNA and FACS analysis, cells were plated at a density of 1550 cells per cm2 in 10-cm tissue culture-treated plates for the times indicated in each experiment, D0-D20. For immunohistochemistry studies, MSC were plated on sterile 22 mm glass cover slips placed on 10 cm plates. For patch clamp experiments, cells were cultured in 35 mm primaria culture dishes at a density of 15 × 103 cells per dish for the times indicated in each experiment, D0-D20.
After the initial 24 h culture in Eagle’s MEM with 15 % BCS, cells were treated with bone morphogenetic protein-4 (BMP4) media, composed of low serum media (Eagle’s MEM with 2 % BCS) and 25 ng/ml of BMP4 [12] (Sigma-Aldrich) for 84 h. The media was then replaced every three to four days with MEM with 5 % BCS and 5 μg/ml of insulin and transferrin, 5 ng/ml of sodium selenite as above.
Skeletal Satellite Cell Isolation and Differentiation
Primary satellite cells were isolated from limb muscles of neonatal mice (0–48 h), as described [13]. Briefly, muscles were finely minced and incubated for a total of 40 min in Ca2 + − and Mg2 + −free rodent Ringer solution (in mM): 155 NaCl, 5 KCl, 10 HEPES, and 11 D-glucose containing 0.3 % trypsin, 0.1 % collagenase, and 0.01 % DNAse. Large debris was removed by centrifugation and filtration. 2.50 × 105 cells were plated onto 35-mm Falcon primaria culture dishes containing 80 % Dulbecco’s modified Eagle’s medium (DMEM) with 4.5 g/L glucose, 10 % horse serum, and 10 % calf serum. After 48 h, the plating media was replaced with DMEM plus 2 % horse serum to promote cell differentiation. Myotube age was recorded starting at this time, corresponding to D0. After 72 h, a group of cells had a second media change to DMEM plus 5 % horse serum. All cultures contained penicillin (100 U/mL) and streptomycin (100 mg/ml). For immunohistochemistry studies, satellite cells were plated on 22 mm collagen-coated cover slips.
Immunofluorescence Staining
Cells were stained for cardiac-specific troponin T isoform Ab-1, (cTnT; Thermo Scientific MS-295-P0, 1:200), L-type calcium channel Cav1.2 subunit (sc-25686, 1:100), Cav1.1 subunit (sc-21782, 1:200), and T-type calcium channel Cav3.2 subunit (sc-25691, 1:100). The last three antibodies for calcium channel subunits were obtained from Santa Cruz Biotechnology. Cells were fixed with 2 % paraformaldehyde for 20 min. We used horse and goat serum (5 %) diluted in 0.1 % Tween in PBS for blocking and permeabilization for 45 min. Cells were incubated overnight in primary antibody at 4 ° C. Biotinylated horse anti-mouse IgG (Vector Labs BA-2001) for 1 h at room temperature, 1:250 and Alexa Fluor 555 streptavidin (Invitrogen: S21381, 1:1000) were used for fluorescent detection of cTnT. For Cav1.2 and Cav3.2, we used an Alexa Fluor 488 goat anti-rabbit secondary antibody (Jackson: 111-546-144, 1:700) and Alexa Fluor 555 goat anti-mouse for Cav1.1. After washing, cells were mounted with DAPI (Vector Laboratories, Inc). As a negative control for fluorescence comparison we used an isotype specific IgG1 (R&D Systems: MAB 002, 1:200).
cDNA Sequencing and RT-qPCR
Total RNA was obtained from satellite cells, control MSC, and BMP4-MSC cultures in the differentiation media described earlier. Extractions for satellite cells were obtained from two 35 mm culture plates for each day (0–6). Extractions for MSC were obtained from two (D0, D3, D6) and one (D9, D12, D15, D20) 10 cm culture plates, and days 0, 3, 6, 12, and 20 for MSC-BMP4. RNA extractions were done following Qiagen RNeasy mini-kit with the addition of DNAse digestion. The RNA density for each sample was measured and normalized to the lowest RNA density found in the sample group and reverse-transcribed to cDNA using ImProm-II kit with a random primer (Promega). Real-time quantitative polymerase chain reaction (RT-qPCR) (Applied Biosystems 7500) was done with a volume of 20 μl, composed of Fast SYBR® Green Master Mix in molecular grade water, 0.5 μM each of forward and reverse gene-specific primer, and 1 μl of reverse-transcribed cDNA solution. All genes, except 18 s, were run between 59 and 60 °C annealing temperature. The 18 s gene was run at 55 °C. Dissociation curves were obtained for all gene/cDNA mixes and for the primer without cDNA for control and comparison. The numbers of independent cell cultures analyzed were n = 3, and each gene/cDNA mix was analyzed in triplicate in 96 well plates. The mRNA fold change was normalized with respect to D0 (or the first day mRNA appeared) in Figs. 1, 2, and 3, and for Table 2, the fold change was normalized with respect to the average expression of α2δ1 in satellite cells at D0. In both cases, the comparative CT method was used as described in Livak & Schmittgen [14] and Schmittgen & Livak [15]. All genes were referenced to the geometric mean of at least two control genes selected from YWHAZ, 18 s, and HPRT1 [16]. The primer sequences are given in Table 1.
