Abstract
In the present study, we investigated the cardioactive glycosides oleandrin and ouabain, and compared them to digoxin in a model of cardiotoxicity induced by doxorubicin. Adult rats were distributed into four experimental groups. Each group was challenged with a single intraperitoneal application of doxorubicin at a dose of 12 mg/kg. Then, they were treated with saline solution and the glycosides oleandrin, ouabain, and digoxin at a dose of 50 µg/kg, for 7 days. They underwent echocardiography, electrocardiography, hematologic, biochemical tests, and microscopic evaluation of the heart. All animals presented congestive heart failure, which was verified by a reduction in the ejection fraction. Oleandrin and digoxin were able to significantly reduce (p < 0.05) the eccentric remodeling caused by doxorubicin. Oleandrin and digoxin were significantly lower (p < 0.05) than the control group in maintaining systolic volume and left ventricular volume in diastole. Other parameters evaluated did not show significant statistical differences. All animals showed an increase in erythrocyte count, and an increase in the duration of the QRS complex on the ECG and myocardial necrosis at the histopathological analysis. It is concluded that the glycosides oleandrin, ouabain, and digoxin in the used dosage do not present therapeutic potential for the treatment of congestive heart failure caused by doxorubicin.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Congestive heart failure is an important syndrome caused by functional and structural abnormalities that impair the heart’s ability to accommodate or eject blood properly [1]. It is one of the major causes of death in humans [2]. The main goal of the treatment is to reduce clinical signs and improve the quality of life of the ones affected. Considering this, it is important to seek new therapeutic modalities [3].
Cardioactive glycosides (CGs) have been historically described as compounds isolated from different plants that can cause clinical signs related to the cardiovascular system. They can inhibit the sodium–potassium pump, which leads to an increase in the intracellular calcium concentration and a positive inotropic effect. Yet the CGs have small differences in their structure, which determine peculiarities in their pharmacokinetics, pharmacodynamics, and effects on cardiovascular physiology [4]. Three of the most studied CGs are digoxin (DIG), oleandrin (OLE), and ouabain (OUA).
DIG was first described in 1785, and it is a metabolite isolated from the plant Digitalis purpurea [5]. Besides the positive inotropic effect, it also has a neuromodulatory action increasing the parasympathetic tonus, which reduces the activity in the sinoatrial and the atrioventricular node, and it is the drug of choice in cases of atrial fibrillation with high ventricular response [6].
OLE is isolated from the plant Nerium oleander. It has a positive inotropic effect and high cytotoxic activity, which can lead to apoptosis. Experimentally, it has been used in the treatment of some types of cancer and HIV therapy. OLE also has an important pro-arrhythmic action [7, 8].
OUA was first isolated from plants of the genus Akocanthera sp. and Strophantus sp. Later it was identified as a metabolite of mammals [9]. It appears to be a stress hormone, and its serum concentration is high in both physiological and pathological events such as pregnancy, hypertension, and congestive heart failure [10, 11]. It also has pro-inflammatory and anti-inflammatory properties depending on its concentration [12].
In order to study therapeutic potential of new cardioactive drugs, many animal models of heart disease may be used. In this regard, doxorubicin (DOX) is an anthracycline antibiotic and a chemotherapy agent widely used in the treatment of various types of cancer, especially hematological ones and breast cancer. It can cause congestive heart failure as one of its side effects, resulting in disease similar to dilated cardiomyopathy [13].
Considering the importance of alternative drugs, the aim of this research was to study the therapeutic effects of the glycosides OLE, OUA, and DIG in the treatment of congestive heart failure induced by DOX, once they could result in good inotropic and neuromodulatory action. This evaluation was made by complementary exams like echocardiography and electrocardiography (ECG) that showed how mechanical and electrical activity of the heart behaved throughout the treatment. The hematological analysis, biochemical panel, and histopathology examination of the heart also contributed to understand better these glycosides mechanisms in the scenario.
Materials and Methods
All experimental procedures in this study were in accordance with the guidelines stated by the Ethical Principles in Animal Research adopted by the Brazilian College of Animal Experimentation (COBEA) and were approved by the Ethics Committee in Animals Use of Universidade Federal de Minas Gerais (Protocol #74/2017). Our research was strictly carried out in accordance with these approved guidelines.
