Abstract
Cisplatin is a first-line chemotherapy drug against ovarian cancer. However, its strong toxic side effects and the development of cisplatin resistance in human cancer cells seriously influence the effects of chemotherapy and quality of life in patients. Noscapine (Nos), a non-toxic benzylisoquinoline alkaloid extracted from opium, has been recently reported to have anti-cancer activity, but the mechanism of that effect has not been clearly established. In the present study, we investigated cytotoxicity of Nos in combination with cisplatin (DDP) in drug-resistant human ovarian cancer cell line SKOV3/DDP in vitro and in vivo null mice xenograft model. Cell proliferation was measured by MTT assay, flow cytometry was used to analyze cell cycle and apoptosis, protein expression of several apoptotic factors was investigated by flow cytometry and immunohistochemical method, and their mRNA expression levels were determined by real-time PCR. In vitro experiments showed that Nos significantly inhibited proliferation of SKOV3/DDP cells. DDP/Nos-combined treatment notably enhanced DDP-induced inhibition of cell proliferation and increased the pro-apoptotic effect of DDP in SKOV3/DDP cells. DDP/Nos administration increased the proportion of G2/M cells, reduced both protein and mRNA expression of anti-apoptotic factors XIAP, surviving and NF-kB, and augmented protein and mRNA levels of pro-apoptotic caspase-3. In vivo experiments revealed that Nos/DDP treatment increased the apoptotic rate of xenograft tumors in null mice. Tumor volume decreased from 1.733 ± 0.155 g in mice treated with DDP alone to 1.191 ± 0.106 g in animals treated with Nos/DDP. These observations suggest that Nos increases the anti-cancer activity of DDP against the drug-resistant ovarian cancer cell line SKOV3/DDP by modulating the cell cycle and activating apoptotic pathways. The study provides a new chemotherapy strategy for the treatment of DDP-resistant human ovarian cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ovarian cancer is one of the three malignant tumors of the female reproductive system, characterized by frequent recurrence, rapid metastasis, and propensity to develop multidrug resistance (MDR) [1–2]. As most patients are clinically diagnosed at an advanced stage, ovarian cancer poses a prominent threat to the lives and health of women, with 5-year survival rate ranging from 30 to 40 %, and the highest mortality rate among gynecologic malignant tumors [3].
Currently, the prevalent treatment of ovarian cancer includes chemotherapy in combination with cytoreductive surgery. Among numerous drugs, platinum compounds such as cisplatin (DDP) are classically used and are most effective. However, multidrug resistance of cancer cells decreases the efficacy of chemotherapy and impairs the quality of life of ovarian cancer patients, considerably reducing the 5-year survival rate [4–7].
Noscapine (Nos) is a non-toxic benzylisoquinoline alkaloid that is similar to colchicine in structure and has long been used as an antitussive drug [8]. In recent years, this drug has been shown to inhibit the dynamic instability of microtubules and promotes apoptosis of tumor cells by disrupting their mitosis [9, 10]. Studies on tumor cells and animal tumor models demonstrated that Nos has anti-cancer proprieties [11, 12]. In addition, several reports indicated that in tumor cells that develop resistance to microtubule inhibitors, such as paclitaxel, Nos and its derivatives still have anti-neoplastic effect and are able to reverse the sensitivity of OVCAR3 cells to vincristine and doxorubicin. Moreover, Nos has insignificant toxic side effects and shows no obvious cross resistance [13].
Given the anti-cancer properties and reversible drug resistance of Nos in some tumors, we used in vitro and in vivo experiments to investigate the effect of Nos on the toxicity of DDP in DDP-resistant ovarian cancer cells and proposed a possible mechanism of this effect.
Materials and Methods
Reagents
DDP, Nos, trypsin, and propidium iodide (PI) were purchased from Sigma-Aldrich, USA. PBS buffer solution, RPMI-l640, dimethyl sulfoxide (DMSO), fetal bovine serum (FBS), and methyl thiazolyl tetrazolium (MTT) were purchased from Gibco®, USA. Rabbit anti-human NF-κb/P65 and XIAP polyclonal antibodies were from Bioworld, USA, and the mouse anti-human caspase-3 and survivin monoclonal antibodies were obtained from Santa Cruz, USA. DAB kits and detection reagents PV-6001 and PV-6002 were from ZSGB-BIO Origene, China. RNA purification, HiFi-MMLV cDNA synthesis and RealSuper Mixture kits were purchased from CWbio, China. All chemicals were of analytical grade and purchased from Sigma, USA unless specified otherwise. Flow cytometer (Epics-XLII) was from Beckman Coulter, USA, and the ELISA reader (HT2-125500) was from Biocell, China. The fluorescence quantitative PCR instrument (AB7500) was from AB SCIEX, USA.
Cell Culture
The epithelial ovarian cancer cell lines SKOV3 and SKOV3/DDP were provided by the Research Center at the Fourth Hospital of Hebei Medical University, China. Cells were cultured at 37 °C in a humidified incubator with 5 % CO2 in RPMI 1640 medium supplemented with 10 % FBS. All the experiments were performed on cells in the logarithmic growth phase.