Temporal mRNA expression of calcium channel subunits in satellite cells in 2 and 5 % serum from day 0 to day 6 (D0-D6). D0 starts after the cells have been 48 h in 20 % serum. a. L-type calcium channel subunit Cav1.1expression increased significantly in the 2 % serum cells, with its highest expression at D5. b. L-type calcium channel subunit Cav1.2 expression was present but unchanged throughout the differentiation period. C. T-type calcium channel subunit Cav3.2 expression increased in both serum groups, with the highest increase at D4 in 2 % serum and a similar increase in 5 % by D6. d. Associated calcium channel subunit α2δ1 expression increased significantly in both serum groups by D4. e. Associated calcium channel subunit β expression increased significantly by D4 in the 2 % serum and remained unchanged in the 5 % group. f. Associated calcium channel subunit γ expression increased significantly in the 2 % serum group by D4 and remained unchanged in the 5 % group. Fold changes were calculated with respect to D0 utilizing the comparative CT method. Average values represent mean ± sem, and significant differences between paired data are represented by solid lines, p < 0.05. The * means that the data point is different from all others except from those that have a #. Three independent cell cultures were analyzed, and each gene was analyzed in triplicate, n = 9. All genes were referenced to the geometric mean of at least two control genes selected among YWHAZ, 18 s, and HPRT1
Temporal mRNA expression of calcium channel subunits in MSC in 2 % or 5 % serum from day 0 to day 20 (D0-D20). D0 starts after the cells have been 24 h in 20 % serum. a. L-type calcium channel subunit Cav1.1 expression was undetected at D0 and appeared by D3. Its expression increased significantly over time in both serum conditions. b. L-type calcium channel subunit Cav1.2 expression significantly decreased during the differentiation period for both serum conditions. c. T-type calcium channel subunit Cav3.2 expression was undetected at D0 and was clearly observed by D3. Its expression decreased during differentiation, most significantly in the 2 % group. d. Associated calcium channel subunit α2δ1 expression increased in the 5 % serum group and was unchanged in the 2 % serum group. D20 with 5 % serum (marked with *-2 %) was significantly higher than all other days in 2 % serum. e. Associated calcium channel subunit β expression increased significantly by D9 and peaked at D15 in the 5 % serum group, but remained unchanged in the 2 % group. f. Associated calcium channel subunit γ expression increased significantly in the 5 % serum group by D9 and remained unchanged in the 2 % group. Fold changes were calculated with respect to D0, except in Cav1.1 and Cav3.2 cases where fold changes were calculated with respect to D3 (marked with a square). Average values represent mean ± sem, and significant differences between paired data are represented by solid lines, p < 0.05. The * means that the data point is different from all others except from those that have a # or &. Three independent cell cultures were analyzed, and each gene was analyzed in triplicate, n = 9. All genes were referenced to the geometric mean of at least two control genes selected among YWHAZ, 18 s, and HPRT1
Temporal mRNA expression comparison between MSC in 5 % serum and BMP4-treated MSC from day 0 to day 20 (D0-D20). D0 starts after the cells have been 24 h in 20 % serum. a. L-type calcium unit Cav1.1 expression was delayed in BMP4 treated cells, having a detectable expression by D12, compared to D3 in untreated cells. Fold changes for the BMP4 treated cells were calculated with respect to D12. Overall, early after BMP4 treatment Cav1.1 expression was significantly attenuated in MSC compared to the untreated case. b. L-type calcium unit Cav1.2 expression significantly increased in the BMP4 treated cells by D3, with nearly a 4-fold increase compared to untreated cells. c. T-type calcium unit Cav3.2 expression in the 5 % case and BMP4 was insignificant at D0, and was expressed by D3 without any significant difference in the expression by D20. D. Calcium subunit α2δ1 expression increased significantly by D12–D20 in the BMP4 treated cells compared to the untreated group. e. Calcium subunit β expression increased significantly by D12 in both BMP4 treated and untreated cells. f. Calcium subunit γ expression increased significantly in the BMP4 treated cells by D12 compared to untreated cells, exhibiting an even greater increase at D20. Fold changes for the 5 % case were calculated as described in Fig. 2. Fold changes for the BMP4 treated cells were calculated with respect to D0, except in Cav1.1 and Cav3.2 cases, where changes were calculated with respect to D12 (for Cav1.1) and D3 (Cav3.2). Average values represent mean ± sem, and significant differences between paired data are represented by solid lines, p < 0.05. The * means that the data point is different from all others except from those that have a #. Three independent cell cultures were analyzed, and each gene was analyzed in triplicate, n = 9. All genes were referenced to the geometric mean of at least two control genes selected among YWHAZ, 18 s, and HPRT1
Fluorescence Activated Cell Sorting (FACS) Analysis
Differentiated MSC and BMP4-treated cells were cultured for 6 days and detached from the plates with 0.05 % trypsin. Each cell group was fixed with 2 % paraformaldehyde. Cells were perforated with cold methanol for 1 h and washed. Thereafter, three groups of MSC-D20 and BMP4-D20 cells were treated with the L-Type Ca2+ channel Cav1.2 antibody (Santa Cruz), another group of MSC-D20 and BMP4-D20 cells were used for controls and treated with IgG antibody (R&D Systems: MAB002, 1:200) and left overnight at 4 °C. The next day, cells were washed and treated with the secondary goat anti rabbit (Jackson: 111-496-144, 1:300). After washing, cells were analyzed with FACS.
Electrophysiological Measurements
Data acquisition and processing were performed with pCLAMP 8.0 software (Axon Instruments). Recording electrodes were pulled from borosilicate glass with resistances between 2 and 3 MΩ when filled with a solution containing (in mM) 140 Cs-aspartate, 5 MgCl2, 2.5 Mg ATP, 0.5 Tris GTP, 10 Cs2EGTA, and 10 HEPES (pH 7.2 adjusted with CsOH). The extracellular solution contained (in mM) 145 tetraethylammonium chloride, 10 CaCl2, 10 HEPES, and 0.001 TTX, (pH 7.4 adjusted with CsOH). I Ca was measured using the whole cell configuration of the patch-clamp technique [17]. Membrane linear components were digitally subtracted by appropriate scaling of the average of eight hyperpolarizing current records obtained with a 10 mV pulse delivered from the membrane holding potential of −80 mV. I Ca was elicited with 250 ms test pulses, which were preceded by a 1-s prepulse to −50 mV to inactivate T-type and sodium currents. To normalize for differences in total membrane area, current densities (in pA/pF) were calculated by dividing the total current by the membrane capacitance of the cell. The current–voltage relationship from a single cell was fit to the Boltzmann equation \( {\mathrm{I}}_{Ca}={G}_{max}\left(V-{V}_{rev}\right)/\left(1+{e}^{\left(\frac{V_{1/2}-V}{k}\right)}\right) \), where G max is the maximum conductance, V is the membrane potential, V rev is the reversal potential, V 1⁄2 is the half-activation potential, and k is the voltage steepness factor [18]. Activation kinetics parameters of I Ca were obtained by fitting the rising phase of the current to the following double exponential equation \( {\mathrm{I}}_{(t)}={A}_{fast}\left({e}^{\left(\frac{-t}{\tau fast}\right)}\right) + {A}_{slow}\left({e}^{\left(\frac{-t}{\tau slow}\right)}\right) + C \), where A fast and A slow are the amplitudes, τ fast and τ slow are the time constants of activation, and C is the steady state current at maximum amplitude.
