Abstract
To study the inhibitory effect of Hedyotis diffusa on cervical cancer and its underlining biomolecular mechanism. Human cervical carcinoma nude mice xenograft was established and the mice were treated by intra-gastric administration of boiled and concentrated Hedyotis diffusa. When the tumor grew to 10 mm in diameter, the mice were randomly divided into Hedyotis diffusa Willd. (HDW) group and control group. The tumor inhibitory rate, survival time, and the expression rate of Ki-67 protein in Hela cells as well as tumor cell apoptosis were compared between these two groups. Hedyotis diffusa had inhibitory effect on cervical cancer cells and induced apoptosis of Hela cells. The expression of Ki-67 protein significantly decreased (P < 0.05) in HDW group, and the mean survival time of the mice was significantly extended (P < 0.05). Hedyotis diffusa directly inhibited the proliferation of cervical cancer cells and induced apoptosis of the tumor cells. It has a positive effect for the treatment of cervical cancer to achieve the goal of clearing the heat, removing the toxins, eliminating the stasis, and dissolving the masses.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Currently, primary treatment for early stage cervical cancer is surgery or radiation therapy, while the treatment options for mid- to late stage cancer, recurrent, and metastatic cervical cancer generally include both radiation therapy and chemotherapy. The individual difference in the sensitivities to chemotherapy leads to the difference in the therapeutic efficacy of the treatment. The efficacy of the therapy can be improved to certain extent by increasing the dosage of medication, which, however, is often coupled with serious toxicity and side effects and severely reduces the quality of life.
Hedyotis diffusa Willd. (HDW), an annual herb belonging to the Rubiaceae family, is a medicinal herb with the functions of clearing the heat, removing the toxins, restoring the circulation, eliminating the stasis and promoting the process of diuresis. Studies have reported that the major chemical constituents include oleanolic acid, ursolic acid, polysaccharide, favone, etc., which render the herb the ability to enhance the immunity and fight against tumor. Clinical studies observed that HDW exhibited significant efficacy against cervical cancer. Ki-67, a biomarker for cell proliferation, is highly expressed in various cancers including the cervical cancer. The high expression of Ki-67 is closely associated with the occurrence, development, recurrence of cancer as well as the resistance to radiation and chemotherapy. In this study, cervical cancer Hela cells were used to examine the expression of Ki-67 in cervical cancer and the effect of HDW on the proliferation and apoptosis of the cancer cells to study the inhibitory effect of HDW on cervical cancer and its underlining biomolecular mechanism, providing the experimental evidence for the further research and the clinical application of HDW.
Materials and Methods
Animals
Forty BALB/C-nu female mice with average weight of 18–20 g were purchased from Shanghai Experimental Animal Center, Chinese Academy of Sciences and housed in laminar flow cabinet under constant temperature (22–25 °C), constant humidity, and specific pathogen-free condition at the Experimental Animal Center of Xuzhou Medical College. Autoclaved food and water were given ad libitum. Animals were fed in stainless cage under natural ventilation. The temperature of laboratory was maintained within the range of 19–29 °C with relative humidity of 40–80 %.
Materials and Reagents
Three-hundred grams of Hedyotis diffusa was supplied by the pharmacy of Xuzhou Central Hospital (affiliation of Integrative Medicine Clinical Institute of Nanjing TCM University, China). RPMI-1640 medium supplemented with 10 % FBS, 100 U/mL penicillin, and 100 μg/mL streptomycin as well as 0.125 % trypsin were purchased from GBICO, USA1. Ki-67 immunohistochemistry kit, TUNEL assay kit, and anti Ki-67 rabbit antibody were obtained from Beijing Zhongshan Biotechnology Inc., China. RNA isolation kit was supplied by Santa Cruz, USA and RT-PCR kit from Promega, USA. Cell cultures and all other experiments were performed in sterile and nontoxic environment at laminar flow laboratory of Institute of Reproductive Medicine, Southeast University, Xuzhou, China.