Experimental Animals
3–4 week-old female BALB/c nu/nu mice, weighing 18–22 g, were purchased from the Animal Experimental Center at the Academy of Military Medical Sciences, China. All mice were housed in a standard pathogen-free facility with a temperature of 25 ± 1 °C and a relative humidity of 40–60 % at the Animal Experimental Center at the Fourth Hospital of Hebei Medical University. Regular ultraviolet irradiation was performed, and the mice had free access to sterile water and food chow. The protocols for the animal studies were approved by the Institutional Animal Care and Usage Committee at Hebei Medical University, and the experiments were in accordance to the rules of the Animal Experimental Center at the Fourth Hospital of Hebei Medical University.
MTT Assay
SKOV3 and SKOV3/DDP ovarian cancer cells in the logarithmic growth phase were diluted to 2.5 × 104/ml, and 200 μl of the diluted single cell suspension was seeded onto 96-well plates and cultured at 37 °C, 5 % CO2 for 48 h. Cells were then incubated with 20 μl of MTT solution (5 mg/ml) per well for 4 h in the dark, followed by removal of supernatant and addition of 0.2 ml DMSO for 10 min with gentle shaking. The absorbance (OD value) was measured at 490 nm with an microplate ELISA reader. The experiment was repeated three times.
Flow Cytometry
SKOV3/DDP cells in the logarithmic growth phase were grown to 60–70 % confluency, fixed with 70 % ethanol, and stored at 4 °C overnight. Cells were then washed three times with PBS, and the cell concentration was adjusted to 1 × 106/ml. DNA staining solution (50 μg/ml PI, 10 μg/ml ribonuclease I, and 1 % Triton-X100) was added to the 0.1 ml cell suspension and incubated in the dark for 30 min at 4 °C. Cells were then analyzed by flow cytometry. Multicycle AV analysis software was applied for the analysis of the DNA cell cycle, and Expo32 ADC software was used to analyze the immunofluorescence data. The sub-diploid peaks represented the peaks of cell apoptosis. The experiment was repeated three times.
For the analysis of XIAP, caspase-3, survivin, and NF-κB expression, 0.1 ml of a solution containing anti-XIAP, caspase-3, survivin, and NF-κB antibodies (1:100 dilution) was added to the 0.1 ml cell suspension. The cells were incubated at room temperature (RT) for 30 min, washed once with PBS, and incubated with 0.1 ml FITC labeled goat anti-rabbit secondary antibody for 30 min. Protein expression was measured by flow cytometry and represented as mean fluorescence intensity. The experiment was repeated three times.
Real-Time PCR
Total RNA was isolated from SKOV3/DDP cells using RNA purification kit according to manufacturer’s instructions and stored at −80 °C. cDNA was synthesized using HiFi-MMLVcDNA synthesis kit and stored at −20 °C. RealSuper Mixture was used for amplification, and the primer sequences used are listed in Table 1. Sample cDNA was diluted by a factor of 5 and 10 μl of each dilution was used as a template and amplified with primers for target or control (actin) genes, respectively. Real-time PCR reaction mixture (total volume of 20 μl) contained 10 μl of 2X RealSuper Mixture, 0.4 μl of forward and reverse primers (10 μM each), and 2 μl of cDNA template. The PCR program was as follows: initial denaturation at 95 °C for 10 min followed by 40 cycles of 95 °C for 15 s and 60 °C for 60 s. AB7500 fluorescence quantitative PCR instrument was used to measure the cDNA amount of target or control genes, establishing a standard curve, and a melting curve analysis was carried out at 60–95 °C. The 2−△△CT method was used for relative quantitative analysis.
Orthotopic Tumor Model in Nude Mice
SKOV3/DDP cells in the logarithmic growth stage were diluted to 3 × 107/ml. Single cell suspension (0.2 ml/mouse) was subcutaneously injected into the right scapular region of nude mice. Tumors were allowed to grow till 5 mm. 2 weeks post injection mice were randomly divided into 4 groups (6 mice per group). The control, Nos, DDP, and Nos + DDP groups received intraperitoneal injection of 0.9 % Sodium Chloride Solution, 40 mg/kg Nos, 3 mg/kg DDP, and 40 mg/kg Nos followed by 3 mg/kg DDP 6 h later, respectively; total volume of each injection was 0.2 ml. Each injection was repeated every 3 days and a total of seven injections were administered to each mouse. Mice were sacrificed 3 days after the last treatment, and the tumors were isolated. The tumors were weighted, their volume was calculated using the formula V(mm3) = ab 2/2 (a length; b width), and tumor growth curves were obtained.
Analysis of Tumor Cells by Flow Cytometry
Tumors were fixed with 70 % ethanol and passed through a mesh nylon strainer to generate a single cell suspension. 1 × 106 cells (0.1 ml) were mixed with 0.1 ml of anti-XIAP, caspase-3, survivin, and NF-κB antibody solution (1:100 dilution for each antibody). The mixture was incubated at RT for 30 min. Cells were then washed once with 10 ml PBS, centrifuged at 1,000 rpm for 5 min, and 0.1 ml of FITC labeled secondary antibody solution was then added to the pellet, followed by incubation at RT for 30 min in the dark. Cells were washed with 10 ml of PBS, centrifuged at 1,000 rpm for 5 min, and the pellet was re-suspended in 1 ml of PBS, filtered through 300 mesh nylon filter. Protein expression (mean fluorescent intensity) was analyzed by flow cytometry. The experiment was repeated three times.