Calcium Transient Measurements
Differentiated cells were incubated with 5 μM Fluo-4-AM (Invitrogen) for 30 min. Thereafter, cells were washed and incubated at 37 °C and allowed to rest for 15 min. Fluo-4-AM was excited at 488 nm and the emission was collected above 515 nm. Cells were electrically stimulated with an extracellular electrode with a 5-ms, 80 V pulse at a frequency of 1.0 Hz. After 15 s, the Ringer’s solution (in mM: 146 NaCl 2, CaCl2, 1 MgCl2, 5 KCl, 10 HEPES, 11 glucose, pH 7.4) was replaced with calcium-free Ringer’s solution. Video was recorded continuously during the test at 30 images per second; however, to reduce bleaching of the fluorescent dye, the microscope light source was blocked during the initial periods of media replacement activity. Areas of pixels within the cell’s active region were selected and analyzed for calcium transient utilizing spatial averaging (defined as the sum of the fluorescence signals of all pixels in the region divided by the number of region pixels) and evaluated as a function of time. The parameters measured included the Ca2+ transient frequency and Ca2+ transient duration at 50 % (TD50) or 90 % (TD90) of the amplitude. Cells were also evaluated for caffeine response by replacing the Ringer’s solution with 10 mM caffeine in calcium-free Ringers. The parameters measured under these conditions included change in amplitude and the rate of rise and rate of decay at 50 % of the amplitude.
Statistical Data
Values are presented as means ± sem. Differences between groups were evaluated using GraphPad Prism One-way analysis of variance (ANOVA) and Tukey’s test for multiple comparisons. For experiments where only two sets of data were compared, we used the unpaired t-test method. A result of p < 0.05 was considered statistically significant.
Results
Expression of Calcium Channel Subunits in Satellite Cells
It is well known that differentiation of satellite cells is promoted when the serum in the culture medium is reduced. We have previously shown that in low serum media, e.g. 2 % or 5 %, satellite cells form contracting myotubes by D6 [4]. We also showed that satellite cells in 2 % serum expressed cardiac-specific mRNA and proteins troponin-T (cTnT), troponin-I (cTnI), and myosin light chain-2 V (MLC2V) at the start of differentiation but by D6 their expression was attenuated. In contrast, the skeletal-specific genes, slow-skeletal-TnI (ssTnI) and skeletal-actin (Sk-Act) expression increased significantly by D5-D6. In the present work, we examined the temporal expression of the skeletal L-type calcium channel subunit Cav1.1, the cardiac L-type calcium channel subunit Cav1.2, and the T-type channel Cav3.2 in satellite cells and MSC. Results obtained with satellite cells are presented in Fig. 1 and show that the expression of Cav1.1 increased significantly over time in 2 % serum (Fig. 1A). Cells had the highest expression of Cav1.1 (9-fold change) at D5. Unexpectedly, the expression of Cav1.1 did not change significantly from D0-D6 in cells exposed to 5 % serum. In contrast, Fig. 1b shows that Cav1.2 was present at low levels in satellite cells and remained unchanged throughout the differentiation period. There were no significant differences in the expression of Cav1.2 in media with 2 % or 5 % serum. The T-type calcium subunit Cav3.2 expression increased in both serum groups over time, with the highest increase (6-fold change) at D4 in 2 % serum and a similar increase in the 5 % group by D6 (Fig. 1c). Additionally, we evaluated the RNA expression of the associated calcium channel subunits: α2δ1, β and γ. Figure 1d shows that α2δ1 expression increased significantly in both serum groups by D4. On the other hand, expression of the β and γ subunits increased significantly by D4 in 2 % serum and remained unchanged in the 5 % group (Fig. 1e and f, respectively).
These results show that satellite cells exhibit RNA expression of both L-type calcium channel subunits Cav1.2 and Cav1.1 early on (D0), but when the media was changed to 2 % serum, there was a significant increase in the expression of only the skeletal subunit Cav1.1; the increase in Cav1.1 subunit levels was maintained throughout the differentiation period. Similarly, there was an increase of the associated subunits α2δ1, β and, γ after D4 in the 2 % group. Increases in calcium channel subunit RNA expression were mostly present in the 2 % serum group and stayed nearly constant in the 5 % serum group, indicating higher myogenic differentiation and calcium channel expression in the lower serum group.
Expression of Calcium Channel Subunits in MSC
MSC undergo myogenic differentiation when cultured in low serum conditions (2 % or 5 % serum). However, MSC need longer time in culture to exhibit calcium transients and action potentials than satellite cells. During differentiation, MSC express cTnT as early as D6 and by D15 some reveal calcium transients and action potentials [4, 10]. Thus, to further understand the similarities and differences between MSC and satellite cell differentiation, we evaluated RNA expression of the calcium channel subunits in 2 and 5 % serum conditions in MSC from D0 to D20. We refer to D0 to the day when serum was reduced to promote differentiation. Figure 2a shows that the expression of Cav1.1 was nearly undetected at D0 and was unequivocally detected by D3 (see also Table 2). After this time, its expression increased significantly over time in both serum conditions. Figure 2b shows that expression of Cav1.2 significantly decreased during the differentiation period in both serum conditions. Cav3.2 subunit expression (Fig. 2c) was nearly insignificant at D0 but was clearly observed by D3. Interestingly, expression of Cav3.2 decreased during differentiation after D3, and most significantly in the group with 2 % serum. In marked contrast, MSC had a robust expression of α2δ1 at D0 without a significant change in its levels for other days in 2 % serum. In contrast, expression of the α2δ1 subunit increased in the 5 % serum group with time. Expression of the β and γ subunits was substantially lower than expression of α2δ1 at D0 (Table 2). However, the β subunit increased significantly by D9 and peaked at D15 in the 5 % serum group, but remained practically unchanged in the 2 % group after D9 (Fig. 2e). Expression of the γ subunit increased significantly in the 5 % serum group by D9 and with a tendency to increase in the 2 % group, but this difference was not statistically significant (Fig. 2f).
Table 2 shows a comparison between the RNA expression of calcium channel subunits in satellite cells with those of MSC at the beginning of differentiation in 2 % serum (D0). Results were normalized with respect to the α2δ1 expression in satellite cells since we have shown that this subunit is present in freshly dissociated cells [7]. The table shows that α2δ1 expression in satellite cells was nearly 1.4 times higher than in MSC. Also, the expression of α2δ1 for MSC was nearly 23, 14, and 1.9 times higher than Cav1.2, β and, γ respectively; there was no detectable expression of Cav1.1 and Cav3.2 for MSC at D0. For satellite cells, α2δ1 was nearly 28, 20, 8, and 1.37 times higher than Cav3.2, Cav1.1, β, and Cav1.2, respectively. However, expression of the γ subunit was nearly 1.6 times higher than α2δ1 expression in satellite cells, reflecting the increase in expression of this subunit in 20 % serum before changing to differentiation medium.
