Abstract
Background
The pawpaw tree has several beneficial effects. However, no studies have been conducted to address the mechanisms underlying the cytotoxic effects of pawpaw extracts against cancer cells, and no study has investigated the anti-inflammatory effects. Hence, in this study, the growth-inhibitory effects of pawpaw (Asimina triloba [L.] Dunal) extracts against gastric (AGS) and cervical (HeLa) cancer cells and the inhibitory effects of pawpaw extracts against inflammatory factors (NO, TNF-α, IL-6, iNOS, and COX-2) were investigated.
Methods and results
The viability of AGS and HeLa cells, the analysis of cell cycle, and the expression of apoptosis marker protein were determined using MTT assay, FACS, western blotting, and TUNEL assays. The inflammatory factors were determined using Griess method, ELISA assay kit, and RAW 264.7 cells. The IC50 values of twig and unripe fruit extracts for AGS cells were 82.01 and 100.61 µg/mL, respectively. For HeLa cells, pawpaw twig extracts exhibited the strongest ability to inhibit cervical cancer cell growth (IC50 = 97.73 µg/mL). Analysis of the cell cycle phase distribution and expression of the apoptosis regulatory proteins BCL-2, BAX, caspase-3, and PARP showed that pawpaw twig, root, and unripe fruit extracts induced Sub G1 cell cycle arrest and apoptosis of AGS and HeLa cells. In addition, the twig, root, and unripe fruit extracts of pawpaw effectively inhibited the inflammatory makers NO, TNF-α, IL-6, and iNOS. Particularly, the twig, root, and unripe fruit extracts at concentrations of 50 µg/mL exhibited > 50% inhibition of TNF-α.
Conclusions
These findings indicate that pawpaw extracts are natural therapeutic agents that may be used for the prevention and treatment of gastric and cervical cancers, and encourage further studies on the anti-inflammatory potential of the pawpaw tree.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cancer is the second leading cause of mortality worldwide, with 8.8 million deaths being reported in 2015 [1]. Breast, colon, lung, cervical, and gastric cancers are the most common types of cancer in women, whereas lung, prostate, colon, gastric, and liver cancers are the most common in men [2]. Incidentally, gastric cancer was the second most common malignancy in both women and men, followed by malignant tumor of the thyroid gland, in 2011, and mortality due to gastric cancer was the highest, at 19.6% [3]. Moreover, cervical cancer is the most common cancer of the reproductive organs in women worldwide, and, with early detection, it can be treated; however, it is becoming a serious social problem, as the age of onset is gradually decreasing from the 40 s to the 30 s and 20 s [4]. The World Health Organization reported that the increase in mortality from cancer is attributable to a number of causes, of which 30% are related to diet. Accordingly, there is a growing interest in, and demand for, functional health foods and various medicines exhibiting anticancer effects, and numerous attempts have been made to develop new anticancer agents from natural products by conducting research into the various physiological activities and antioxidant/anticancer mechanisms of natural products. Important anticancer effects include the inhibition of cancer cell division and proliferation through various mechanisms, and the selective removal of cancer cells [5], whereas the induction of apoptosis by these specific drug treatments is recognized as crucial for the development of anticancer agents [6, 7].
Inflammatory responses are caused by inflammatory mediators, including nitric oxide (NO), tumor necrosis factor-α (TNF-α), and interleukin-6 (IL-6), and enzymes associated with immune cells, such as inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2). NO reacts readily with superoxide anions and causes inflammation-related tissue damage, including arthritis, whereas TNF-α meditates inflammation by regulating immune cells. IL-6 is a cytokine produced by macrophages, and its expression is closely associated with immunological abnormalities, inflammatory disease, and lymphoid tumors. Increased iNOS accelerates the formation of NO which induces the inflammatory reaction, and COX-2 is a prostaglandin-producing enzyme that causes pain and inflammation [8,9,10]. Therefore, inhibition of inflammatory factors, such as NO, TNF-α, IL-6, iNOS, and COX-2, is very important for the prevention and treatment of inflammatory diseases.
Pawpaw (Asimina triloba [L.] Dunal) is one of nine species belonging to the genus Asimina, which is the only temperate climate member of the Annonaceae family to inhabit the tropics [11]. Pawpaw is extensively cultivated in 26 states of the USA, including California, Maryland, Michigan, Missouri, North Carolina, Kentucky, West Virginia, and Ohio; in addition, these trees are widely distributed in Asia, including in Korea, China, and Japan [12, 13]. Recently, since the pawpaw tree was highlighted as a functional material likely to increase the income of farm households over an approximately 10-year period in Korea, some farms began to cultivate pawpaw trees, leading to a gradual increase in its range distribution and areas of cultivation. Many studies have shown that the extracts from pawpaw roots, twigs, leaves, fruit, and seeds have beneficial antioxidant and anticancer effects [13,14,15,16,17,18]. In addition, the bioactive polyphenols and acetogenins isolated from several pawpaw tissues have growth inhibitory effects on various cancer cells [19,20,21]. However, to the best of our knowledge, few studies have been conducted to address the mechanisms underlying the cytotoxic effects of pawpaw tree extracts against cancer cells [22], and no study has investigated the anti-inflammatory effects of the pawpaw tree.