The DNA staining solution (50 μg/ml PI, 10 μg/ml ribonuclease I, and 1 % Triton-X100) was added into 0.1 ml cell suspension. The cells were stained at 4 °C for 30 min and washed twice with PBS. Expo32 ADC software was used to analyze cell apoptosis. The experiment was repeated 3 times.
Immunohistochemistry
mRNA expression in tumor tissue was evaluated by immunohistochemistry PV method. In brief, the tumors were all fixed with 10 % formalin, embedded in regular paraffin, and sectioned. 5-μm-thick sections were deparaffinized and dehydrated, and soaked in 3 % hydrogen peroxide at RT for 10–15 min to eliminate the activity of endogenous peroxidases. Heat-induced epitope retrieval was performed in a pressure cooker for 2 min. Slides were then blocked in 1 % bovine serum albumin (BSA) at RT for 10–15 min, incubated with anti-XIAP, caspase-3, survivin, and NF-κB primary antibodies (1:50 dilution) overnight at 4 °C, washed three times with 0.01 M PBS (5 min each time), and finally incubated with the secondary antibodies, PV-6001 and PV-6002, at 37 °C for 60 min. The slides were incubated with the developing solution composed of 4 ml PBS, 5 g DAB, and 25 μl H2O for 5 min (the time was determined by observation under the microscope). The slides were washed with distilled water five times, and re-stained with hematoxylin for 1 min. After dehydration, xylene transparentizing, and neutral gum mounting, stained specimens were observed under a light microscope. XIAP and NF-κB staining showed brown granules in the cytoplasm. Survivin and caspase-3 staining was detected as brown granules in both the nucleus and the cytoplasm. Immunohistochemical scores (IHSs) were determined as previously described [14] according to the percentage of positive cells (c) and the intensity of positive staining (d), using the following equation: IHS = c × d. c is 0 for no positive cells, 1 for 1–10 %, 2 for 11–50 %, 3 for 51–80 %, and 4 for 81–100 % positive cells. d is 0 for negative staining, 1 for weak positive staining, 2 for positive staining, and 3 for strong positive staining.
Statistical Analysis
SPSS13.0 software was used for statistical analysis and the results were presented as (\(\bar{x} \pm s\)). A variance analysis was used to compare three or more groups. The LSD method was used to further compare differences between the two groups. T tests were used to compare the two sets of data and P < 0.05 was considered as statistically significant.
Results
Noscapine Inhibits Proliferation of Ovarian Cancer Cells
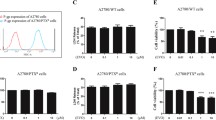
In order to determine the effect of Nos on the proliferation of ovarian cancer cells, DDP-sensitive SKOV3 and DDP-resistant SKOV3/DDP ovarian cancer cells were treated with 2.5, 5, 10, 20, and 40 μM of Nos for 48 h, and the rate of cell proliferation inhibition was measured by MTT assay. Nos significantly inhibited SKOV3 and SKOV3/DDP proliferation in a dose-dependent manner. While at the lowest concentration (2.5 μM) Nos had no obvious inhibitory effect on either cell line (P > 0.05), it significantly inhibited proliferation of both cell lines at 40 μM (P < 0.05) as compared to untreated control (Fig. 1a). Treatment with Nos led to similar cytotoxicity in DDP-sensitive and resistant ovarian cancer cells (P > 0.05). We next incubated SKOV3/DDP cells with 2.5 μM Nos and various concentrations of DDP (0, 2, 4 and 8 μg/ml) for 48 h, and evaluated cytotoxicity by MTT assay. Even at the lowest concentration, Nos significantly increased DDP-induced cell proliferation inhibition rate as compared to cells treated with DDP alone (Fig. 1b).
Noscapine increases the sensitivity of ovarian cancer cells to cisplatin. a Inhibition curves of SKOV3 and SKOV3/DDP cells. Cells were treated with Nos at different concentrations (2.5, 5, 10, 20, and 40 μM) for 48 h. MTT assay was used to detect the inhibition rate of cell proliferation. b The inhibition curve of SKOV3/DDP cells. Cells were treated with Nos (2.5 μM), DDP alone (0, 2, 4, and 8 μg/ml) or Nos + DDP for 48 h. Cytotoxicity was determined by MTT assay. Values are presented as mean ± SD based on three independent experiments
Noscapine Increases the Sensitivity of Drug-Resistant Ovarian Cancer Cells to Cisplatin
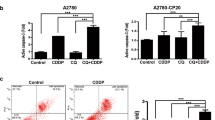
In order to further investigate whether Nos is able to affect DDP cytotoxicity, we studied the effect of Nos/DDP combination on DDP-resistant ovarian cancer cells. SKOV3/DDP cells were treated with low concentration of Nos (2.5 μM), DDP (0, 2, 4, and 8 μg/ml) alone or a combination of Nos and DDP for 48 h. The cytotoxicity was assessed by flow cytometry as described in “Materials and Methods” section. Combined treatment of SKOV3/DDP cells with Nos and DDP resulted in a significant increase in the inhibition rate of cell proliferation, comparing to cells treated with DDP alone (P < 0.05, Fig. 2a). Cells treated with low concentration of Nos (2.5 μM) in conjunction with DDP (4 μg/ml) exhibited markedly higher apoptosis rate (over 1.9-fold increase comparing to cells treated with DDP alone, P < 0.05, Fig. 2b). There was no significant difference between the percentage of apoptotic cells treated with Nos alone (2.956 ± 0.932) % and control cells (2.851 ± 0.849) % (P > 0.05, Fig. 2b). We further tested the effect of DDP/Nos treatment on cells using inverted microscopy. Combined DDP/Nos treatment resulted in dramatic changes in cell morphology comparing to cells, treated with DDP alone, with cells exhibiting typical features of apoptosis (Fig. 2c). Together these results suggest that while Nos alone is not toxic for the cells, low dose of Nos (2.5 μM) increases the toxicity of DDP to SKOV3/DDP cells and promotes cell apoptosis.