The MSC RNA results show that the largest RNA increase was in the Cav1.1 subunit, with a larger fold increase compared to satellite cells in 2 % serum at D20 (compare Figs. 2a and 1a). However, MSC exhibited a decrease in expression of Cav1.2 by D9 (Fig. 2b). Additionally, expression of Cav3.2 remained low and even decreased after its transient appearance at D3 (Fig. 2c). Similar to satellite cells, α2δ1, β and γ were present in MSC at D0 in 2 % serum; however, their expression at D0 was low compared to the levels found in satellite cells (Fig. 2d-f and Table 2). Nevertheless, MSC RNA expression of α2δ1, β and, γ exhibited a significant increase in 5 % serum between D9-D15. Throughout MSC differentiation, there was an increasing expression of Cav1.1 RNA and a loss of Cav1.2 expression, indicating an inclination of MSC to enter the skeletal-like pathway.
Expression of Calcium Channel Subunits in MSC treated with BMP4
Our RNA calcium channel data in MSC show a weak commitment of the myogenic cells towards the cardiac-like calcium channel pathway, showing a decrease in Cav1.2 RNA expression (Fig. 2b). Thus, we questioned whether treating MSC with BMP4 will alter the RNA expression of the calcium channel subunits since it has been shown that BMP4 induces cardiac differentiation in embryonic and inducible pluripotent stem cells [19, 20]. In our previous experiments, we observed increased RNA and protein expression of Nkx2.5 and cTnI in BMP4-treated MSC compared to untreated MSC [4]. Thus, we modified our protocol to include the transient addition of BMP4 during the first days of the differentiation period (D0 to D3) when the 2 % serum was first applied. After D3, the medium was switched to 5 % serum as described in the methods section. Figure 3a shows that there was a delay in the expression of Cav1.1 in BMP4-treated cells. Cav1.1 expression was first detected by D12 in BMP4-treated cells compared to D3 in untreated cells. Fold changes for Cav1.1 were calculated with respect to D12 in BMP4-treated cells. Figure 3b shows that the RNA expression of Cav1.2 significantly increased in the BMP4-treated cells by D3, with nearly a 4-fold increase compared to untreated cells. Figure 3c shows that there were no significant differences in Cav3.2 expression between the BMP4-treated and untreated cells. BMP4-treated cells expressed Cav3.2 by D3 and by D20 its expression was not significantly different than D3. In contrast, the α2δ1 subunit was present in both treated and untreated MSC and its expression increased significantly by D12 in the BMP4 treated cells compared to the untreated group (Fig. 3d). There was no difference in the expression of the β subunit between treated and untreated MSC, although in both conditions it showed an increase by D12 (Fig. 3e). In contrast, expression of the γ subunit increased significantly in BMP4-treated cells by D12 compared to untreated cells, and this increase was even larger at D20.
Overall, the transient BMP4 treatment results show a modification of RNA expression of calcium channels with an increased expression of the cardiac subunit Cav1.2, and an early attenuation of the skeletal subunit Cav1.1 compared to untreated MSC. These results indicate a transient shift towards the cardiac-like pathway and an early attenuation of the skeletal-like pathway in MSC with transient BMP4 treatment. Similarly, BMP4 treatment also caused an increase in the associated calcium channel subunits α2δ1 and γ. Whereas the α2δ1 subunit showed only a 4-fold (control MSC) or 8-fold (BMP4-treated MSC) increase at D12 compared to D0, the β subunit increased by more than 20-fold under both conditions at D12 compared to D0 and the γ subunit increased by more than 80-fold (control MSC) or 120-fold (BMP4-treated MSC) after D12. The ratios of increase of the associated subunits in MSC resemble those previously reported for satellite cells [7] although the nature of the α1 subunit is different between satellite cells (Cav1.1) and MSC (Cav1.2).
Calcium Channel Protein Expression
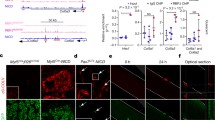
Our studies indicate modifications of expression of the RNA calcium channel subunits when MSC are treated with BMP4. Thus, we complemented these studies with protein expression studies using immunohistochemistry and flow cytometry for quantification. From our RNA results from Fig. 3b, we noticed that Cav1.2 RNA expression had the highest fold increases between D3-D6 for the BMP4-treated cells. Thus, we performed a quantitative protein analysis using FACS with D6 differentiated MSC and BMP4-treated MSC, stained with a Cav1.2 antibody. The results given in Fig. 4a show a positive distribution of the Cav1.2 protein expression compared to the control labeling with IgG. In addition, the BMP4-treated MSC had a higher number of cells with increased fluorescent expression, represented by a right shift in the mean fluorescence value of 2.24 ± 0.82 compared to the mean fluorescent value of untreated MSC. Along with the FACS data, we obtained immunohistochemistry data of Cav1.2 protein expression in MSC and BMP4-treated MSC at D6 (Fig. 4b and c, respectively). cTnT was used to identify cells undergoing cardiac-like myogenic differentiation as previously reported [4].
Protein expression of Cav1.2 and Cav3.2. a. Quantitative comparison of Cav1.2 protein expression in MSC and BMP4 treated MSC at D6 using FACS analysis. The results show a positive distribution of the Cav1.2 expression compared to the IgG expression. BMP4-treated MSC had a higher number of cells with increased fluorescent expression, represented by a right shift in the mean fluorescence value compared to the mean fluorescence value of untreated MSC. b-c. Immunohistochemistry data of Cav1.2 protein expression (green) in MSC (B) and BMP4-treated MSC (C) at D6. cTnT (in red) was used to identify cells undergoing cardiac-like myogenic differentiation. d-f. Immunohistochemistry data of Cav1.2 protein expression (green) in Sk-sat 2 % at D6 (D), and MSC (E) and BMP4-treated MSC (F) at D20. Staining patterns for Cav1.2 are more defined around the cell compared to D6. They also show that Cav1.2 protein expression presence is not exclusive to cTnT positive cells. g-i. Immunohistochemistry data of Cav3.2 protein expression (green) in Sk-sat 2 % at D6 (G), and MSC (H) and BMP4-treated MSC (I) at D20. Staining patterns are more defined in Sk-sat 2 % and MSC compared to BMP4 treated MSC at D20
Figure 4d-f show immunohistochemistry data for Cav1.2 protein expression in satellite cells 2 % at D6 (D), and MSC (E) and BMP4-treated MSC (F) at D20. Staining patterns for Cav1.2 are more defined around the cell compared to D6 in Fig. 4b and c. These results also show that Cav1.2 protein expression presence is not limited to the cells expressing cTnT. Figures 4g-i show immunohistochemistry data of Cav3.2 protein expression and cTnT in satellite cells 2 % at D6 (G), MSC (H), and BMP4-treated MSC (I) at D20. Staining patterns are more defined in satellite cells in 2 % and MSC compared to BMP4 treated MSC at D20.