Glycosides and Reagents
The CGs OLE, OUA, and DIG were obtained by Sigma Laboratory® and were stored in an appropriate refrigerator, at the average temperature of 1.1 to 3.3 °C. DOX used was Fauldoxo®, the commercial version present in hospitals for chemotherapy. Commercial kits from Bioclin® were used to measure aspartate aminotransferase (AST), lactate dehydrogenase (LDH), creatinine, and urea. IDEXX® kits were used to measure symmetric dimethylarginine (SDMA) and a semi-quantitative immunoenzymatic test from Biocon® evaluated the cardiac troponin type I (cTnI).
Experimental Design
Twenty healthy rats underwent ECG exam (T0). After, all the animals were challenged with a single intraperitoneal shot of DOX at the dose of 12 mg/kg. The animals were randomly divided into four groups, with five animals each, and started to receive treatment with the CGs: the control group (G1) received just 0.1 mL of saline and other groups (2, 3, and 4) received cardiac glycosides (50 μg/kg), dissolved in 0.1 ml of saline by subcutaneous via, daily, at for 7 days, as follows: group 2—OLE; group 3—OUA; group 4—DIG. Selected dose was based on previous study of our research group [14].
Clinical Exam
All rats were clinically evaluated by veterinarians before and after the treatment. Behavior, spontaneous bleeding, bruises, dehydration, diarrhea, loss of appetite, and alterations in the breathing pattern were the parameters observed with proposed evaluation score (Table 1). The minimum score is 0 and 10 is the highest.
Electrocardiogram and Echocardiography
All rats were evaluated daily regarding their overall state and possible clinical alterations. Rats were anesthetized by isoflurane (Isoflurine®, at 2.5% during induction and 1.5% for maintenance; VetCase, Brasmed®) and placed in supine position in a wooden board. A six-channel non-invasive electrocardiograph (ECG-PC version 2.07, Brazilian Electronic Technology—TEB, Belo Horizonte, MG, Brazil) was used. All procedures were performed in a quiet room to minimize stress. Readings were made in DII, at 50 mm/s, and 2 N. Recordings were made before therapy administration (T0), and seven days (T1) after.
Cardiac morphology and its mechanical function were evaluated in vivo, with a non-invasive high-resolution echocardiographic system (VEVO 2100, VisualSonics®, Toronto, Canada). The equipment had two sector transducers with frequencies between 24 and 40 mHz. Animals were anesthetized and positioned on dorsal recumbence. Images were obtained on B mode and M mode by three transducers locations: right parasternal location, left cranial parasternal location, and left caudal (apical) parasternal location. Ejection fraction (EF), the fraction of shortening (FS), cardiac output (CO), systolic volume (SV), heart rate (HR), left ventricular volume in diastole (VOLd), left ventricular volume in systole (VOLs), left ventricular diameter in diastole (DIAMd), and left ventricular diameter in systole (DIAMs) were the parameters measured. The EF was obtained by Teicholz’s method on M mode. Recordings were made seven days (T1) after and compared with rats healthy.
Hematological and Biochemical Analysis
After 7 days of treatment (T1), all rats were anesthetized with isoflurane followed by intraperitoneal injection of Thiopental ® (100 mg/kg), and blood samples were collected from abdominal aorta. The blood was stored in tubes containing EDTA 10% to hematological evaluation, was performed by an automatic analyzer (PoCH100i®) and stored in tubes without anticoagulant for serum samplings. The biochemical serum profile was obtained through evaluation of the enzymes aspartate aminotransferase (AST) and lactate dehydrogenase (LDH), urea, and creatinine by spectrophotometry (TP Analyser®) using Bioclin® kits. SDMA was measured using IDEXX® kits in an appropriated.
IDEXX® analyzer and Troponin I (cTnI) were measured with immunochromatography kit (Biocon®).
Euthanasia
After the ECG, echocardiography, and collecting blood samples, the rats were euthanized by overdose of isoflurane.