Noscapine increases pro-apoptotic effect of cisplatin on ovarian cancer cells. a Apoptosis of SKOV3/DDP cells. DDP-resistant cells were treated with Nos (2.5 μM) alone, DDP (4 μg/ml) alone, or a combination of Nos (2.5 μM) and DDP (4 μg/ml) for 48 h. Apoptotic rate was detected by flow cytometry. The sub-diploid peaks represented the apoptotic peaks. b Statistical analysis of apoptotic rate in SKOV3/DDP cells. **Compared with the DDP group (P < 0.05), *compared with the control group (P > 0.05). c The morphology of SKOV3/DDP cells under a light microscope (×100). Values are presented as mean ± SD of three independent experiments
Noscapine Changes the Protein and mRNA Levels of Apoptotic Genes in Ovarian Cancer Cells
We next studied the possible mechanism of Nos effect on cytotoxicity of DDP in DDP-resistant cells. We treated drug-resistant SKOV3/DDP ovarian cancer cells with Nos (2.5 μM) and DDP (4 μg/ml) alone, or a combination of Nos + DDP for 48 h, and evaluated protein and mRNA expression of anti-apoptotic proteins XIAP, survivin, and NF-κB, and pro-apoptotic caspase-3 using flow cytometry and real-time PCR, respectively. As compared to the DDP group, Nos-treated cells exhibited lower protein levels of XIAP, survivin, and NF-κB, but increased caspase-3 levels (P < 0.05, Fig. 3a). However, there was no significant difference in the expression of these proteins between the DDP and control groups (P > 0.05) (Fig. 3a). Changes in protein expression levels in SCOV3/DDP cells correlated with the changes in the levels of mRNA expression, as indicated by real-time PCR (Fig. 3b). Combined DDP+Nos treatment markedly reduced mRNA expression levels of XIAP, survivin, and NF-κB, but increased caspase-3 mRNA expression (Fig. 3b). These results implicate that Nos may improve the sensitivity of drug-resistant ovarian cancer cells to DDP by regulating apoptotic factors.
Noscapine changes protein and mRNA levels of apoptotic genes. a Protein expression of XIAP, caspase-3, survivin, and NF-κB in SKOV3/DDP cells. Cells were treated with Nos (2.5 μM), DDP (4 μg/ml) alone, or combined Nos (2.5 μM) + DDP (4 μg/ml) for 48 h. Flow cytometry was used to detect protein expression, which was presented as the fluorescent intensity. **Compared with the DDP group (P < 0.05), *compared with the control group (P > 0.05). b mRNA levels of XIAP, caspase-3, survivin, and NF-κB in SKOV3/DDP cells detected by real-time PCR. The 2−∆∆CT method was applied to perform relative quantitative analysis. **Compared with the DDP group (P < 0.05), *compared with the control group (P > 0.05). Values are presented as mean ± SD of three independent experiments
Noscapine Increases the Proportion of Drug-Resistant Ovarian Cancer Cells in the G2/M Phase
Previous studies show that the effect of DDP on cell cycle of ovarian cancer cells is mainly manifested in cells arresting in the S phase [15]. In order to explore how Nos influences growth of SKOV3/DDP cells, we treated this cell line with Nos (2.5 μM), DDP (4 μg/ml), or Nos (2.5 μM) + DDP (4 μg/ml) for 48 h, and the analyzed developmental stage of the cells using flow cytometry. Comparing to control group, DDP treatment led to 1.2-fold decrease in the percentage of G0/G1 cells and over 1.6-fold increase in the percentage of S phase cells (P < 0.05, Fig. 4a, b). Nos alone and a combined DDP+Nos treatment significantly reduced the proportion of SKOV3/DDP cells in the S phase while increasing the percentage of G2/M cells (P < 0.05) as compared to DDP-treated cells (Fig. 4a, b). These findings indicate that in SKOV3/DDP cells, Nos in conjunction with DDP modulates cell cycle, arresting the cells in G2/M phase.