Our RNA studies also show that for Cav1.1, the highest expression occurred in satellite cells with 2 % serum. In addition, the RNA results for the BMP4-treated MSC show a delay in the expression of Cav1.1 until D12. Thus, we performed Cav1.1 immunohistochemistry studies for satellite cells (2 and 5 %), MSC, and BMP4-treated MSC as shown in Fig 5a-d, respectively. Satellite cells with 2 % serum at D4 had the largest protein expression compared to the other cell groups. This pattern of expression is in agreement with the temporal RNA expression described for Cav1.1.
Immunohistochemistry data for Cav1.1. a-d. Immunohistochemistry data of Cav1.1 protein expression (red) in satellite cells with 2 % serum at D4 (A), Sk-sat with 5 % serum at D6 (B), MSC in 5 % serum at D12 (C), and BMP4-treated MSC at D12 (D). Sk-sat with 2 % serum show the brightest protein expression compared to the other cell groups, this expression characteristic being consistent with the temporal RNA expression described for Cav1.1 in Figs. 1, 2, and 3
Overall, these experiments show that the protein expression data are in agreement with the RNA data, and allow us to conclude that there is an enhancement of both Cav1.2 RNA and protein expression in MSC when treated with BMP4. In addition, it corroborates the presence of the Cav1.1 subunit in satellite cells and its high expression in 2 % serum compared to the other cell groups.
Calcium currents of Satellite Cells, MSC, and BMP4-treated MSC
Our observations in the changes in calcium channel subunits RNA and protein expression in satellite cells and in MSC with or without BMP4 treatment led us to evaluate the changes in calcium currents. We measured calcium currents in all three cell groups and examined whether there were distinguishable modifications on the BMP4-treated MSC compared to untreated cells.
Calcium current (I Ca) densities were measured at membrane potentials ranging from −40 to 60 mV. Figures 6a-c show the current densities and I Ca records for satellite cells in 2 % serum obtained from D4-D6 cells, and MSC and BMP4-treated MSC from D15-D22. The maximum current in satellite cells (−6.23 ± 1.08, n = 12 pA/pF) occurred at 20 mV, whereas the maximum current for MSC (−7.79 ± 2.78, n = 7 pA/pF) and BMP4-treated MSC (−8.04 ± 0.93, n = 11 pA/pF) occurred at 10 mV. The maximum values of I ca for MSC had the largest dispersion of all three cell groups, and no statistically significant difference was found between MSC and satellite cells. We also performed a best fit analysis to the Boltzmann equation as described in Methods, and the resultant parameters for each cell type are given in Fig. 6e. Analysis of variance on all Boltzmann parameters showed that the mid-point potential of activation (V1/2) had statistical differences between satellite cells and MSC and this difference remained significantly different after BMP4 treatment (p < 0.05). The kinetics of activation of I ca were also determined in the three cell groups by fitting a biexponential function to the rising phase of the current at 30 mV, a membrane potential were maximum conductance has already been attained. Although the I ca activated slighlty faster in BMP4-treated MSC than in the other two groups, the difference was not statistically significant. The average values for the time constants of activation for satellite cells were τ fast 13.3 ± 2.1 msec and τ slow 74.0 ± 15.0 msec; for MSC cells, τ fast 8.9 ± 1.9 msec and τ slow 78.2 ± 19.5 msec; for BMP4-treated MSC cells, τ fast 8.9 ± 2.4 msec and τ slow 49.1 ± 6.4 msec. While we measured the capacitance for each cell to normalize the I Ca, we noticed that BMP4-treated MSC had significantly lower capacitance compared to untreated cells (Fig. 6d). This reduction in capacitance value can be explained by observations from the immunohistochemistry data for cTnT. By D20, cTnT positive cells stay mononucleated as opposed to forming long and wide multinucleated myotubes as in the untreated condition, consistent with our previous observations [4]. The difference in morphology can also be appreciated by comparing untreated MSC (panels E and H) with BMP4-treated MSC (panels F and I) shown in Fig. 4.
Electrophysiological data. A-C. Maximum I Ca densities and traces at membrane potentials ranging from −40 to 60 mV. (A) Sk-sat 2 % from D4-D6. (B) MSC and (C) BMP4 treated MSC from D15-D22. Maximum current amplitude in satellite cells (−6.23 pA/pF) occurred at 20 mV, whereas the maximum current for MSC (−7.79 pA/pF) and BMP4-treated MSC (−8.5 pA/pF) occurred at 10 mV. D. Average capacitance value for each cell group. BMP4 treated MSC had a significant decrease in the capacitance value when compared to the untreated case. E. Boltzmann parameter values calculated from current measurements shown in A-C, using the best fit for the Boltzmann equation. Among the Boltzmann parameters, V1/2 was significantly different between BMP4 treated MSC compared to satellite cells. The numbers of cells analyzed were n = 12 for satellite cells between D4-D6, n = 7 for MSC between D14-20, and n = 11 for MSC-BMP4 between D14-D20. Average values represent mean ± sem, p < 0.05
These results show that BMP4-treated MSC had a modification of the maximum calcium current density waveform with the maximum value occurring at 10 mV as opposed to the 20 mV in satellite cells. This shift in the current waveform is consistent with publications on L-type I Ca recordings from cardiac cells, where the maximum I Ca density occurs at membrane potentials between 0 mV and 10 mV [21, 22], recordings from satellite cells where the maximum density occurs at 20 mV [23, 24], and recordings of dysgenic myotubes expressing the skeletal or cardiac α1 subunit [25]. The faster activation kinetics in BMP4-treated MSC, although not statistically significant, is likely due to the presence of the cardiac I Ca, since the cardiac calcium channel has faster kinetics of activation than the skeletal calcium channel [25].