In a preliminary experiment, we determined that the pawpaw tree exhibits increased levels of antiproliferative activity against gastric and cervical cancer cells compared to that against lung, liver, prostate, colon, and breast cancer cells [21]. Therefore, this study was conducted to investigate the following hypothesis: extracts from the pawpaw tree inhibit the growth of gastric and cervical cancer cells by increasing apoptosis and have anti-inflammatory effects.
Materials and methods
Samples and reagents
Leaves, twigs, and roots of pawpaw (A. triloba), obtained from Okchon, South Korea, were cultivated under the following conditions: average annual temperature, 13.0 °C; relative humidity, 66.7%; wind velocity, 1.9 m/s; total annual rainfall, 1458.7 mm. Pawpaw fruit, purchased from Cheongyang, South Korea, were grown under the following conditions: average annual temperature, 11.9 °C; relative humidity, 83.1%; wind velocity, 1.3 m/s; total annual rainfall, 861.5 mm. Leaves, twigs, and roots were removed from 150 2 year-old pawpaw trees (height, 1–2 m; diameter, 1–2 cm), and unripe fruits (length, 12–14 cm; weight, 120–130 g; diameter, 4–5 cm; °brix, 8.0–10.0) were gathered from 30 pawpaw trees that were 8–10 years old. A voucher specimen of A. triloba was authenticated by Dr. Otto Jahn (United States Department of Agriculture/Agricultural Research Service) and was deposited in the herbarium by the U.S. National Plant Germplasm System. Samples were immediately washed with water, and fruits were peeled, pipped, and chopped, and then lyophilized using a freeze-dryer (LP100, Ilshin Lab Co. Ltd., Daejeon, Korea). Samples were ground using a food mixer (Blixer®, Robot Coupe USA, Inc., Jackson, MS, USA), sieved through a 40 mesh to generate fine powder, and then stored at -70 °C for use in subsequent experiments.
DMEM, RPMI, FBS, trypsin–EDTA were acquired from Welgene (Gyeongsan, Korea). PBS, MTT, DMSO, PI, BCA, β-actin, and LPS were obtained from Sigma-Aldrich (Schnelldorf, Germany). Primary antibodies including BAX, BCL-2, PARP, cleaved PARP, and cleaved caspase-3 were obtained from Santa Cruz Biotechnologies (Santa Cruz, CA, USA), and secondary antibodies were purchased from Bio-Rad (Hemel, Hempstead, UK). A Griess reagent kit was purchased from Promega (Madison, WI, USA), and an ELISA kit, to determine the levels of TNF-α, was acquired from BioVision Research Products (Mountain View, CA, USA). IL-6 was supplied by Cloud-Clone Corp. (Wuhan, Hubei, China). BSA, skim milk, and COX-2 were purchased from BD Biosciences (San Jose, CA, USA), and iNOS was obtained from Aviva systems biology (La Jolla, CA, USA). A TUNEL assay kit was acquired from Roche Molecular Biochemicals (Basel, Switzerland), and DAPI was obtained from Vector Laboratories (Burlingame, CA, USA).
Preparation of sample extracts
Our preliminary results indicated that 80% methanol extracts from leaves, twigs, and roots and 95% ethanol extracts from unripe fruits exhibited higher levels of antiproliferative activity against gastric and cervical cancer cells than other sample extracts. Therefore, leaf, twig, and root powders were extracted with 80% methanol, and unripe fruit powder was extracted with 95% ethanol. Briefly, samples were added to 80% methanol or 95% ethanol at a ratio of 10:1 (v/w), followed by incubation in a shaking water bath (BS-21, Jeio Tech., Daejeon, Korea) at 25 °C for 24 h. Collected extracts were centrifuged at 11,000 × g for 30 min at 4 °C, and the supernatants were combined. The supernatants were concentrated in a rotary evaporator (R-210, Buchi, Flawil, Switzerland), lyophilized using a freeze dryer (FD5512, Ilshin Lab Co. Ltd., Daejeon, Korea), and stored at − 70 °C in a Forma 900 Series deep freezer (Thermo Fisher Scientific Inc., Waltham, MA, USA) prior to use. A. Triloba extract contains the ‘polyphenolic compounds’ catechins, chlorogenic acid, caffeic acid, p-coumaric acid, ferulic acid, rutin [13].
Cell lines and cell culture
The cancer cell lines AGS (human gastric cancer; KCLB No. 21739), HeLa (human cervical cancer; KCLB No. 10002), and RAW 264.7 (murine macrophage; KCLB No. 40071) were purchased from the Korean Cell Line Bank (Seoul, Korea). The cells were cultured in complete DMEM or RPMI supplemented with 10% FBS (v/v) and 1% penicillin/streptomycin (v/v) in a 5% CO2 incubator atmosphere at 37 °C (BB15, Thermo Fisher Scientific Inc., Langenselbold, Germany). The cells were transferred to a new plate once every 1–2 days, and subjected to no more than 20 passages.