Noscapine affects cell cycle of ovarian cancer cells. a The percentage of SKOV3/DDP cells in different stages of cell cycle. Cells were treated with Nos (2.5 μM), DDP (4 μg/ml), or Nos (2.5 μM) + DDP (4 μg/ml) for 48 h. Flow cytometry was used to detect different stages of cell development. b Statistical analysis of SKOV3/DDP cell cycle. **Compared with the DDP group (P < 0.05), *compared with the control group (P > 0.05). Values are presented as mean ± SD of three independent experiments
Noscapine Inhibits Tumor Growth in Nude Mice
Since Nos enhanced the cytotoxic effects of DDP in vitro, we further investigated the effect of a combined Nos/DDP treatment on the ovarian cancer xenograft model in nude mice. Nude mice with tumors induced by subcutaneous injection of SKOV3/DDP cells were treated with intraperitoneal injection of Nos (40 mg/kg), DDP (3 mg/kg), or with a combination of 40 mg/kg DDP and 3 mg/kg DDP as described above, and the volume and weight of tumors were measured. As predicted, tumor growth was markedly reduced in the Nos + DDP mice (Fig. 5a). After Nos/DDP administration, the average weight of tumors isolated from the Nos + DDP mice was 1.191 ± 0.106 g, significantly smaller than that of the DDP mice (1.733 ± 0.155 g) (P < 0.05) (Fig. 5b, c). Administration of Nos in conjunction with DDP did not affect the body weight of the treated mice (Fig. 5d), suggesting that Nos treatment had no apparent cytotoxic side effects and the combined approach may be considered a potentially suitable strategy for treating ovarian cancer.
Noscapine coupling with cisplatin significantly inhibits tumor growth in nude mice. a The growth curve of tumors in nude mice. Nude mice (six per group) received intraperitoneal injection of Nos (40 mg/kg), DDP (3 mg/kg), or Nos (40 mg/kg) + DDP (3 mg/kg). The control mice received 0.9 % sodium chloride. b The average weight of tumors after administration. *Compared to the DDP group (P < 0.05). c Tumors isolated from each group. d Body weight of nude mice during the treatment
Noscapine Increases Apoptosis of Tumor Cells in Nude Mice
The efficiency of Nos in promoting apoptosis in vitro prompted further study of its pro-apoptotic effect in vivo. Nude mice with tumors induced by subcutaneous injection of SKOV3/DDP cells were treated with 40 mg/kg Nos, 3 mg/kg DDP, or a combination of Nos/DDP as described above, and cell apoptosis of the tumors was evaluated 3 days after the last treatment by flow cytometry (Fig. 6a). Combined Nos/DDP treatment significantly increased apoptosis rate in tumors of nude mice as compared to animals treated with DDP alone (P < 0.05, Fig. 6b), while Nos had no marked effect on the apoptosis rate of tumor cells as compared to control (P > 0.05, Fig. 6b). These results indicate that Nos increases the sensitivity of xenografts to DDP.
Noscapine increases apoptosis rate of tumor cells isolated from nude mice. a Apoptosis of tumor cells. Nude mice received intraperitoneal injections of Nos (40 mg/kg), DDP (3 mg/kg), or Nos (40 mg/kg) + DDP (3 mg/kg). The control mice received 0.9 % sodium chloride. Orthotopic tumors were isolated, and the transplanted tumor cell suspension was analyzed by flow cytometry to determine apoptotic rate. Sub-diploid peaks represent cell apoptosis peaks. b Statistical analysis of apoptosis in tumor cells. **Compared to the DDP group (P < 0.05), *compared to the control group (P > 0.05)
Noscapine Regulates Protein Expression of Apoptotic Factors in Xenografts
We further investigated the effect of Nos on in vivo expression of apoptotic factors survivin, XIAP, NF-κB, and caspase-3 in the nude mouse xenograft model using immunohistochemistry. Tumor tissues from mice treated with Nos, DDP, and Nos/DDP were embedded in paraffin, sectioned and stained by immunohistochemistry PV staining as described in “Materials and Methods” section (Fig. 7a). IHSs were determined according to the percentage of positive cells and the intensity of positive staining. While levels of survivin, XIAP, and NF-κB were all reduced in the tumor tissues of Nos/DDP-treated mice, caspase-3 expression was markedly increased as compared to animals treated with DDP alone (P < 0.05, Fig. 7b, c). Compared to the control group, Nos treatment led to reduced levels of survivin, XIAP, and NF-κB, and elevated caspase-3 protein levels (P < 0.05, Fig. 7b, c). There were no significant differences in protein expression of XIAP, caspase-3, survivin, and NF-κB between the DDP-treated and control mice (P > 0.05) (Fig. 7b, c).