Histopathological Evaluation
Necropsy was performed immediately after euthanasia. For microscopic analysis, myocardial samples from four animals of each group were fixed in buffered formalin solution and embedded in paraffin. Serial Sects. (4 μm) were stained with hematoxylin and eosin. Myocardial samples from the remaining three animals of each group were frozen at − 80 °C for Western blot analysis and oxidative stress estimation as described below.
Statistical Analysis
Data are presented as means ± SD. Normality of data distribution was evaluated using the Kolmogorov–Smirnov test. Statistical significance of parametric data was determined by ANOVA, followed by Tukey’s test. Non-parametric data were compared by Kruskal Wallis’ test. Significance was set at p < 0.05. Data were analyzed using GraphPad Prism v.7.0 (Systat Software Inc., USA).
Results
Clinical Examination
On the second day of the treatment, the animals of G2, treated with OLE, presented nasal bleeding. This group was the first to present clinical signs, suggesting that OLE has a faster effect compared to the other drugs. From the third day on, all animals from G1, G2, G3, and G4 presented modifications to their behavior, such as prostration and reduced appetite. Diarrhea, nasal bleeding, and ocular bleeding, opaque, and goosebump fur, indicating dehydration, were also observed. All the rats received the maximum score on the clinical examination.
ECG
ECG records made on T0 did not show any abnormality. On T1, none of the rats presented any type of arrhythmia, and they all had sinus rhythm. All animals showed prolongation of QRS complex duration after treatments. QT interval duration and an increase in T-wave amplitude were observed only in G1.
G1, G2, and G3 presented normal HR, and G4 presented HR above the limit established for the species [15]. Table 2 shows the results.
Echocardiography
HR, DIAMs, and VOLs remained within the reference ranges for the species in all groups [16]. Groups 2, 3, and 4 presented reduced EF, FS, DIAMd, VS, and VOLd. The CO was normal only in G2, treated with OLE. There was a significant difference between the groups in the CO, DIAMd, VOLs and SV. G2, treated with OLE, was capable of maintaining a normal CO and was better than G4, treated with digoxin when it comes to this parameter. G2 and G4 were superior to control on reducing the DIAMd, which shows an effect of these glycosides on the cardiac remodeling. G2 and G4 also showed significantly reduced SV and VOLd when compared to control. The results are expressed in Fig. 1.
Echocardiographic parameters of Wistar rats challenged with doxorubicin and treated with saline (CON), oleandrin (OLE), ouabain (OUA), and digoxin (DIG). A Mean and standard deviation for cardiac output (CO). B Mean and standard deviation for left ventricle diameter in diastole (DIAMd). C Mean and standard deviation for stroke volume (SV). D Mean and standard deviation for left ventricle volume in diastole (VOLd). *p < 0.05
Hematology
None of the rats developed anemia. Red cells count and hematocrit (Ht) were within the reference ranges in all groups, except in G3 that presented hematocrit (Ht) above the limits [16]. Hemoglobin (Hb) and the concentration of mean corpuscular hemoglobin (CMCH) were elevated in all groups. Mean corpuscular volume (MCV) was reduced only in G4. The mean corpuscular hemoglobin (MCH) was elevated in the control group and G4. All groups showed platelet count and white blood cells count extremely reduced (Table 3). Neutrophils (NEU), lymphocytes (LYMP), and eosinophils (EOS) were reduced in all groups when compared with reference ranges for the species [16]. (Fig. 2).
Serum Biochemistry Exams
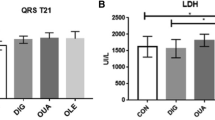
Creatinine and SDMA levels were normal in all rats. Urea, AST, and LDH were elevated in all groups (Table 4). The cTnI was positive in only one rat of G4.
Histopathological Evaluation
Lesions observed macroscopically and microscopically were similar between the four groups, varying in extension and severity. G2, G3, and G4 were affected by DOX and by the CGs. During gross examination, the main findings were congestion in the heart, lungs, liver, kidneys, and spleen. Serum-hemorrhagic fluids were present in the abdominal cavity of all rats. Microscopically, it was possible to observe intracytoplasmic vacuoles in all groups, a common feature of DOX intoxication [17]. Myocardial edema, fragmentation in cardiomyocytes, myocardial pallor, eosinophilic and amorphous cytoplasm, nuclear pyknosis, cell nuclei of different sizes, and free red blood cells, indicating myocardial hemorrhage, were also observed in all groups (Fig. 3). The most extensive and serious injuries were in G2. OLE had pronounced effects on cardiac muscle cells resulting in multifocal-to-coalescing lesions and more hemorrhagic areas. G1, G3, and G4 showed similar lesions, but they were less severe and had focal to multifocal distribution. G1 had damages caused exclusively by DOX, such as intracytoplasmic vacuoles and acute myocardial necrosis. G3 had disorganization of cardiac tissue and cell nuclei of different sizes as principal lesions. In G4, myocardial pallor and necrosis were observed.