Methods
The Establishment of Nude Mouse Cervical Cancer Xenograft Model
Forty female nude mice (aged 4–6 weeks, average weight of 18–20 g) were purchased from Shanghai Experimental Animal Center, Chinese Academy of Sciences and housed in laminar flow cabinet under constant temperature (22–25 °C), constant humidity, and specific pathogen-free condition at the Experimental Animal Center of Xuzhou Medical College. Autoclaved food and water were given ad libitum. Cultured Hela cells were digested by trypsin followed by centrifugation and being washed twice by PBS; cell clumps were dispersed and cells were verified of having 95 % viability via trypan blue exclusion method; a 0.2-mL suspension of 2 × 106 cells was injected subcutaneously at the back of nude mice. Animals continued to be housed in the laminar flow cabinet for 2 weeks and resulted in a 100 % engraftment rate.
Administration of Medication
When the tumor grew to 10 mm in diameter, the mice were randomly divided into two groups (n = 20), the HDW group with the treatment of HDW and the control group. Each group was further randomized into two subgroups to study the efficacy for tumor inhibition and survival time:
-
1.
Control group: mice in this group did not receive HDW and were allowed to live until the end of experiment (n = 10).
-
2.
HDW group: mice were given intra-gastric administration of 5.0 mL/kg of HDW, once daily for 10 days (n = 10). The administered HDW was prepared by adding 3,000 mL water to 300 g HDW, heated, and boiled for 2 h.
Observation and Examination
Tumor Inhibition Rate
The tumor-bearing mice in control group (n = 10) were not treated with any medication and continued to be survived for 10 days. The mice in HDW group were given intra-gastric administration of HDW once daily for 10 days (n = 10). On the next day, mice in both groups were anesthetized and killed by cervical dislocation. Tumors were removed under sterile condition, weighed, and the tumor inhibition rate was calculated according to the following formula: (C − T)/C × 100 %. T represents the average tumor weight of HDW group and C represents the average tumor weight of control group. Tumors were then fixed and underwent pathological examination.
The observation of survival time: Mice in both subgroups (n = 10) were allowed to live until death and the average survival time of tumor-bearing mice was calculated.
Immunohistochemistry Study of Ki-67 Expression in Cervical Cancer Cells
Tumor samples were processed conventionally for paraffin-embedded tumor sections. The tumor sections were then deparaffinized and rehydrated via treatment with a series of xylenes and graded alcohols, then immersed in citric acid/sodium citrate solution and incubated in 95 °C for 10 min. Tumor samples were fixed in 10 % buffered formalin for 12 h, and subsequently processed conventionally for paraffin-embedded tumor sections (4-μm thick). The tumor sections were then deparaffinized and dehydrated via treatment with a series of xylenes and graded alcohols. Endogenous peroxidase activity of the sections was quenched by incubating in water containing 0.3 % H2O2 for 30 min at room temperature. Tumor sections were then washed by PBS, sealed in serum, and incubated for 1 h at room temperature. After being washed by PBS, samples were incubated with primary anti-Ki-67 antibody (1:100) at 4 °C for 48 h. The redundant primary antibodies were then aspirated and samples were washed with PBS for three times. The rest of the procedure was performed using VectABC kit according to the manufacture’s instruction. Briefly, the samples were incubated with biotinylated secondary antibodies at 4 °C overnight and then washed with PBS for three times followed by being probed with horseradish peroxidase-conjugated biotin–avidin complex at 37 °C for 1 h. Samples were then washed in PBS for four times and finally treated with Diaminobenzidine (DAB). After being verified under microscope, samples were dehydrated through graded alcohol, cleared with xylene, mounted with neutral resin medium, and then studied under microscope. Positive and negative controls were used for controlling the quality of each stain. The known positive slides were served as positive controls. For control group, samples were incubated with PBS instead of being probed with the primary antibody and the rest of the procedures were same as the HDW group.
In Situ Apoptosis Detection by TUNEL Staining
The assay was performed using ApopTag® Peroxidase In Situ Apoptosis Detection Kit according to manufacturer’s instruction. The 5-μm-thick sections of paraffin-embedded tumor samples were deparaffinized and dehydrated through treatment with a series of xylenes and graded alcohols followed by digestion with protease K (20 μg/mL) at room temperature for 20 min, then incubated in the mixture of TDT enzyme and nucleotide at 37 °C for 1 h and reaction was stopped by using stop/wash buffer. Samples were then incubated with anti-digoxigenin conjugate at room temperature for 30 min and stained with DAB, followed by hematoxylin staining. Samples were finally dehydrated through graded alcohol, cleared with xylene, mounted with neutral resin medium, and then studied under the microscope. The nuclei of apoptotic cells were stained brown.