Calcium transients of Satellite Cells, MSC, and BMP4-treated MSC
The α1 subunits of the skeletal and cardiac calcium channels mediate very different mechanisms of excitation-contraction coupling in muscle cells. Thus, we were interested in examining differences in calcium transients in satellite cells and MSC and whether the transients in MSC are altered by BMP4 treatment. For consistency in the transient measurements, we stimulated the cells electrically with a 5-ms pulse at a 1 Hz frequency and 80 V. Calcium transients were first measured in Ringers solution with calcium followed by calcium-free Ringers (0 Ca). Recorded calcium transient data for all cell groups were analyzed for the average amplitude (F/F0) and average time duration at 50 and 90 % amplitude (TD50, TD90) (Fig. 7a-c). The largest differences in the Ringers with calcium were found between the satellite cells with 2 and 5 % medium conditions. Interestingly, the satellite cells in 2 % serum followed the electrical stimulation signal closely, and exhibited greater TD50 and TD90 than in 5 % serum. The 5 % satellite cells had spontaneous calcium transients and on average cycled at a faster pace (2 Hz) than the electrically stimulating frequency (1 Hz) as shown in Fig. 7e; consequently the TD50 and TD90 were lower for this condition. These calcium transient results agree with the increase in Cav1.1 mRNA and protein expression in satellite cells in 2 % serum compared to 5 % serum, suggesting a more developed skeletal-type ECC mechanism. In the presence of calcium, BMP4-treated cells show a significant increase in the calcium transient amplitude when compared to MSC. In addition, both control and BMP4-treated MSC had significantly longer TD50 and TD90 when compared to both groups of satellite cells. When calcium was removed from the extracellular solution, there was a significant difference between the control and BMP4-treated MSC. Untreated MSC or satellite cells did not exhibit any significant changes in the time durations when the media was changed from calcium to calcium-free. In contrast, BMP4-treated MSC had a significant reduction in their TD50 and TD90 when calcium was removed from the media, although in this case the amplitude was not significantly affected. These results indicate that BMP4-treated MSC have a higher dependence on extracellular calcium than the other cell groups, reminiscent of the calcium-induced calcium release mechanism in native cardiac cells. The results also agree with our previous results showing that nifedipine almost completely blocked calcium transients in the majority of MSC while the addition of cadmium to the extracellular solution eliminated the transients in all the cells [10]. Representative calcium transient signals for each cell group in Ringers with and without calcium are shown in Fig. 7d-g and movies are shown in supplementary material.
Calcium transient measurements. a. Average calcium transient amplitude F/Fo for all cell groups. b. Average time duration at 50 % amplitude (TD50) for all cell groups. c. Average time duration at 90 % (TD90) for all cell groups. d. Average frequency value in Hertz; satellite cells in 5 % serum cycled at higher frequency than the stimulating frequency. e-h. Representative calcium transients for each cell group; traces on the left were obtained when cells were in Ringers with calcium while those on the right were recorded in the absence of calcium. Satellite cells were examined between D5-D6 while all MSC and BMP4-treated MSC were examined between D15-D22. The numbers of cycling cells analyzed in Ringers with calcium were (134, 141, 364) and without calcium were (42, 54, 151) for satellite cells, MSC, and MSC-BMP4 respectively. The asterisk represents a significant difference between one experimental condition and all the others; a solid line represents a statistical difference between two experimental conditions. The statistical analysis of data was done using Mann–Whitney and Tukey’s methods for p < 0.05
To determine whether calcium loading was similar in the different cell types, we measured calcium release in response to caffeine application (10 mM) (Fig. 8). We observed a significantly larger calcium transient magnitude for the BMP4-treated MSC compared to untreated MSC and satellite cells in 2 % serum (Fig. 8d). Similarly, BMP4-treated MSC had a significant increase in the average rate of rise (rising slope at 50 % value) and the average rate of decay (falling slope at 50 % value) of the caffeine induced calcium transient compared to untreated MSC (Fig. 8e-f). The changes observed with BMP4 treatment of MSC indicate an increase in calcium storage of the cell and a higher ability to release and remove calcium upon caffeine exposure. Overall these results show that BMP4-treated MSC are in a greater myogenic development compared to the untreated cells.
Calcium transient measurements in the presence of caffeine. a-c. Representative calcium transient during caffeine exposure in satellite cells, MSC, and BMP4-treated MSC, respectively. d. Calcium transient amplitude measurement (F-F0)/F0. e. Maximum rising slope at 50 % of the amplitude of calcium release for each cell group. f. Maximum decay slope at 50 % of the amplitude for each cell group. The dotted lines represent the 50 % amplitude of the transients. Satellite cells were examined between D5-D6 while all MSC and BMP4-treated MSC were examined between D15-D22. The numbers of cells analyzed were 7, 5, 24 for satellite cells, MSC, and MSC-BMP4 respectively. Significant differences were analyzed using Mann–Whitney method for p < 0.05
Discussion
MSC are able to differentiate into several cell types, including adipocytes, osteocytes, chondrocytes, myocytes and neural cells [1–3]. We are interested in examining the striated muscle phenotypes and in determining the time course of expression of skeletal- or cardiac-specific markers. We have seen that in our culture conditions, MSC tend to differentiate into a muscle phenotype that favors a skeletal-like type, evidenced by the up-regulation of MyoD and skeletal actin proteins and a delay in the expression of Nkx2.5, GATA4, and cTnT [4]. Since calcium channels are a crucial component of the ECC mechanism in both skeletal and cardiac muscles, we sought to measure the expression of the different subunits of calcium channels as MSC differentiate into skeletal- or cardiac-like cells. In addition, we steered differentiation of MSC into the cardiac phenotype with BMP4 treatment as previously reported [4] to determine the effect on the expression of cardiac calcium channel subunits. There are only a few previous studies with human MSC that have looked at the expression of calcium channels [26–28]. However, those studies examined only α1 subunits, at only one time after plating, and did not establish a relationship with the fate of MSC into the muscle phenotype.
In the present study we found that the first calcium channel subunits that were expressed in MSC were the α2δ1 and Cav1.2 subunits. The early appearance of α2δ1 in MSC is consistent with our previous studies with satellite cells, where this subunit was the first one to be expressed [7]. As with satellite cells, this suggests that α2δ1 may have other functions independent from calcium channels in MSC as well. Expression of the β and γ subunits was delayed in comparison to α2δ1 in MSC, but their levels showed a large fold increase with time and remained elevated up to D20, resembling the pattern of expression found with 2 % serum in satellite cells. However, the levels of expression of the associated channel subunits remained high in the presence of 5 % serum in MSC, while their levels decreased slightly in satellite cells. The increase in associated subunit levels in MSC was more robust in the presence of BMP4. These results indicate that MSC are undergoing differentiation, which is more prominent after treatment with BMP4.