Noscapine changes protein expression in xenografts. a Nude mice received intraperitoneal injections of Nos (40 mg/kg), DDP (3 mg/kg), or Nos (40 mg/kg) + DDP (3 mg/kg). The control mice received 0.9 % sodium chloride. Protein levels of XIAP, caspase-3, survivin, and NF-κB in tumor tissue were analyzed by immunohistochemical PV straining as described in “Materials and Methods” section. b The statistical analysis of XIAP and caspase-3 immunohistochemical scores. **compared with the DDP group (P < 0.05), *compared with the control group (P > 0.05). c The statistical analysis of survivin and NF-κB immunohistochemistry scores. **compared with the DDP group (P < 0.05)
Discussion
Drug resistance is the main cause of treatment failure and mortality in cancer patients [16]. DDP is a widely used chemotherapeutic drug in a case of malignant tumors such as ovarian cancer. However, long-term efficacy of the treatment and survival rates of patients undergoing DDP therapy are seriously reduced due to the toxic side effects of DDP that affect blood cells and nervous system, as well as the development of drug resistance and cancer recurrence [17]. It has been reported that DDP-induced drug resistance in ovarian cancer may be related to drug metabolism disorders, DAN repair dysfunction, abnormal cell cycle progression, and inhibition of apoptosis. Studies showed that DDP resistance can be stimulated in tumor cells by reducing expression of p53 and Bax, and increasing Bcl-2 and XIAP expression [18, 19].
Nos has been reported to have in vitro anti-cancer activity for a spectrum of neoplasies, including glioma, myeloid leukemia, lymphoma, prostate cancer, etc. Animal tests showed that oral or intravenous administration of Nos does not have toxic side effects on heart, thymus, kidney, blood, bone marrow, and other organs [20]. Nos has been also shown to enhance the cytotoxicity of chemotherapeutic agents to various tumor cells, effectively inhibiting tumor growth. However, the molecular mechanism of these anti-tumor effects is not unclear [21, 22]. Our current results clearly showed that Nos significantly inhibited proliferation of both DDP-sensitive ovarian cancer cell line SKOV3 and DDP-resistant cell line SKOV3/DDP. There was a similar observed cytotoxicity of Nos to both cell lines. Low doses of Nos in conjunction with DDP significantly increased the toxicity of DDP to SKOV3/DDP cells, changed cell morphology, and promoted cell apoptosis. In addition, tumor weight was notably less in nude mice treated with a combination of Nos/DDP as compared to animals treated with DDP alone without any evident side effects. It shows that the anti-cancer activity of Nos is probably not due to the inhibition of DDP-resistant mechanisms, but rather to enhanced cytotoxicity, which may be caused by a coordinated effect of Nos and DDP.
Many anti-cancer drugs regulate the progression of the cell cycle by reactivating certain cell cycle checkpoints and blocking cell division that eventually leads to cancer growth inhibition. Newcomb et al. showed that Nos increases expression of MPM-2 and p-H3 in glioma cells, indicating its critical role in regulating mitosis [23]. Compared to the DDP treatment group, the percentage of SKOV3/DDP cells in the S phase were reduced after treating the cells with a combination of Nos and DDP. The decreased number of S cells and increased number of G2/M cells suggest possible effect of Nos/DDP on the progression of cell cycle, and may, therefore, present a mechanism by which Nos improves cytotoxicity of DDP in SKOV3/DDP cells.
A successful chemotherapeutic treatment necessitates inducing apoptotic factors in cancer cells [24], and the rate of apoptosis can be regulated by increasing anti-neoplastic and reducing pro-neoplastic factors. XIAP belongs to the family of IAPS (inhibitor of apoptosis proteins) and plays an important role in immune evasion and anti-apoptosis. It is highly expressed in many malignant tumors and is associated with poor prognosis [25–28]. Survivin is also a member of the IAPS family, playing a key role in mitosis and proliferation. It can control cell proliferation by increasing XIAP activity and inhibiting the activity of caspase-3 and caspase-7. Consequently, survivin inhibits apoptosis of tumor cells induced by numerous factors [29–31]. Caspase-3 plays an integral role in apoptotic pathways by transducing intracellular signals through protease hydrolysis. In multiple DDP-resistant ovarian cancer cell lines, caspase-3 expression is reduced, indicating that it may be involved in the resistance mechanism in these cell lines [32–34]. NF-κB is a multi-directional, multi-functional nuclear transcription factor. It has anti-apoptosis function and is able to inhibit apoptosis by promoting expression of anti-apoptotic genes. It is also closely related to occurrence and development of tumor and drug resistance, playing a key role in the anti-apoptotic mechanism in cancers [35, 36].
In the present study, we investigated the role of Nos in regulating expression of anti-apoptotic factors XIAP, survivin, and NF-κB and the pro-apoptotic factor caspase-3 in drug-resistant ovarian cancer cells, and studied the relationship between Nos and DDP resistance. Both in vitro and in vivo results showed that Nos increased DDP-mediated apoptosis, reduced protein and mRNA levels of XIAP, survivin, and NF-κB, while increasing mRNA expression of caspase-3. Our results are in agreement with previous reports that showed that Nos down-regulates survivin expression and up-regulates caspase-3 expression in non-small cell lung cancer A549 and H460 cells, and reduces NF-κB protein expression in leukemia cells [37, 38]. Our results indicate that the combined treatment with Nos and DDP may promote apoptosis in drug-resistant cells by regulating expression of XIAP, caspase-3, survivin, and NF-κB.