Determination of cell viability
The MTT assay was used as a relative measure of cell viability. For MTT assays, cells were seeded in 96-well plates at a density of 1 × 104 cells/well and allowed to adhere for 24 h. Subsequently, the medium was removed, and the cells washed with PBS, and treated with pawpaw extracts (0–200 μg/mL) dissolved in DMSO for 24 h at 37 °C. Next, 20 μL of MTT solution (1 mg/mL in PBS) was added to each well, and the plates incubated at 37 °C for 3 h. After incubation, media were aspirated, and 100 µL of DMSO was added. Absorbance was then determined at 570 nm, using a microplate spectrophotometer (Tecan Infinite M200, Tecan Group Ltd., Männedorf, Switzerland). Viability was calculated, with the control cells considered to be 100% viable.
Cell cycle analysis
Cancer cell lines were seeded in 6-well plates at a final density of 4 × 105 cells/well, and incubated at 37 °C in a 5% CO2 incubator (Thermo Fisher Scientific Inc.). After 24 h stabilization, pawpaw extracts were added, and the cells were maintained at 37 °C in a 5% CO2 incubator (Thermo Fisher Scientific Inc.) for 24 h. The cells were harvested and fixed using 70% ethanol, washed with PBS, and then treated with 1 mg/mL RNase at 37 °C for 1 h. Next, the cells were stained with PI, filtered using a cell strainer, and apoptotic cells were measured based on deoxyribonucleic acid (DNA) fragmentation using a fluorescence activated cell sorter (FACS) Calibur flow cytometer (Becton–Dickinson, Mountain View, CA, USA).
Western blotting
Cancer cell lines were seeded in 6-well plates at a final density of 4 × 105 cells/well, and incubated at 37 °C in a 5% CO2 incubator (Thermo Fisher Scientific Inc.) for 24 h. Subsequently, pawpaw extracts were added, and the cells were maintained at 37 °C in a 5% CO2 incubator (Thermo Fisher Scientific Inc.) for 24 h. The cells were harvested using trypsin–EDTA solution, washed with PBS, and centrifuged at 135 × g for 3 min (Union 32R Plus, Hanil Science Industrial Co. Ltd., Seoul, Korea). Supernatants were removed, and 40 µL of lysis buffer was added to the pellets to extract proteins.
The protein content of sample lysates was measured using the BCA method. Briefly, 20 μL of lysates and 160 μL of BCA reagent (bicinchoninic acid solution: copper (II) sulfate pentahydrate 4% solution = 50:1, v/v) were mixed, and then reacted at 37 °C for 30 min. The absorbance of the reacted mixtures was measured using a microplate spectrophotometer (Tecan Group Ltd.) at 560 nm.
Protein extracts were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), according to protein size, and electro-transferred onto a nitrocellulose membranes (Bio-Rad, Hemel, Hempstead, UK). The membranes were blocked with 5% skim milk for 1 h, and then probed with primary antibodies against BAX, BCL-2, PARP, cleaved PARP, cleaved caspase-3, and β-actin overnight at 4 °C. Subsequently, the membranes were probed with secondary antibodies at 25 °C for 1 h. Specific proteins was detected by western blotting using an x-ray film in an automated developer (SRX 101A, Konica Minolta, Tokyo, Japan).
TUNEL (Terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling) assay
TUNEL assays were performed to determine the effect of pawpaw extracts on apoptosis using a kit. The cells were grown in 8-well plates (2 × 104 cells/well) and treated with pawpaw extracts for 24 h, washed twice with PBS, and fixed by addition of 3.5% paraformaldehyde for 20 min at 4 °C. Next, the cells were washed twice with PBS, and permeabilized with 0.1% Triton X-100 solution in 0.1% sodium citrate for 5 min on ice. The cells were then incubated with TUNEL assay kit mixture for 60 min and treated with DAPI mounting solution. TUNEL-stained cells were visualized by confocal microscopy (Olympus, Tokyo, Japan).
Determination of nitrite production
A Griess reagent system kit was used to evaluate nitrite accumulation. Briefly, RAW 264.7 cells were seeded into 6-well plates at a density of 1 × 106 cells/well. After incubation for 24 h, medium was removed and cells washed with PBS. Cells were exposed to pawpaw extracts (25 and 50 μg/mL) and LPS (1 μg/mL) including medium, then incubated at 37 °C for 24 h. Supernatants (100 μL) were mixed with an equal volume of Griess reagent and incubated at 25 °C for 10 min. Then absorbance at 540 nm was measured in an ELISA plate reader (Tecan Group Ltd.) and the amount of nitrite was calculated using a nitrite standard curve.
Determination of IL-6 and TNF-α expression
The expression levels of IL-6 and TNF-α were determined using an ELISA assay kit, according to the manufacturer’s instructions. Specifically, RAW 264.7 cells were plated at a density of 1 × 106 cells/well in 6-well plates and incubated at 37 °C in 5% CO2 for 24 h. Medium was removed from plates and fresh media containing different concentrations of pawpaw extracts added to the plate. After 24 h incubation, 100 μL aliquots of supernatants were used for assays.