The L- and T-type cardiac calcium channel α1 subunits, Cav1.2 and Cav3.2 respectively, were present in satellite cells from D0. However, their levels did not increase as much as those of the other subunits examined. The presence of the cardiac subunits in satellite cells was expected since it has been reported that embryonic and early postnatal skeletal muscle express Cav1.2 [29] and Cav3.2 [30]. Furthermore, a cardiac-like L-type calcium current has been described in myotubes from dysgenic skeletal muscle [31] that can mediate calcium influx-dependent ECC when potentiated [32].
In MSC, Cav1.2 was detected at D0, while Cav3.2 and Cav1.1 were first measured reliably at D3 and D6, respectively. The presence of Cav1.2 in mouse MSC agrees with previous results showing its expression in human MSC as well [27, 28]. Kawano et al. [26] did not look for Cav1.2 but they found expression of Cav2.1, Cav3.2 and an absence of Cav2.3 in human MSC. Other α1 subunits that were found at very low levels or were undetectable in human MSC include Cav1.3, Cav3.2, and Cav1.1 [27] and Cav3.1 [27, 28]. The lack of detection in those earlier studies may be due to the fact that Cav1.2 and Cav3.2 tend to decrease with time as we show in Fig. 2. The decrease in level of both of these cardiac α1 subunits was apparent even in the presence of BMP4. BMP4 caused a large, transitory fold increase in the level of Cav1.2 by D3 and a suppression of expression of Cav1.1 (compare Figs. 2 and 3). This is in agreement with our previous results showing that treatment of MSC with BMP4 leads to expression of cardiac genes [4]. The results with the cardiac calcium channel subunits further indicate that it is harder to differentiate MSC into cardiac cells and suggest that other factors or environmental cues are necessary to lead these cells into a cardiac phenotype, such as the supporting matrix, external loads, or cyclic contraction. The measured calcium currents and calcium transients confirmed that all the different subunits of a calcium channel were expressed in the cells and that they formed functional channels. The properties and voltage dependence of calcium currents recorded from myotubes derived from satellite cells were similar to those reported previously for L-type currents [23, 24]. The calcium currents in untreated MSC showed a larger variability with regards to the amplitude, indicating that these cells represent a more heterogeneous group with cells in different states of differentiation. In contrast, BMP4 treated MSC had larger currents with a lower dispersion than untreated cells. In this case, cells were in a more differentiated state and therefore more homogeneous. Interestingly, the currents recorded from MSC (untreated or exposed to BMP4) had properties resembling more closely the skeletal than the cardiac L-type calcium current, since they were slowly activating, did not decay for the duration of the depolarizing pulses, and the activation kinetics were not statistically different. The four groups of cells examined here displayed myoplasmic calcium transients in response to electrical stimulation (Fig. 7) indicating that intracellular calcium release channels had also been expressed and that they had a functional interaction with the plasma membrane calcium channels. The properties of calcium transients in satellite cells remained unchanged when the extracellular calcium was removed from the media, which corresponds to the skeletal type of e-c coupling. Although the durations of the transients in differentiated MSC (D15-D22) were longer than in satellite cells, the properties of the transients in untreated MSC also remained unchanged upon removal of calcium, suggestive of a skeletal type e-c coupling. In contrast, the durations of the calcium transients recorded from BMP4-treated MSC experienced a significant reduction without appreciable changes in amplitude in calcium-free media compared to calcium-containing media. This indicates that calcium release in BMP4-treated MSC depends on extracellular calcium to a greater extent than untreated cells and suggests the possibility of a mixed (skeletal and cardiac) type of e-c coupling.
Since calcium release in all four groups of cells was elicited with electrical stimulation, it is likely that the intracellular channel mediating release belongs to the ryanodine receptor (RyR) family. In addition, our experiments with caffeine showing release in all cells indicate this to be the case, since RyRs are sensitive to this drug, while the inositol 1,4,5 trisphosphate receptor (IP3R), which is another intracellular release channel, is not. These results are in sharp contrast with the study by Kawano et al. [26] in which they did not find any change in calcium levels when human MSC were exposed to 10 mM caffeine; however, they did find that calcium release was blocked in the presence of 2-APB, a blocker of IP3R. It would be interesting to examine the temporal expression of intracellular channels in control and BMP4-treated MSC and determine the type(s) of RyR and/or IP3R mediating calcium release.
In summary, our data show that the α2δ1 subunit is the first subunit of calcium channels expressed in MSC and confirms our previous results with satellite cells [7], suggesting that the α2δ1 subunit may be involved in signaling mechanisms that do not involve ion channels. We also showed that the mRNA and protein of several α1 subunits is expressed simultaneously in the same cell type, but that the recorded calcium currents have predominantly characteristics of the slowly-activating skeletal type current. The calcium release in all four groups was also more closely related to the skeletal-type of ECC, although BMP4-treated MSC showed a marked effect on transient duration in the absence of extracellular calcium. Exposure of MSC to BMP4 also showed that, although transiently, the cardiac α1 subunits were up-regulated and that the cells are more differentiated than untreated MSC.
References
Chamberlain, G., Fox, J., Ashton, B., & Middleton, J. (2007). Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells, 25(11), 2739–2749.
Williams, A. R., & Hare, J. M. (2011). Mesenchymal stem cells: biology, pathophysiology, translational findings, and therapeutic implications for cardiac disease. Circulation Research, 109, 923–940.
Otto, W.R., Sarraf, C.E. (2012). Culturing and differentiating human mesenchymal stem cells for biocompatible scaffolds in regenerative medicine. In: Human Cell Culture Protocols, Methods Mol Biol, vol. 806, chapter 27.
Grajales, L., Garcia, J., & Geenen, D. (2012). Distinct temporal patterns of myogenic lineage development exist in bone marrow-derived mesenchymal stem cells compared to skeletal muscle satellite cells and are enhanced after BMP4 treatment. Journal of Molecular and Cellular Cardiology, 53(3), 382–391.