Heart histological sections of Wistar rats challenged with doxorubicin and treated with saline, oleandrin, ouabain, and digoxin. A Heart histology of a Wistar rat challenged with doxorubicin and treated with saline, showing intracytoplasmic vacuoles. B Heart histology of a Wistar rat challenged with doxorubicin and treated with oleandrin, showing an extensive hemorrhagic area. C Heart histology of a Wistar rat challenged with doxorubicin and treated with oleandrin, showing eosinophilic and amorphous cytoplasm, indicating necrosis. D Heart histology of a Wistar rat challenged with doxorubicin and treated with oleandrin, showing nuclear pyknosis and intracytoplasmic vacuoles. E Heart histology of a Wistar rat challenged with doxorubicin and treated with ouabain, showing disorganization of cardiac muscle cells and nuclei of different sizes. F Heart histology of a Wistar rat challenged with doxorubicin and treated with digoxin, showing nuclear pyknosis. HE staining. Bars = 50 µm
Discussion
The congestive heart failure was successfully induced by DOX that was verified by the clinical signs and reduced EF and FS on the echocardiographic exam. The used dose of 12 mg/kg of doxorubicin was based on previous studies of the group [18] and is endorsed by other experiments [19, 20]. The single dose was administered in order to cause severe and rapid onset on cardiotoxicity. In our previous studies, we observed an important response of single dose, especially regarding heart failure with low death cases in compared to previous attempts at chronic doses. The glycosides dosage of 50 µg/kg was also based on previous studies of the group [14], in which the glycosides OLE, OUA, and DIG were administered to healthy Wistar rats during 21 days and did not cause clinical signs, arrhythmias, or death. Considering this, the dose was chosen to continue the research about the effects on the cardiovascular physiology and the therapeutic potential.
Clinical signs presented by the animals were thought to be caused by DOX, once the drug’s effects are widely known. DOX has a myelosuppressive action, diminishing the platelet count and other cellular types count [21], resulting in bleedings and bruises. Besides that, this chemotherapy agent is also capable of inducing diarrhea and dehydration by consequence [22]. Otherwise, it is important to notice that the clinical signs related to the digestive system could be caused by the glycosides as a manifestation of digitalis intoxication. Congestive heart failure itself could worsen the clinical signs.
At the moment, the main use of DIG in cardiology is due to its neuromodulatory effect, which can reduce HR and convert atrial fibrillation into sinus rhythm [23]. ECG analysis did not show any arrhythmias. The HR of the rats in G1, G2, and G3 was normal, and it was above the limits in G4. None of the drugs appears to have a good neuromodulatory effect in the congestive heart failure caused by DOX, once rats that did not receive any treatment also presented normal HR. Even tough, OLE can act directly on the sinoatrial node and can enhance the parassimpatic activity [24]. OUA is also capable of increasing parasympathetic effects on the heart [25]. This can explain the normal HR in the groups treated with these drugs. DIG was not capable of reducing the rats HR.
Besides the effects on HR, all animals showed changes in the ECG that can be associated with congestive heart failure. QRS complex widening observed in the four groups can be explained by ventricular overload or possible branch block [26]. The QT interval prolongation and the increase in T-wave amplitude observed only in G1 show that there was a greater impairment of the ventricular function in this group. The normal QT interval and normal T-wave in the treated groups can be explained by the direct effect of the glycosides on the electrical activity of the heart. CGs increase the intracellular content of calcium when they block the NKA, what modifies the action potential of the cardiac cells and reduces the duration of the phase 2, diminishing the QT , interval [27]. That was a beneficial effect of the treatment applied, mainly because a prolonged QT interval is related to greater chances of development of serious arrhythmias, such as torsades des pointes [28].