Determination of iNOS and COX-2 expression
RAW 264.7 cells (1 × 106 cells/well), plated in 6-well plates, were harvested by gentle scraping, and washed with PBS. Extraction of protein, determination of protein content, loading, and electrotransfer to membranes were performed according to the methods described in above. Membranes were blocked in 5% skim milk, and primary antibodies, including against iNOS and COX-2, were added at the manufacturer’s recommended dilutions and incubated at 4 °C for 24 h. After incubation, membranes were washed and secondary antibodies added at the manufacturer’s recommended dilutions. After incubation for 1 h at 25 °C, membranes were washed three times for 10 min each, and protein bands detected using western blotting detection and x-ray film in an automated developer (Konica Minolta).
Statistical analyses
Statistical analyses were performed using the statistical package for social sciences (SPSS; Version 10.0, SPSS Inc, Chicago, IL, USA). The results are presented as means ± standard deviation. Statistical differences between two groups were evaluated by unpaired t-test (p < 0.05).
Results
The inhibitory effects of pawpaw on human cancer cells
The effects of pawpaw leaf, twig, root, and unripe fruit extracts on the growth of AGS (gastric) and HeLa (cervical) cell lines were investigated; the concentrations of pawpaw required for 50% cell growth inhibition (IC50) are presented (Table 1). The IC50 values of twig and unripe fruit extracts for AGS cells were 82.01 and 100.61 µg/mL, respectively, whereas those of leaf and root extracts were > 200 µg/mL. For HeLa cells, the potency (IC50) of pawpaw extracts ranged from 97.73 to 181.55 µg/mL, with pawpaw twig extracts exhibiting the strongest ability to inhibit cervical cancer cell growth.
The effects of pawpaw on cell cycle arrest and apoptosis induction
For AGS cells, pawpaw root (200 µg/mL) and unripe fruit (100 µg/mL) extracts remarkably increased the Sub G1 cell population, and there was a corresponding significant decrease in the percentage of cells in the G1/G2 phase (data not shown). Treatment with leaf extract (200 µg/mL) significantly elevated the Sub G1 cell population to 4.00% (p < 0.05); however, it did not have any statistically significant effect on the percentage of G1/S/G2 phase cells (data not shown). Similar results were observed in HeLa cells; pawpaw root (200 µg/mL) and unripe fruit (100 µg/mL) extracts caused a considerable increase in the Sub G1 cell population, whereas the population of cells in the G1/G2 phase decreased significantly compared to that of control cells (p < 0.05); however, the leaf and twig extracts did not significantly influence HeLa cell cycle progression (data not shown).
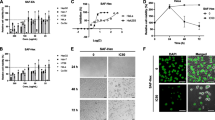
Accordingly, we analyzed the cell cycle arrest caused by twig, root, and unripe fruit pawpaw extracts, according to the concentration, to further understand the characteristics of apoptosis caused by these substances. The results presented in Fig. 1A and Fig. 1B demonstrate that twig, root, and unripe fruit extracts of pawpaw not only significantly increased the proportion of the AGS cell population in the Sub G1 in a dose-dependent manner, but also exhibited a remarkable concentration-dependent increase in the percentages of HeLa cells in the same cell cycle phase.
Cell cycle distribution of AGS (A) and HeLa (B) cells treated with different doses of pawpaw extracts. PTM, 80% methanol extract from pawpaw twigs; PRM, 80% methanol extract from pawpaw roots; URFE, 95% ethanol extract from unripe fruit. Results are presented as representative distribution charts of DNA content in cells incubated without or with different concentrations of PTM (100 and 200 µg/mL), PRM (100 and 200 µg/mL), and URFE (50 and 100 µg/mL), and as percentages of cell populations in sub G1, G1, S, and G2 phases. Data are presented as means ± standard deviation of triplicate experiments. Significant differences between control and extract-treated samples are indicated by *p < 0.05, **p < 0.01, and ***p < 0.001
The effects of pawpaw extracts on apoptosis regulatory proteins
The expression of apoptosis regulatory proteins was determined by immunoblotting (Fig. 2). Exposure of AGS cells to twig, root, and unripe fruit pawpaw extracts led to downregulation of BCL-2 and PARP and induced BAX, cleaved caspase-3, and cleaved PARP, in a dose-dependent manner (Fig. 2A). Twig, root, and unripe fruit extracts also decreased the expression of BCL-2, and increased that of BAX, cleaved caspase-3, and cleaved PARP in HeLa cells in a dose-dependent manner (Fig. 2B). However, none of the extracts had a substantial impact on PARP expression.