Statistical Analysis
All data was presented as mean ± SD. Statistical analysis was carried out using t test or ANOVA test and performed on SPSS 14.0 software. P < 0.05 was considered significantly different.
Results
The Efficacy of HDW in Inhibiting Tumor Growth in Hela Cell Xenograft Mice
Intra-gastric administration of boiled HDW significantly inhibited the growth of cervical cancer Hela cells. The average tumor weight of control group was 1.61 ± 0.3 g while that of HDW group was 1.03 ± 0.26 g with tumor inhibition rate of 36.1 %. The tumor decreased significantly in HDW group than control group (P < 0.05) (Fig. 1).
The Effect of HDW on the Survival Time of Hela Cell Xenograft Mice
The average survival time of mice in control group was 13.3 ± 1.1 days while that in HDW group was 19.4 ± 1.9 days; this resulted in a significant extension of survival time by up to 46.6 % (P < 0.05). The longest survival time in control group was 25 days while that of HDW was 34 days (Fig. 2).
The Effect of HDW on Ki-67 Expression in Hela Cells
The Ki-67 expression rate was detected through immunohistochemistry study. The cells with Ki-67 expression or positive cells show yellow or brown staining in nuclei; the cells without Ki-67 expression or negative cells show no staining or staining of keratinocyte <5 % (Fig. 3).
In control group, Ki-67 expression is identified by the presence of yellow to brown particles in nuclei. The expression rate is 72 %; in HDW group, the Ki-67 expression is only 21.2 % (×400).
The Ki-67 was quantified by counting respective positive cells and total number of cells in five high-power fields (×400) randomly selected in each slide and calculated according to the following formula: The rate of positive cells = positive cells/total number of cells × 100 %. Samples were scored by percentage of nuclear positive cells and scoring method for positive cells was: 5–25 % as (+); 26–50 % as (++); 51–75 % as (+++), and >75 % as (++++). The Ki-67 expression rate was significantly lower in HDW group (21.2 %) than that of control group (72 %) (P < 0.05) (Fig. 4).
Effect of HDW on the Apoptosis of Hela Cells
Results of TUNEL assay demonstrated that no significant change was observed in tumors of control group; however, a large area of necrosis was detected in tumors of HDW group. A well-defined necrosis was noticed in tumors including degenerated intracellular structure, strongly stained nuclear cell shrinking as well as karyopyknosis, and other characteristics of apoptosis (Fig. 5).
Views under transmission electron microscopy showed no significant cellular change occurred in tumor cells of control group while large areas of necrosis were found in tumor cells of HDW group. Signs of necrosis were noticed in tumors including degenerated intracellular structure, strongly stained nuclear cell shrinking as well as karyopyknosis, and other characteristics of apoptosis were also found.
The apoptotic rate of cancer cells of HDW group (60.43 ± 0.17 %) was significantly higher than that of control group (3.09 ± 0.21 %) (P < 0.05) (Fig. 6).
Discussion
Cancer is a common disease that severely threatens human life. In modern Western medicine, surgery, radiation, and chemotherapy are the primary, effective, and curative treatment for cancer. However, these interventions usually cause significant damage and toxic side effects to human body. Therefore, finding safer and more effective anti-tumor treatment is the major challenge in the field of biomedical research. Chinese herbal medicine, as a traditional medicine in China, has been commonly used to treat against cancer with significant efficacy and fewer side effects. In Chinese medicine, it is believed that tumors are the consequence of unmitigated accumulations of qi, moisture, and blood that have become toxic, transforming what is healthy into morbid tissue, simultaneously obstructing and usurping normal circulation. Prolonged stagnation eventually leads to depletion of qi and blood, and ultimately the essence. Because essence governs growth and maturation, loss of or damage to it can result in a disregulation of growth typical of cancer, a process of uncontrolled proliferation of immature, undifferentiated, and malformed cells. Patients with cancer usually present symptoms of fever, enlargement of tumor, burning, pain, thirst, dark urine, dry stool, red tongue, and fast pulse rate all of which indicate the accumulation of toxicity. Therefore, treatment that supplements qi, moisture, and blood restores circulation and eliminates stasis; removes toxins; replenishes essence; and dissolves masses is critical in the treatment of cancer [1]. Chinese medicine has gradually drew world’s attention and recognition in its efficacy in balancing the internal environment of human body, supplementing the qi, boosting the immune system, improving the quality of life, prolonging the survival period and controlling the development of disease.