In the echocardiographic examination, EF and FS were reduced in all groups, showing that the heart failure was induced and that none of the treatments was capable of improving these variables of systolic function. DOX depresses the cardiac function and causes a disease similar to dilated cardiomyopathy [29], which explains the impaired contractility.
Another important feature of heart failure caused by DOX is increased ventricular diameter [30] that was not observed in this study. The groups that received glycosides had a DIAMd smaller than before the treatment. G2 and G4 were statistically superior to control G1 in reducing the DIAMd, suggesting a possible protective effect of these glycosides against myocardial dilation after DOX administration. A smaller DIAMd was observed in a donkey accidentally intoxicated with extracts of Nerium oleander, plant that contains OLE [31]. In another study, rats treated exclusively with DIG also showed myocardial hypertrophy [32]. OUA is also capable of reducing DIAMd [33], as observed. The VOLd and SV were significantly smaller in G2 and G4 when compared to control, these can be explained by the reduced DIAMd and consequently, accommodation of less volume in the heart cavity. Besides that, the SV is related to EF and CO, parameters that were also diminished. The VOLs and DIAMs did maintained the normal values and seem to be variables that take a longer period of exposure to DOX to suffer modifications [20, 34, 35]. The normal CO observed in G2 may be due to increased contractility. OLE can enhance the cardiac contraction [36], although there is not much information about that in the literature.
Normal Ht and red blood cells count probably occurred because there was not enough time to observe these cell type destruction, once their half-life is long [16]. Dehydration could also be responsible for the relative polycythemia. The increase in Hb and CMHC is compatible with hemolysis and oxidation that can be a result of DOX’s action in the cells as well as the glycoside’s action. The NKA is present in all cell membranes, including the erythrocytes, which allows glycosides to bind to them and cause oxidative damage [37]. Reduced MCV and elevated CMH in G4 indicate greater oxidative damage in this group.
All animals showed marked thrombocytopenia. DOX has a negative action on the platelet’s count, once it promotes oxidation of the platelet’s membranes, causing lesions [38]. Besides that, OUA and DIG can activate and modulate platelets, reducing their circulation [39, 40]. There are also case reports showing that DIG can cause thrombocytopenia [41, 42]. This action is directly associated with the presentation of diarrhea, nasal, and ocular bleeding, confirming the disturbance of hemostasis caused by DOX in all groups.
Leukopenia observed is a direct effect of DOX action [43]. All the white blood cells types count were reduced. That may have been a synergistic effect between DOX and the glycosides, worsening the condition. Both OUA and DIG are capable of increasing the leukocytes migration to the tissues, reducing their serum concentration [44, 45]. OLE reduces the expression of interleukins involved in the synthesis of cyclooxygenase-2 (COX-2), resulting in anti-inflammatory action [46]. G2 had an EOS’s count significantly smaller than control group, showing that OLE has a negative impact on this cell type. Lymphopenia is a common alteration associated with chemotherapy. DOX causes impairment of the bone marrow and several inflammatory cytokines [47]. Our study presented low levels of LYMP with no significant differences between groups, suggesting that CGs and DOX may have a synergistic action in reducing lymphocyte count. This finding may implicate in a worse prognosis once the immune system response is impaired [48].
Creatinine and SDMA levels were within the reference ranges for the species. Urea was above the limits in all groups. Dehydration diagnosed on clinical examination can explain the elevated urea. The rats were followed up for a week, a short period of time, which may have made it difficult to assess kidney damage.
AST and LDH were above the reference limits in all groups, showing that all rats had muscle injuries. The rise in the levels of these enzymes also indicates liver damage. DOX can diminish cytochrome P-450 and glutathione levels in rat’s liver [49], which can explain the results found. OUA has direct action in the liver [50], and OLE is also capable of inducing liver damage [51], what may have intensified DOX’s effects in this organ. The cTnI, a specific cardiac biomarker of cardiac necrosis, was positive in only one animal of G4. The test used to measure the cTnI only gives positive results if the protein concentration is more than 0.5 ng/mL, which explains the negative results. Besides, DOX does not seems to cause significant elevation in cTnI levels [52, 53].