In conclusion, in vitro tests in cancer cells and in vivo experiment in nude mice revealed that Nos induces apoptosis of ovarian cancer cells and enhances the sensitivity of SKOV3/DDP cells to DDP. Nos promotes DDP-induced apoptosis in drug-resistant ovarian cancer cells by controlling the cell cycle, down-regulating anti-apoptotic factors, and up-regulating pro-apoptotic factors to consequently inhibit tumor growth. This study provides a basis for improving chemotherapy effect on ovarian cancer and puts new insights into the development of new clinical strategies.
References
Jemal, A., Siegel, R., Ward, E., Hao, Y., Xu, J., & Thun, M. J. (2009). Cancer statistics, 2009. CA: A Cancer Journal for Clinicians, 59(4), 225–249.
Perez-Tomas, R. (2006). Multidrug-resistance: Retrospect and prospects in anti-cancer drug treatment. Current Medicinal Chemistry, 13(16), 1859–1876.
Parkin, D. M., Bray, F., Ferlay, J., & Pisani, P. (2001). Estimating the world cancer burden: Globocan 2000. International Journal of Cancer, 94(2), 153–156.
McGuire, W. P., Hoskins, W. J., Brady, M. F., Kucera, P. R., Partridge, E. E., Look, K. Y., et al. (1996). Cyclophosphamide and cisplatin compared with paclitaxel and cisplatin in patients with stage III and stage IV ovarian cancer. New England Journal of Medicine, 334(1), 1–6.
Hogberg, T. (2005). A systematic overview of chemotherapy effects in ovarian cancer. Acta Oncologica, 40(2), 340–360.
Takara, K., Sakaeda, T., & Okumura, K. (2006). An update on overcoming MDR1-mediated multidrug resistance in cancer chemotherapy. Current Pharmaceutical Design, 12(3), 273–286.
Bose, R. N. (2002). Biomolecular targets for platinum antitumor drugs. Mini-Reviews in Medicinal Chemistry, 2, 103–111.
Ye, K., Ke, Y., Keshava, N., Shanks, J., Kapp, J. A., Tekmal, R. R., et al. (1998). Opium alkaloid noscapine is an antitumor agent that arrests metaphase and induces apoptosis in dividing cells. Proceedings of the National Academy of Sciences of the United States of America, 95(4), 1601–1606.
Zhou, J., Panda, D., Landen, J. W., Wilson, L., & Joshi, H. C. (2002). Minor alteration of microtubule dynamics causes loss of tension across kinetochore pairs and activates the spindle checkpoint. Journal of Biological Chemistry, 277(19), 17200–17208.
Ke, Y., Ye, K., Grossniklaus, H. E., Archer, D. R., Joshi, H. C., & Kapp, J. A. (2000). Noscapine inhibits tumor growth with little toxicity to normal tissues or inhibition of immune responses. Cancer Immunology, Immunotherapy, 49(4–5), 217–225.
Tulpule, A., Lavine, A. M., Berman, N. E., Smith, L., Auerbach, A., & Douer, D. (2005). Phase I study of noscapine for patients with non-Hodgkin’s lymphoma or chronic lymphocytic leukemia refractory to chemotherapy. Blood, 106(11), 3341.
Jackson, T., Chougule, M. B., Ichite, N., Patlolla, R. R., & Singh, M. (2008). Antitumor activity of noscapine in human non-small cell lung cancer xenograft model. Cancer Chemotherapy and Pharmacology, 63(1), 117–126.
Mahmoudian, M., & Rahimi-Moghaddam, P. (2009). The anti-cancer activity of noscapine: A review. Recent Patents on Anti-Cancer Drug Discovery, 4(1), 92–97.
Soslow, R. A., Dannenberg, A. J., Rush, D., Woerner, B. M., Khan, K. N., Masferrer, J., & Koki, A. T. (2000). Cox-2 is expressed in human pulmonary, colonic, and mammary tumors. Cancer, 89(12), 2637–2645.
Guo, Z., Ni, H., Li, B., Xing, W. G., Liu, F., Yu, H. P., et al. (2006). The effect of the MDM2-p53 loop on the sensitivity of ovarian cancer cells to cisplatin. Chinese Journal of Clinical Oncology, 3(2), 87–91.
Rauch, C. (2011). The “Multi” of drug resistance explained by oscillating drug transporters, drug–Membrane physical interactions and spatial dimensionality. Cell Biochemistry and Biophysics, 61(1), 103–113.
Harries, M., & Gore, M. (2002). Part 1: Chemotherapy for epithelial ovarian cancer-treatment at first diagnosis. Lancet Oncology, 3(9), 529–536.
Lowe, S. W., & Lin, A. W. (2000). Apoptosis in cancer. Carcinogenesis, 21(3), 485–495.
Goyal, L. (2001). Cell death inhibition: Keeping caspases in check. Cell, 104(6), 805–808.
Chougule, M. B., Patel, A. R., & Jackson, T. (2011). Antitumor activity of noscapine in combination with doxorubicin in triple negative breast cancer. PLoS One, 6(3), e17733.
Zhou, J., Gupta, K., Yao, J., Ye, K., Panda, D., Giannakakou, P., & Joshi, H. C. (2002). Paclitaxel-resistant human ovarial cancer cells undergo c-Jun NH2-terminal kinase-mediated apoptosis in response to noscapine. The Journal of Biological Chemistry, 277(42), 39777–39785.