Cervical cancer, as one of the most common malignant tumor in female population, was usually treated by radiation, surgery, or combined therapy [8] with poor prognosis. Recurrence and metastasis often occur after surgery. Surgery is the most effective therapy for early stage cervical cancer due to its small size and the absence of invasion or metastasis. The primary surgical procedures involve extensive hysterectomy with pelvic lymphadenectomy to eliminate the adjacent cancerous tissue. Chemotherapy and Chinese medicine can be used after the surgery to prevent recurrence. However, the treatment for advanced cervical cancer is not optimistic. The appropriated treatment options should be selected according to the progression of cancer and patients’ condition. Most common treatment options include radiation, chemotherapy, and Chinese medicine. The combined internal and external radiation is chosen according to patients’ condition. Chemotherapy is often combined with radiation. In particular, cisplatin is most widely used and more effective chemotherapy regimen due to its dual action in chemotherapy and radiation. However, the extension of the time of treatment increases the toxicity. Therefore, the response of tumor, patients’ condition as well as the treatment-related toxicity, and side effects limited the clinical use of chemotherapy.
With the further research in Tradition Chinese Medicine, the efficacy of Chinese medicine in managing cervical cancer has received considerable attention. Finding effective anti-cancer compounds with fewer side effects in herbs has become a major topic in worldwide drug discovery. For mid- and late-stage cervical cancer, Chinese medicine can be combined with routine treatment in order to potentiate the effects of conventional radiation and chemotherapy and reducing the side effects. For patients with mid- or late-stage cancer with metastasis and low tolerance to chemotherapy, Chinese medicine can be used as conservative treatment to preserve the immunity and enhance the anti-tumor function of internal organs. Although the short-term effect of Chinese medicine is less significant than chemotherapy, it shows significant long-term effect including improving the quality of life and prolonging the survival period.
HDW, an annual herb belonging to the Rubiaceae family, is a medicinal herb widely distributed in northeast Asia. It is harvested in summer and autumn. The whole plant can be used either in fresh or dried. The herb is sweet and bland in taste, cool in nature, and attributive to lung, liver, urinary bladder, and large intestine with the functions of clearing the heat, removing the toxins, restoring the circulation, and anti-tumor activity. Recently, extensive research has been conducted especially on the chemical components, pharmacology, and clinical application of HDW. Studies revealed that Hedyotis diffusa has various pharmacological activities among which its efficacy in enhancing the immunity and anti-tumor activity has drawn considerable attention of researchers and clinicians. HDW, as an effective adjuvant therapy of cancer, has been widely used in various cancers including the primary bronchial cancer, lung cancer, colorectal cancer, nasopharyngeal cancer, liver cancer, esophageal cancer, lymphoma, ovarian and endometrial cancer, and gastric cancer [2]. In vitro study also showed that HDW can inhibit the proliferations of varieties of cancer cell lines [3, 4]. However, the underlining anti-tumor mechanism of HDW is not well elucidated.
In this study, the cervical cancer Hela cell xenograft mice were used to investigate the efficacy of the water-extracted HDW in inhibiting the proliferation of cancer cells through the observation of Ki-67 expression in cervical cancer to study and analyze the underlining biomolecular mechanism of the inhibitory effect of HDW on cervical cancer, providing the experimental evidence for the clinical application of HDW and further cancer drug R&D.
This study showed that intra-gastric administration of boiled HDW significantly inhibited the growth of cervical cancer Hela cells (P < 0.05) with tumor inhibition rate of 36.1 %. No significant change was observed in tumors of control group; however, large areas of necrosis were detected in tumors of HDW group. A well-defined necrosis was noticed including the degenerated intracellular structure, strongly stained nuclear cell shrinking as well as karyopyknosis, and other characteristics of apoptosis. The apoptotic rate of cancer cells of HDW group (60.43 ± 0.17 %) was significantly higher than that of control group (3.09 ± 0.21 %) (P < 0.05). Besides, the average survival time of mice in control group was 13.3 ± 1.1 days while that in HDW group was 19.4 ± 1.9 days. Using HDW resulted in a significant extension of survival time by up to 46.6 % (P < 0.05). The longest survival time in control group was 25 days while that of HDW was 34 days.