Effects of pawpaw extract on apoptosis regulatory proteins in AGS (A) and HeLa (B) cells. PTM, 80% methanol extract from pawpaw twigs; PRM, 80% methanol extract from pawpaw roots; URFE, 95% ethanol extract from unripe fruit. AGS and HeLa cells were treated with different concentrations of PTM (100 and 200 µg/mL), PRM (100 and 200 µg/mL), and URFE (50 and 100 µg/mL) for 24 h. Levels of the apoptosis regulatory proteins, BCL-2, BAX, cleaved caspase-3 (C-Cas3), PARP, and cleaved PARP (C-PARP), were determined by immunoblotting. β-actin was used as a loading control
Analysis of apoptosis using TUNEL assays
After incubation with twig (200 µg/mL), root (200 µg/mL), and unripe fruit (100 µg/mL) extracts for 24 h, TUNEL assays were performed to visualize apoptosis in AGS (Fig. 3A) and HeLa cells (Fig. 3B). Control AGS cells did not produce a positive TUNEL reaction, while cell treated with twig, root, and unripe fruit extracts contained labeled and stained DNA nucleotide fragments. Likewise, control HeLa cells did not generate a positive TUNEL reaction, while treatment with pawpaw twig, root, and unripe fruit extracts resulted in positive TUNEL reactions.
TUNEL nuclear staining of AGS (A) and HeLa (B) cells treated with pawpaw extracts. PTM, 80% methanol extract from pawpaw twigs; PRM, 80% methanol extract from pawpaw roots; URFE, 95% ethanol extract from unripe fruit. AGS and HeLa cells were treated with PTM (200 µg/mL), PRM (200 µg/mL), and URFE (100 µg/mL) for 24 h. Cells were washed with PBS, fixed, permeabilized, subjected to TUNEL nuclear staining, and visualized by confocal microscopy
The anti-inflammatory activities of pawpaw extracts
The effects of pawpaw extracts on NO production in LPS-stimulated RAW 264.7 macrophages are presented in Fig. 4A. LPS considerably stimulated the production of NO in RAW 264.7 cells at a concentration of 32.07 µM. Treatment with LPS-stimulated RAW 264.7 cells with pawpaw leaf, twig, root, and unripe fruit extracts led to a dose-dependent decrease in the production of NO. In particular, pawpaw root extract exhibited the strongest inhibition of NO production. In addition, LPS (59.71 pg/mL) remarkably induced the production of TNF-α by macrophages, while pawpaw extracts reduced TNF-α production in a dose-dependent manner, with similar effects to those on NO production (Fig. 4B). Twig extract (50 µg/mL) inhibited TNF-α production by > 50%, while 25 µg/mL root and unripe fruit extracts inhibited its production by > 60%. Similarly, IL-6 was significantly inhibited by pawpaw extracts (p < 0.05), except those from leaves, with 50 µg/mL twig extract inducing an approximately 50% decrease in IL-6 cytokine production (Fig. 4C). The leaf, twig, root, and unripe fruit extracts of pawpaw considerably reduced iNOS expression compared to the positive control cells (Fig. 4D). However, it did not affect the levels of COX-2.
Effect of pawpaw on NO production (A), and TNF-α (B), IL-6 (C), iNOS and COX-2 (D) expression in LPS-stimulated RAW 264.7 cells. PLM, 80% methanol extract from pawpaw leaves; PTM, 80% methanol extract from pawpaw twigs; PRM, 80% methanol extract from pawpaw roots; URFE, 95% ethanol extract from unripe fruit. RAW 264.7 cells were treated with PLM, PTM, PRM, and URFE (25 and 50 µg/mL) for 24 h. Data are presented as means ± standard deviation of triplicate experiments. Differences were evaluated by comparisons with the LPS-treatment group (##). *p < 0.05. β-actin was used as a loading cont
Discussion
In previously study, the viabilities of AGS and HeLa cells treated with 500 µg/mL mango (flesh) ethanol extract were approximately 80% and 100%, respectively [23]; hence, our data indicate that pawpaw has superior anticancer properties compared to that of mango. In addition, the IC50 values of leaf methanol extracts from Gnaphalium luteoalbum and Lannea coromandelica for AGS cells were 980 and 670 µg/mL, respectively [24], whereas the stem, root, leaf, unripe fruit, and ripe fruit methanol extracts from Cudrania tricuspidata had had minimal effects on AGS and HeLa cell growth [25]. Another study showed that the IC50 values of Pistacia atlantica extract for AGS and HeLa cells were 382.3 and 332.3 µg/mL [26]. These results are considerably different from those of the present study, and our data demonstrate that pawpaw tree extracts exhibit superior cytotoxicity against AGS and HeLa cells.
The results of cell cycle analysis demonstrated that twig, root, and unripe fruit pawpaw extracts can cause the Sub G1 cell cycle arrest, thereby obstructing the growth of AGS and HeLa cells. In addition, pawpaw induced the activation of BAX, cleaved caspase-3, and cleaved PARP, which contribute to apoptosis. These results confirm that twig, root, and unripe fruit pawpaw extracts can clearly induce apoptosis of AGS and HeLa cells. However, the proteins expression of extracts in AGS and HeLa cells were showed a relatively small difference. Briefly, the effect of PTM and PRM on the protein expression of BAX is not clear and the same thing with cleaved caspase-3 for URFE extract in AGS cells while a clear effect of BAX in HeLa cells was noticed. These findings are thought to the pawpaw extracts were induce apoptosis via the other apoptosis pathway in AGS and HeLa cells. Actually, Saralamma et al. [27] demonstrated that Poncirin a form of flavonoid induced apoptosis though an extrinsic pathway in AGS cells, which is independent of mitochondrial-related apoptotic pathways. On the other hand, Mane et al. [28] proved that ascorbyl searate formed from ascorbic acid and stearic acid induces apoptosis though an extrinsic as well as intrinsic pathways in HeLa cancer cells. This implies that the apoptosis pathway varies depending on the substance and cell line, and pawpaw also suggests that the apoptosis pathway may vary depending on the cell line.