DOX and glycosides caused important myocardial necrosis. The intracytoplasmic vacuoles are common lesions observed in DOX’s cardiotoxicity [54]. OLE, OUA, and DIG also cause severe damage to the cardiomyocytes [55,56,57], as noted in the rats of the study.
In the conditions that the experiment was conducted, it is possible to conclude that the glycosides OLE, OUA, and DIG do not have a therapeutic potential in the treatment of heart failure induced by DOX. These drugs were not able to optimize the cardiac parameters evaluated on the echocardiography and ECG. In fact, they caused serious damages to the hematological and biochemical analysis, as well as to the cardiac tissue, and early sign of kidney and liver damage. Our results indicate that cardiac glycosides, even at low doses, may be prejudicial to heart failure treatment, and further research is necessary to understand both pharmacokinetics and pharmacodynamics of these digitalis-like drugs.
Data Availability
The datasets generated and/or analyzed during the current study are not available in any database repository. They are available from the corresponding author on reasonable request.
References
Mangini, F., Pires, P. P., Braga, F. G. M., & Bacal, F. (2013). Decompensated heart failure. Einstein, 11, 383–391.
Savarese, G., & Lund, L. H. (2017). Global public health burden of heart failure. Cardiac Failure Review, 3, 7–11.
Borlaug, A. B., & Paulus, W. J. (2010). Heart failure with preserved ejection fraction: Pathophysiology, diagnosis, and treatment. European Heart Journal, 32, 670–679.
Botelho, A. F. M., Pierezan, F., Soto-Blanco, B., et al. (2019). A review of cardiac glycosides: Structure, toxicokinetics, clinical signs, diagnosis and antineoplastic potential. Toxicon, 158, 63–68.
Whayne Jr, T. F. (2018). Clinical use of digitalis: A state of art review. American Journal of Cardiovascular Drugs, 18, 427–440.
Formiga, F., & Ariza, A. (2018). Digoxin in reduced heart failure and sinus rhythm. When sould it be indicated in 2018? Revista Espanola de Geriatria y Gerontologia, 53, 119–120.
Pedroza, H. P., Ferreira, M. G., Carvalho, J. G., Melo, K. D. A., Keller, K. M., Melo, M. M., & Soto-Blanco, B. (2014). Concentrações de oleandrina nas folhas de Nerium oleander de diferentes cores de floração. Ciência Rural, 45, 864–866.
Kumar, A., Tamnoy, D., Mishra, A., et al. (2013). Oleandrin: A cardiac glycoside with potent cytotoxicity. Pharmacognosy Review, 7, 131–139.
Hamlyn, J., Blaustein, M. P., Bova, S., et al. (1991). Identification and characterization of an ouabain-like compound form human plasma. Proceedings of the National Academy of Sciences of the United States of America, 88, 6259–6263.
Hamlyn, J. M., & Manunta, P. (2015). Endogenous cardiotonic steroids in kidney failure: A review and a hypothesis. Advances in Kidney Chronic Disease, 22, 232–244.
Manunta, P., Ferrandi, M., Bianchi, G., et al. (2009). Endogenous ouabain in cardiovascular function and disease. Journal of Hypertension, 27, 9–18.
Cavalcante-Silva, L. H. A., Lima, E. A., Carvalho, D. C. M., et al. (2017). Much more than a cardiotonic steroid: Modulation of inflammation by ouabain. Frontiers in Physiology, 8, 1–8.
Henriksen, P. A. (2018). Anthracycline cardiotoxicity: An update on mechanism, monitoring and prevention. Heart, 104, 971–977.
Botelho, A. F. M., Miranda, A. L. S., Freitas, T. G., et al. (2020). Comparative cardiotoxicity of low doses of digoxin, ouabain, and oleandrin. Cardiovascular Toxicology, 20, 539–547.
Botelho, A. F. M., Joviano-Santos, J. V., Santos-Miranda, A., et al. (2019). Noninvasive ECG recording and QT interval correction assessment in anesthetized rats and mice. Pesquisa Veterinária Brasileira, 39, 409–415.