Aneja, R., Ghaleb, A. M., Zhou, J., Yang, V. W., & Joshi, H. C. (2007). p53 and p21 determine the sensitivity of noscapine-induced apoptosis in colon cancer cells. Cancer Research, 67(8), 3862–3870.
Newcomb, E. W., Lukyanov, Y., Smirnova, I., Schnee, T., & Zagzag, D. (2008). Newcomb noscapine induces apoptosis in human glioma cells by an apoptosis-inducing factor-dependent pathway. Anti-Cancer Drugs, 19, 553–563.
Mitsios, N., Gaffney, J., Krupinski, J., Mathias, R., Wang, Q., Hayward, S., et al. (2007). Expression of signaling molecules associated with apoptosis in human ischemic stroke tissue. Cell Biochemistry and Biophysics, 47(1), 73–85.
Fulda, S., & Vucic, D. (2012). Targeting IAP proteins for therapeutic intervention in cancer. Nature Reviews Drug Discovery, 11(2), 109–124.
Kluger, H. M., McCarthy, M. M., Alvero, A. B., Sznol, M., Ariyan, S., Camp, R. L., et al. (2007). The X-linked inhibitor of apoptosis protein(XIAP)is up- regulated in metastatic melanoma, and XIAP cleavage by phenoxodiol is associated with carboplatin sensitization. Journal of Translational Medicine, 5, 6.
Schimmer, A. D., Dalili, S., Batey, R. A., & Riedl, S. J. (2006). Targeting XIAP for the treatment of malignancy. Cell Death Differentiation, 13(2), 179–188.
Kashkar, H. (2010). X-linked inhibitor of apoptosis: A chemoresistance factor or a hollow promise. Clinical Cancer Research, 16(18), 4496–4502.
Yang, L., Cao, Z., Yan, H., & Wood, W. C. (2003). Coexistence of high levels of apoptotic signaling and inhibitor of apoptosis proteins in human tumor cells: Implication for cancer specific therapy. Cancer Research, 63, 6815–6824.
Tong, Q. S., Zheng, L. D., Chen, F. M., Zeng, F. Q., Wang, L., Dong, J. H., & Lu, G. C. (2005). Selection of optimal antisense accessible sites of survivin and its application in treatment of gastric cancer. World Journal of Gastroenterology, 11(5), 634–640.
Dohi, T., Okada, K., Xia, F., Wilford, C. E., Samuel, T., Welsh, K., & Marusawa, H. (2004). An IAP–IAP complex inhibits apoptosis. Journal of Biological Chemistry, 279(33), 34087–34090.
Yang, X. H., Sladek, T. L., Liu, X., Butler, B. R., Froelich, C. J., & Thor, A. D. (2001). Reconstitution of caspase-3 sensitizes MCF-7 breast cancer cells to doxorubicin- and etoposide-induced apoptosis. Cancer Research, 61(1), 348–354.
Tsang, W. P., & Kwok, T. T. (2008). Let-7a microRNA suppresses therapeutics-induced cancer cell death by targeting caspase-3. Apoptosis, 13(10), 1215–1222.
McGonigal, K., Tanha, J., Palazov, E., Li, S. H., Gueorguieva-Owens, D., & Pandey, S. (2009). Isolation and functional characterization of single domain antibody modulators of Caspase-3 and apoptosis. Applied Biochemistry and Biotechnology, 157(2), 226–236.
Kim, Y., Kang, H., & Jang, S. W. (2011). Celastrol inhibits breast cancer cell invasion via suppression of NF-kappa B-mediated matrix metalloproteinase-9 expression. Cellular Physiology and Biochemistry, 28(2), 175–184.
Shishodia, S., Potdar, P., Gairola, C. G., & Aggarwal, C. G. (2003). Curcumin (diferuloylmethane) down-regulates cigarette smoke-induced NF-κB activation through inhibition of IκBα kinase in human lung epithelial cells: Correlation with suppression of COX-2, MMP-9 and cyclin D1. Carcinogenesis, 24(7), 1269–1279.
Chougule, M., Patel, A. R., Sachdeva, P., Jackson, T., & Singh, M. (2011). Anticancer activity of noscapine, an opioid alkaloid in combination with cisplatin in human non-small cell lung cancer. Lung Cancer, 71(3), 271–282.
Sung, B., Seok, K. S., & Aggarwal, B. (2010). Noscapine, a benzylisoquinoline alkaloid, sensitizes leukemic cells to chemotherapeutic agents and cytokines by modulating the NF-κB signaling pathway. Cancer Research, 70(8), 3259–3268.
Acknowledgments
This work was supported by the Key Project of Medical Scientific Research of Hebei Province, China (NO. 20110477).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shen, W., Liang, B., Yin, J. et al. Noscapine Increases the Sensitivity of Drug-Resistant Ovarian Cancer Cell Line SKOV3/DDP to Cisplatin by Regulating Cell Cycle and Activating Apoptotic Pathways. Cell Biochem Biophys 72, 203–213 (2015). https://doi.org/10.1007/s12013-014-0438-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12013-014-0438-y