González-Montoya et al. [29] reported that peptides derived from soy bean protein inhibited NO production by 45% at a concentration of 10 mg/mL. In addition, Xu et al. [30] demonstrated that ethanol extracts of pomegranate flower reduce TNF-α and IL-6 production by 50% at concentrations of 62.5 and 48.7 µg/mL, respectively. Furthermore, TNF-α and IL-6 were inhibited by buckwheat sprout extract (100 µg/mL); however, the rate of inhibition was < 50% [31]. Overall, even at low concentrations, pawpaw extracts had stronger inhibitory effects on inflammatory markers than those reported for other plant extracts in previous studies. NO is a free radical that is very unstable, and it is involved in lipid oxidation reactions thereby affecting on inflammatory reactions in tissue [32]. These findings suggest that the leaves, twigs, roots, and unripe fruit of pawpaw have enormous potential as natural agents exhibiting antioxidant and anti-inflammatory activity. On the other hand, Liu et al. [33] reported that the alkaloids reduced the production of NO though down-regulation of iNOS protein expression, however, have no effect on the expression of COX-2. Therefore, our results are also anticipated that the anti-inflammatory effect of pawpaw was caused via down-regulation of iNOS protein expression irrelevant to COX-2 pathway.
Phenolic compound extracted from natural substance are potent bioactive materials, which possess anticancer and anti-inflammatory activities. They inhibits the development and progression of cancer by inducing cell cycle arrest and apoptosis, and have potential to inhibit the inflammatory response by modulating various inflammatory factors such as NO, IL-6, iNOS, and COX-2 [34, 35]. These phenolic compound is widely known to be rich in the various tissue of pawpaw. In particular, the roots and twigs extract from pawpaw were contained a lot of phenolic compound and there was variety of phenolic compound, than other tissue of pawpaw [13]. Therefore, we are anticipated that the anticancer and anti-inflammatory activities of pawpaw are due to various phenolic compounds extracted from pawpaw.
Inflammation is induce before a malignant occurs in some cancers. In addition, an oncogenic change present an inflammatory environment that accelerate the development of tumors [36]. Briefly, there is close correlation between inflammation and cancer. Accordingly, many studies on molecular pathways of cancer-related inflammation are now being performed, these results helps to identify the new target molecules lead to better diagnosis and treatment of cancer [36]. In this regard, this study on anticancer and anti-inflammatory activities of pawpaw is expected that is encourage further studies on molecular pathways of cancer-related inflammation.
Conclusion
In conclusion, our data confirm our initial hypothesis that extracts from the pawpaw tree both inhibit the growth of gastric and cervical cancer cells by increasing their apoptosis and have anti-inflammatory effects. Extracts from the twigs, roots, and unripe fruits of pawpaw, but not those from leaves, induced Sub G1 cell cycle arrest. Our data clearly demonstrate that pawpaw extracts are inducers of AGS and HeLa cell apoptosis, and possess increased levels of antitumor activity on human gastric and cervical cancer cells. Furthermore, this is the first study to investigate the anti-inflammatory activity of pawpaw extracts, and the results prove that extracts from this plant have great potential as anti-inflammatory agents. These results highlight the preventive and therapeutic effects of pawpaw against gastric and cervical cancer and encourage further studies on the anti-inflammatory effects of the pawpaw tree.
Data availability
All data generated or analyzed during this study are included in this published article.