Teixeira, M. A., Chaguri, L. C. A. G., & Carissimi, A. S. (2000). Hematological and biochemical profiles of rats (Rattus novergicus) kept under microenvironmental ventilation system. Brazilian Journal of Veterinary Research and Animal Science, 37, 341–347.
Pontes, J. C. D. V., Gomes, J. F., Jr., Silva, G. V. R., et al. (2010). Estudo anatomopatológico da miocardiopatia induzida pela doxorrubicina em ratos. Acta Cirúrgica Brasileira, 25, 137–143.
Oliveira, M. S., Melo, M. B., Carvalho, J. L., et al. (2013). Doxorubicin cardiotoxicity and cardiac function improvement after stem cell therapy diagnosed by strain echocardiography. Journal of Cancer Science & Theraphy, 5, 52–57.
O’connell, J. L., Romano, M. M. D., Pulici, E. C. C., et al. (2017). Short-term and long-term models of doxorubicin-induced cardiomyopathy in rats: A comparison of functional and histopathological changes. Experimental and Toxicologic Pathology, 69, 213–219.
Polegato, B. F., Minicucci, M. F., Azevedo, P. S., et al. (2015). Acute doxorubicin-induced cardiotoxicity is associated with matrix metalloproteinase-2 alterations in rats. Cellular Physiology and Biochemistry, 35, 1924–1933.
Kim, E., Lim, K., Kim, K., et al. (2009). Doxorubicin-induced platelet cytotoxicity: A new contributory factor for doxorubicin-mediated thrombocytopenia. Journal of Thrombosis and Haemostasis, 7, 1172–1183.
Rau, S. E., Barber, L. G., & Burgess, K. E. (2010). Eficacy of maropitant in the prevention of delayed vomiting associated with administration of doxorubicin to dogs. Journal of Veterinary Internal Medicine, 24, 1452–1457.
Campbell, J. T., & Macdonald, P. S. (2003). Digoxin in heart failure and cardiac arrhythmias. The Medical Journal of Australia, 179, 98–102.
Langford, S. D., & Boor, P. J. (1996). Oleander toxicity: An examination of human and animal toxic exposures. Toxicology, 109, 1–13.
Wallick, D. W., Valencic, F., Fratianne, R. B., et al. (1984). Effects of ouabain and vagal stimulation on heart rate in dog. Cardiovascular Research, 18, 75–79.
Murkofsky, R. L., Dangas, G., Diamond, J. A., et al. (1998). A prolonged QRS duration on surface electrocardiogram is a specific indicator of left ventricular dysfunction [see comment]. Journal of The American College of Cardiology, 32(2), 476–482.
Stemmer, P., & Tai, A. (1998). Sodium-pump activity and its inhibition by extracellular calcium in cardiac myocytes of guinea pigs. Biochimica et Biophysica Acta, 940, 188–196.
Alexandre, J., Moslehi, J. J., & Bersell, K. R. (2018). Anticancer drug-induced cardiac rythm disorders: Current knowledge and basic underlying mechanisms. Pharmacology & Therapeutics, 189, 89–103.
Chatterjee, K., Zhang, J., Honbo, N., et al. (2010). Doxorubicin cardiomyopathy. Cardiology, 115, 155–162.
Mathew, T., Williams, L., Navaratnam, G., et al. (2017). Diagnosis and assessment of dilated cardiomyopathy: A guideline protocol from the British Society of Echocardiography. Echo Research and Practice, 4, 1–13.
Smith, P. A., Aldridge, B. M., & Kittleson, M. D. (2003). Oleander toxicosis in a donkey. Journal of Veterinary Internal Medicine, 17, 111–114.
Neves, C. H., Tibana, R. A., Prestes, J., et al. (2017). Digoxin induces cardiac hypertrophy without negative effects on cardiac function and physical performance in trained normotensive rats. International Journal of Sports Medicine, 38, 263–269.
Simonini, M., Pozzoli, S., Bignami, E., et al. (2015). Endogenous ouabain: An old cardiotonic steroid as a new biomarker of heart failure and a predictor of mortality after cardiac surgery. Biomed Research International. https://doi.org/10.1155/2015/714793
Silva, C. E. V., & Camacho, A. A. (2005). Alterações ecocardiográficas em cães sob tratamento prolongado com doxorrubicina. Arquivo Brasileiro de Medicina Veterinária e Zootecnia, 57, 300–306.