Previous studies proved that the occurrence of cervical cancer was the result of complex interactions among the multiple factors. The cancer progresses gradually, in terms of histological changes, from hyperplasia of squamous epithelia, dysplasis, and in situ carcinoma to invasive cancer. Human papilloma viruses (HPVs) are small DNA viruses of type A genera of the papovavirus family. HPV–DNA integration into genome is a key step in the pathogenesis of HPV-related cancer. Once HPV infected the host cells, it stays in the basal cells at the surface of mucosa, then replicates in acanthosis cells and in the differentiated keratinocytes in granular layer. The integration of virus DNA into host cells causes the mutation of host cells. The integration of HPV–DNA leads to the expression of several oncogenes including E6, E7, and E2. E6 and E7 modify the cycle of cell proliferation leading to indefinite proliferation. All of which is believed to be the molecular mechanisms of HPV-induced cervical carcinoma. Studies also showed that the infection of HPV was significantly associated with Ki-67 expression [5–7], indicating the occurrence of cervical cancer was related to cell proliferation. Based on this theory, we speculated that the inhibition of Ki-67 expression might inhibit the cancer cell proliferation and finally lead to cell apoptosis in cervical cancer.
Ki-67 is a cell proliferation-related gene. The encoding Ki-67 protein is an essential DNA-binding protein for cancer cell proliferation. Ki-67 expresses at the mid- to late-G1 phase, increases through S and G2 phase, peaks at M phase and rapidly degrades at afterwards. Ki-67 is a nuclear protein expressed in proliferating cells [8]. The short half-life of Ki-67 allows accurate detection of proliferative activity of cells. The degree of Ki-67 expression is closely correlated with the degree of differentiation, invasion, and metastasis of varieties of cancers. Studies have shown that Ki-67 expression was significantly increased in cervical cancer cells. Chen et al. demonstrated that almost no Ki-67 expression was observed in normal cervical epithelia and low-grade cancer epithelia, while the positive Ki-67 expression in cancer cells significantly increased (P < 0.01) reaching up to 98 % [9], which indicated that cervical cancer is due to excessive cell proliferation.
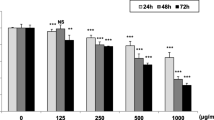
The Ki-67 mRNA was detected by RT-PCR. The results showed that the serum with various concentrations of HDW significantly inhibited the expression of Ki-67 mRNA in Hela cells compared to control group: In Hela cells of xenograft mice with 10, 20, and 40 % of HDW in serum, the expression rate of Ki-67 mRNA at 24 h was 82.0 ± 3.17 %, 68.5 ± 2.25 %, and 54.5 ± 3.7 %, respectively; the expression rate was 76 ± 1.78 %, 57.7 ± 1.56 %, and 41.7 ± 1.5 %, respectively, at 48 h, and the expression rate was 61.3 ± 2.27 %, 51.4 ± 1.83 % and 34.1 ± 1.7 %, respectively, at 72 h. The expression rate of Ki-67 mRNA in Hela cells of control group at above time points was all at 100 %, which was significantly higher than that of HDW group (P < 0.05). Besides, the expression of Ki-67 mRNA was inversely correlated with the concentration of HDW and the time of treatment. The expression rate significantly decreased with the increase of HDW concentration and the extension of the time of treatment.