References
Feigin V (2016) Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the global burden of disease study 2015. Lancet 388:1459–1544. https://doi.org/10.1016/S0140-6736(16)31012-1
Wild CP, Stewart BW (2014) World Cancer Report 2014. World Health Organization, Lyon, France
Han HH, Park JW, Na JC, Chung BH, Kim CS, Ko WJ (2015) Epidemiology of prostate cancer in South Korea. Prostate Int 3(3):99–102. https://doi.org/10.1016/j.prnil.2015.06.003
Kim JH, Park MK (2010) Effects of preventive sexual education of HPV on HPV knowledge, cervical cancer preventive behaviors, and sexual autonomy in female university students. J Korean Acad Nurs 16(2):257–264. https://doi.org/10.5977/jkasne.2010.16.2.257
Kim EJ, Kim GT, Kim BM, Lim EG, Kim SY, Kim YM (2016) Apoptosis-induced effects of extract from Artemisia annua Linné by modulating Akt/mTOR/GSK-3β signal pathway in AGS human gastric carcinoma cells. J Korean Soc Food Sci Nutr 45(9):1257–1264. https://doi.org/10.3746/jkfn.2016.45.9.1257
Kasibhatla S, Tseng B (2003) Why target apoptosis in cancer treatment? Mol Cancer Ther 2(6):573–580
Kerr JF, Wyllie AH, Currie AR (1972) Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer 26(4):239–257. https://doi.org/10.3736/jintegrmed2013036
Hseu YC, Wu FY, Wu JJ, Chen JY, Chang WH, Lu FJ, Lai YC, Yang HL (2005) Anti-inflammatory potential of Antrodia camphorata through inhibition of iNOS, COX-2 and cytokines via the NF-κB pathway. Int Immunopharmacol 5(13):1914–1925. https://doi.org/10.1016/j.intimp.2005.06.013
Reinecker HC, Steffen M, Witthoeft T, Pflueger I, Schreiber S, MacDermott RP, Raedler A (1993) Enhand secretion of tumour necrosis factor-alpha, IL-6, and IL-1β by isolated lamina ropria monouclear cells from patients with ulcretive cilitis and Crohn’s disease. Clin Exp Immunol 94(1):174–181. https://doi.org/10.1111/j.1365-2249.1993.tb05997.x
Umesalma S, Sudhandiran G (2010) Differential inhibitory effects of the polyphenol ellagic acid on inflammatory mediators NF-κB, iNOS, COX-2, TNF-α, and IL-6 in 1, 2-dimethylhydrazine-induced rat colon carcinogenesis. Basic Clin Pharmacol Toxicol 107(2):650–655. https://doi.org/10.1111/j.1742-7843.2010.00565.x
Callaway MB (1993) Pawpaw (Asimina triloba). A “tropical” fruit for temperate climates. Wiley, New York, pp 505–515
Brannan RG, Peters T, Talcott ST (2015) Phytochemical analysis of ten varieties of pawpaw (Asimina triloba [L.] Dunal) fruit pulp. Food Chem 168:656–661. https://doi.org/10.1016/j.foodchem.2014.07.018
Nam JS, Jang HL, Rhee YH (2017) Antioxidant activities and phenolic compounds of several tissues of pawpaw (Asimina triloba [L.] Dunal) grown in Korea. J Food Sci 82(8):1827–1833. https://doi.org/10.1111/1750-3841.13806
Brannan RG, Salabak DE (2009) Ability of methanolic seed extracts of pawpaw (Asimina triloba) to inhibit n-3 fatty acid oxidation initiated by peroxyl radicals and reactive oxygen, nitrogen, and sulfur. Food Chem 114(2):453–458. https://doi.org/10.1016/j.foodchem.2008.09.071
Coothankandaswamy V, Liu Y, Mao SC, Morgan JB, Mahdi F, Jekabsons MB, Nagle DG, Zhou YD (2010) The alternative medicine pawpaw and its acetogenin constituents suppress tumor angiogenesis via the HIF-1/VEGF pathway. J Nat Prod 73(5):956–961. https://doi.org/10.1021/np100228d
Farag MA (2009) Chemical composition and biological activities of Asimina triloba leaf essential oil. Pharm Biol 47(10):982–986. https://doi.org/10.1080/13880200902967995
Harris GG, Brannan RG (2009) A preliminary evaluation of antioxidant compounds, reducing potential, and radical scavenging of pawpaw (Asimina tribloba) fruit pulp from different stages of ripeness. LWT-Food Sci Technol 42(1):275–279. https://doi.org/10.1016/j.lwt.2008.05.006
Kobayashi H, Wang C, Pomper KW (2008) Phenolic content and antioxidant capacity of pawpaw fruit (Asimina triloba L.) at different ripening stages. HortScience 43(1):268–270. https://doi.org/10.21273/HORTSCI.43.1.268
Woo MH, Cho KY, Zhang Y, Zeng L, Gu ZM, McLaughlin JL (1995) Asimilobin and cis-and trans-murisolinones, novel bioactive annonaceous acetogenins from the seeds of Asimina triloba. J Nat Prod 58(10):1533–1542. https://doi.org/10.1021/np50124a009
Zhao GX, Miesbauer LR, Smith DL, McLaughlin JL (1994) Asimin, asiminacin, and asiminecin: Novel highly cytotoxic asimicin isomers from Asimina triloba. J Med Chem 37(13):1971–1976. https://doi.org/10.1021/jm00039a009
Nam JS, Park SY, Lee HJ, Lee SO, Jang HL, Rhee YH (2018) Correlation between acetogenin content and antiproliferative activity of pawpaw (Asimina triloba [L.] Dunal) fruit pulp grown in Korea. J Food Sci. 83(5):1430–1435. https://doi.org/10.1111/1750-3841.14144