Lin, Z., Witschas, K., Garcia, T., et al. (2008). A critical GxxxA motif in the γ6 calcium channel subunit mediates its inhibitory effect on Cav3.1 calcium current. Journal of Physiology, 586(22), 5349–5366.
García, K., Nabhani, T., & García, J. (2008). The calcium channel α2/δ1 subunit is involved in extracellular signaling. Journal of Physiology, 586(3), 727–738.
Tamayo, T., Grajales, L., & García, J. (2012). Commitment of satellite cells expressing the calcium channel α2δ1 subunit to the muscle lineage. J Signal Transduction. doi:10.1155/2012/460842.
Dulhunty, A. F. (2006). Excitation-contraction coupling from the 1950s into the new millennium. Clinical and Experimental Pharmacology and Physiology, 33(9), 763–772.
Boomsma, R. A., Swaminathan, P. D., & Geenen, D. L. (2007). Intravenously injected mesenchymal stem cells home to viable myocardium after coronary occlusion and preserve systolic function without altering infarct size. International Journal of Cardiology, 122(1), 17–28.
Grajales, L., Garcia, J., Banach, K., & Geenen, D. (2010). Delayed enrichment of mesenchymal cells promotes cardiac lineage and calcium transient development. Journal of Molecular and Cellular Cardiology, 48(4), 735–745.
Kassem, M. (2004). Mesenchymal stem cells: biological characteristics and potential clinical applications. Cloning and Stem Cells, 6(4), 369–374.
Mazaheri, Z., Movahedin, M., Rahbarizadeh, F., & Amanpour, S. (2011). Different doses of bone morphogenetic protein 4 promote the expression of early germ cell-specific gene in bone marrow mesenchymal stem cells. In Vitro Cellular and Developmental Biology - Animal, 47(8), 521–525.
Alden, K. J., & Garcia, J. (2001). Differential effect of gabapentin on neuronal and muscle calcium currents. Journal of Pharmacology and Experimental Therapeutics, 297(2), 727–735.
Livak, K. J., & Schmittgen, T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔC T Method. Methods, 25, 402–408.
Schmittgen, T. D., & Livak, K. J. (2008). Analyzing real-time PCR data by the comparative CT method. Nature Protocols, 3(6), 1101–1108.
Vandesompele, J., De Preter, K., Pattyn, F., et al. (2002). Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biology 3(7) RESEARCH0034.
Hamill, O. P., Marty, A., Neher, E., Sakmann, B., & Sigworth, F. J. (1981). Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflügers Archiv, 391, 85–100.
Dubois, J.-M., Ouanounou, G., & Rouzaire-Dubois, B. (2009). The Boltzmann equation in molecular biology. Progress in Biophysics and Molecular Biology, 99(2–3), 87–93.
Laflamme, M. A., Chen, K. Y., Naumova, A. V., et al. (2007). Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nature Biotechnology, 25(9), 1015–1024.
Yang, L., Soonpaa, M. H., Adler, E. D., et al. (2008). Human cardiovascular progenitor cells develop from a KDR + embryonic-stem-cell-derived population. Nature, 453(7194), 524–528.
Zhou, Y. Y., Cheng, H., Song, L.-S., Wang, D., Lakatta, E. G., & Xiao, R.-P. (1999). Spontaneous β2-adrenergic signaling fails to modulate L-type Ca2+ current in mouse ventricular myocytes. Molecular Pharmacology, 56(3), 485–493.
Hatano, S., Yamashita, T., Sekiguchi, A., et al. (2006). Molecular and electrophysiological differences in the L-type Ca2+ channel of the atrium and ventricle of rat hearts. Circulation Journal, 70(5), 610–614.
García, J., & Beam, K. G. (1994). Measurement of calcium transients and slow calcium current in myotubes. Journal of General Physiology, 103, 107–123.
Alden, K. J., & García, J. (2002). Dissociation of charge movement from calcium release and calcium current in skeletal myotubes by gabapentin. American Journal of Physiology - Cell Physiology, 283, C941–C949.
García, J., Tanabe, T., & Beam, K. G. (1994). Relationship of calcium transients to calcium currents and charge movements in myotubes expressing skeletal and cardiac dihydropyridine receptors. Journal of General Physiology, 103, 125–147.
Kawano, S., Shoji, S., Ichinose, S., Yamagata, K., Tagami, M., & Hiraoka, M. (2002). Characterization of Ca2+ signaling pathways in human mesenchymal stem cells. Cell Calcium, 32(4), 165–174.
Heubach, J. F., Graf, E. M., Leutheuser, J., et al. (2003). Electrophysiological properties of human mesenchymal stem cells. Journal of Physiology, 554(3), 659–672.
Li, G. R., Sun, H., Deng, X., & Lau, C. P. (2005). Characterization of ionic currents in human mesenchymal stem cells from bone marrow. Stem Cells, 23(3), 371–382.
Chaudhari, N., & Beam, K. G. (1993). mRNA for cardiac calcium channel is expressed during development of skeletal muscle. Developmental Biology, 155(2), 507–515.
Berthier, C., Monteil, A., Lory, P., & Strube, C. (2002). Alpha(1H) mRNA in single skeletal muscle fibres accounts for T-type calcium current transient expression during fetal development in mice. Journal of Physiology, 539(3), 681–691.
Adams, B. A., & Beam, K. G. (1989). A novel calcium current in dysgenic skeletal muscle. Journal of General Physiology, 94, 429–444.
Adams, B. A., & Beam, K. G. (1991). Contractions of dysgenic skeletal muscle triggered by a potentiated, endogenous calcium current. Journal of General Physiology, 97, 687–696.
Acknowledgements
The authors want to thank Santipongse Chatchavalvanich for his help with the initial experiments. This work was funded by NIH training grant (2 T32 HL7692-21; PI: Solaro, R. J.) and by the Illinois Department of Public Health (P.I. D.L. Geenen), NHLBI (R01 HL071046; P.I. D.L. Geenen), and Muscular Dystrophy Association, Inc. (P.I. J. García).
Disclosures
The authors indicate no potential conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
David L. Geenen and Jesús García Co-senior authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
(MPG 15450 kb)
(MPG 10804 kb)
(MPG 12534 kb)
Rights and permissions
About this article
Cite this article
Grajales, L., Lach, L.E., Janisch, P. et al. Temporal Expression of Calcium Channel Subunits in Satellite Cells and Bone Marrow Mesenchymal Cells. Stem Cell Rev and Rep 11, 408–422 (2015). https://doi.org/10.1007/s12015-014-9566-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-014-9566-4