Meanwhile, the immunohistochemistry study demonstrated that the expression of Ki-67 protein in Hela cells was significantly inhibited by various serum concentration of HDW. The expression rate of Ki-67 protein at 24, 48, and 72 h in Hela cells cultured in serum with 10 % HDW was 60 ± 3.17 %, 56 ± 1.78 %, and 51.3 ± 2.27 %, respectively; the expression rate in Hela cells cultured in serum with 20 % HDW was 53.5 ± 2.25 %, 46.7 ± 1.56 %, and 42.4 ± 1.83 %, respectively; the expression rate in serum with 40 % HDW was 45.78 ± 1.34 %, 38.1 ± 2.13 %, and 31.9 ± 1.15 %, respectively. However, the expression rate in control group was 78 ± 0.4 %, 80 ± 0.3 %, and 79 ± 0.5 %, significantly higher than those of HDW group (P < 0.05). The expression rate was correlated with the dosage of the medicine and the time of treatment. The expression of Hi-67 protein significantly decreased with the increase of HDW dosage and the extension of the time of treatment.
This study demonstrated that Hedyotis diffusa significantly inhibited the proliferation of cervical cancer Hela cell (P < 0.05) in a dose- and time-dependent manner. With the increase of the dosage and extension of the time of treatment, the inhibitory efficacy was enhanced. The significant inhibitory effect of HDW on the in vivo and in vitro expression of Ki-67 protein in cancer cell indicated the underlining mechanism of inhibition of cancer cell proliferation by HDW. The cervical cancer cells cultured in vitro in HDW containing serum underwent the significant process of apoptosis (P < 0.05) and also in a dose- and time-dependent manner. The cancer cells in mice of HDW group displayed significant characteristics of apoptosis (P < 0.05). The intra-gastric administration of HDW significantly inhibited the tumor growth and extended the survival time of tumor-bearing mice.
Taken together, we believe that the mechanism of tumor inhibition by HDW was carried out through the direct inhibiting proliferation, inducing, and promoting the cancer cell apoptosis to achieve the goal of clearing the heat, removing the toxins, eliminating the stasis, and dissolving the masses.
References
Meng, L. Y., Shi, J., & Zhou, H. L. (2009). Advances in the treatment of cervical cancer. Medical Recapitulate, 15(20), 3089–3091.
Du, Y., & Wu, X. (2006). The clinical application of Hedyotis diffusa. Chinese Journal of Trauma and Disability Medicine, 14(5), 93.
Yu, C. Y., Li, W., Liu, Y. H., et al. (2004). The study of the anti-tumor effect of Hedyotis diffusa on human cancer multiresistant Bel-7402 cells. Journal of Beihua University (Natural Science), 5(3), 221–223.
Shan, B. E., Zhang, J. Y., Du, X. N., et al. (2001). The immunity regulatory activity and anti-tumor effect of Hedyotis diffusa. Chinese Journal of Integrated Traditional and Western Medicine, 21(5), 370.
Slavesen, H. B., Lversen, O. E., & Akslen, L. A. (1999). Prognostic significance of angiogenesis and Ki-67, p53 and p21 expression: A population-based endometrial carcinoma study. Journal of Clinical Oncology, 17(5), 1382–1390.
Guo, D. H., Shi, Y. Q., Fan, J. D., et al. (2002). The value of using p53 and Ki-67 to evaluate difficult diagnosis of uterus smooth muscle tumor. Chinese Journal of Clinical and Experimental Pathology, 18(3), 286–289.
Zhai, Y. L., Kohayashi, Y., Mori, A., et al. (1999). Expression of steroid receptors, Ki267 and p53 in uterine leiomyosarcomas. International Journal of Gynecology and Pathology, 18(1), 20–28.
Wong, F. W. (1994). Immunohistochemical detection of proliferating tumor cells in cervical cancer using monoclonal antibody Ki-67. Gynecologic and Obsteric Investigation, 37(2), 1–3.
Chen, W., & Zhao, Y. (2007). Expression of MCM5 and Ki67 in cervical intraepithelial neoplasia and cervical cancer and their clinical significance. Acta Academiae Medicinae Militaris Tertiae, 29(14), 1436–1439.
Author information
Authors and Affiliations
Corresponding author
Additional information
Peiying Zhang and Bei Zhang contributed equally to this work as co-first authors.
Rights and permissions
About this article
Cite this article
Zhang, P., Zhang, B., Gu, J. et al. The Study of the Effect of Hedyotis diffusa on the Proliferation and the Apoptosis of the Cervical Tumor in Nude Mouse Model. Cell Biochem Biophys 72, 783–789 (2015). https://doi.org/10.1007/s12013-015-0532-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12013-015-0532-9