Ko YM, Wu TY, Wu YC, Chang FR, Guh JY, Chuang LY (2011) Annonacin induces cell cycle-dependent growth arrest and apoptosis in estrogen receptor-α-related pathways in MCF-7 cells. J Ethnopharmacol 137(3):1283–1290. https://doi.org/10.1016/j.jep.2011.07.056
Kim H, Moon JY, Kim H, Lee DS, Cho M, Choi HK, Kim YS, Mosaddik A, Cho SK (2010) Antioxidant and antiproliferative activities of mango (Mangifera indica L.) flesh and peel. Food Chem 121(2):429–436. https://doi.org/10.1016/j.foodchem.2009.12.060
Akter R, Uddin SJ, Grice ID, Tiralongo E (2014) Cytotoxic activity screening of Bangladeshi medicinal plant extracts. J Nat Med 68(1):246–252. https://doi.org/10.1007/s11418-013-0789-5
Choi SR, You DH, Ahn MS, Song EJ, Seo SY, Choi MK, Kim YS, Kim MK, Choi DG (2012) Cytotoxicity of methanol extract from Cudrania tricuspidata bureau. Korean J Med Crop Sci 20(3):153–158. https://doi.org/10.7783/kjmcs.2012.20.3.153
Hashemi L, Asadi-Samani M, Moradi MT, Alidadi S (2017) Anticancer activity and phenolic compounds of Pistacia atlantica extract. Int J Pharm Phytopharm Res 7(2):26–31. https://doi.org/10.24896/eijppr.2017725
Saralamma VVG, Nagappan A, Hong GE, Lee HJ, Yumnam S, Raha S, Heo JD, Lee SJ, Lee WS, Kim EH, Kim GS (2015) Poncirin induces apoptosis in AGS human gastric cancer cells through extrinsic apoptotic pathway by up-regulation of Fas ligand. Int J Mol Sci 16(9):22676–22691. https://doi.org/10.3390/ijms160922676
Mane SD, Thoh M, Sharma D, Sandur SK, Naidu KA (2016) Ascorbyl stearate promotes apoptosis through intrinsic mitochondrial pathway in HeLa cancer cells. Anticancer Res 36(12):6409–6417. https://doi.org/10.21873/anticanres.11238
González-Montoya M, Hernández-Ledesma B, Silván JM, Mora-Escobedo R, Martínez-Villaluenga C (2018) Peptides derived from in vitro gastrointestinal digestion of germinated soybean proteins inhibit human colon cancer cells proliferation and inflammation. Food Chem 242:75–82. https://doi.org/10.1016/j.foodchem.2017.09.035
Xu J, Zhao Y, Aisa HA (2017) Anti-inflammatory effect of pomegranate flower in lipopolysaccharide (LPS)-stimulated RAW264. 7 macrophages. Pharm Biol 55(1):2095–2101. https://doi.org/10.1080/13880209.2017.1357737
Karki R, Park CH, Kim DW (2013) Extract of buckwheat sprouts scavenges oxidation and inhibits pro-inflammatory mediators in lipopolysaccharide-stimulated macrophages (RAW264. 7). J Integr Med 11(4):246–252. https://doi.org/10.3736/jintegrmed2013036
Bloodsworth A, O’Donnell VB, Freeman BA (2000) Nitric oxide regulation of free radical-and enzyme-mediated lipid and lipoprotein oxidation. Arterioscler Thromb Vasc Biol 20(7):1707–1715. https://doi.org/10.1161/01.ATV.20.7.1707
Liu P, Li H, Luan R, Huang G, Liu Y, Wang M, Chao Q, Wang L, Li D, Fan H, Chen D, Li L, Matsuzaki K, Koike K, Zhao F (2019) Identification of β-carboline and canthinone alkaloids as anti-inflammatory agents but with different inhibitory profile on the expression of iNOS and COX-2 in lipopolysaccharide-activated RAW 264.7 macrophages. J Nat Med. 73(1):124–130. https://doi.org/10.1007/s11418-018-1251-5
Abbaszadeh H, Keikhaei B, Mottaghi S (2019) A review of molecular mechanisms involved in anticancer and antiangiogenic effects of natural polyphenolic compounds. Phytother Res 33(8):2002–2014. https://doi.org/10.1002/ptr.6403
Zhu F, Du B, Xu B (2018) Anti-inflammatory effects of phytochemicals from fruits, vegetables, and food legumes: A review. Crit Rev Food Sci Nutr 58(8):1260–1270. https://doi.org/10.1080/10408398.2016.1251390
Mantovani A, Allavena P, Sica A, Balkwill F (2008) Cancer-related inflammation. Nature 454(7203):436–444. https://doi.org/10.1038/nature07205
Acknowledgements
Not applicable.
Funding
The research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
J-SN performed the study and wrote the manuscript, S-YP, S-OL and H-JL helped experiment and data arrangement, H-LJ and YHR planed the study, analyzed the results, and aided writing the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
This paper does not contain any studies with animals or human participants. Therefore no ethical approval is required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nam, JS., Park, SY., Lee, SO. et al. The growth-inhibitory effects of pawpaw (Asimina triloba [L.] Dunal) roots, twigs, leaves, and fruit against human gastric (AGS) and cervical (HeLa) cancer cells and their anti-inflammatory activities. Mol Biol Rep 48, 2173–2181 (2021). https://doi.org/10.1007/s11033-021-06226-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-021-06226-y