Markiewicz, W., Robinson, E., Peled, B., et al. (1980). Early detection of doxorubicin cardiotoxicity by M-mode echocardiography. Cancer Chemotherapy and Pharmacology, 5, 119–125.
Deavers, S., Rosborough, J. P., & McCrady, J. D. (1979). The effects of oleandrin on cardiac contractility in the normal dog. Archives Internacionales de Pharmacodynamie et de Therapie, 239, 283–289.
Van Boxtel, C. J., Koopmans, R., Oosterhuis, B., et al. (1985). Red blood cell electrolytes for monitoring digoxin therapy in adults. Therapeutic Drug Monitoring, 7, 191–196.
Sleijfer, S., Rizzo, E., Litiére, S., et al. (2018). Predictors for doxorubicin- induced hematological toxicity and its association with outcome in advanced soft tissue sarcoma patients; a retrospective analysis of the EORTC-soft tissue and bone sarcoma group database. Acta Oncologica, 57, 1117–1126.
Abdou, R. H., Basha, W. A., & Khalil, W. F. (2019). Subacute toxicity of Nerium oleander ethanolic extract in mice. Toxicological Research, 35, 233–239.
Roevens, P., & Courcelles, D. C. (1990). Ouabain increases the calcium concentration in intracellular stores involved in stimulus-response coupling in human platelets. Circulation Research, 67, 1494–1502.
Young, R. C., Nachman, R. L., & Horowitz, H. I. (1966). Thrombocytopenia due to digoxin. The American Journal of Medicine, 41, 605–614.
Medenica, R., Hatam, K., Hatam, V., et al. (1972). Digitoxin-induced thrombocytopenia. International Archives of Allergy and Applied Immunology, 43, 1–8.
Henry, C. J., Brewer, W. G., & Stutler, S. A. (1993). Early onset leukopenia and severe thrombocytopenia following doxorubicin chemotheraphy for tonsillar squamous cell carcinoma in a dog. The Cornell Veterinarian, 82, 163–168.
De Vasconcelos, D. I., Leite, J. A., Carneiro, L. T., et al. (2011). Anti-inflammatory and antinociceptive activity of ouabain in mice. Mediators of Inflammation. https://doi.org/10.1155/2011/912925
Esposito, A. L. (1985). Digoxin disrupts the inflammatory response in experimental pneumococcal pneumonia. The Journal of Infectious Diseases, 152, 14–23.
Dey, P. (2020). The pharmaco-toxicological conundrum of oleander: Potential role of gut microbiome. Biomedicine & Pharmacotherapy, 129, 1–13.
Tolaney, S. M., Najita, J., Winer, E. P., et al. (2008). Lymphopenia associated with adjuvant anthracycline/taxane regimens. Clinical Breast Cancer, 8, 352–356.
Vecchi, A., Mantovani, A., Taglibue, A., et al. (1976). A characterization of the immunosuppressive activity of Adriamycin and daunomycin on humoral antibody production and tumor allograft rejection. Cancer Research, 36, 1222–1227.
Marchand, D. J., & Renton, K. W. (1984). Depression of cytochrome P-450-dependent drug biotransformation by adriamicyn. The Journal of Pharmacology and Experimental Therapeutics, 229, 299–304.
Graf, J., & Peterlik, M. (1976). Ouabain mediated sodium uptake and bile formation by isolated perfused rat liver. American Journal of Physiology, 230, 876–885.
Ceci, L., Girolami, F., & Cappuchio, M. T. (2020). Outbreak of oleander (Nerium oleander) poisoning in dairy cattle: Clinical and food safety implication. Toxins, 471, 1–11.
Adamcova, M., Skarkova, V., Seifertova, J., et al. (2019). Cardiac troponins are among targets of doxorubicin-induced cardiotoxicity in hiPCS-CMs. International Journal of Molecular Sciences, 20, 1–13.
Polena, S., Shikara, M., Naik, S., et al. (2005). Troponin I as a marker of doxorubicin induced cardiotoxicity. Proceeding of the Western Pharmacology Society, 48, 142–144.
Shivakumar, P., Rani, M. U., Reddy, A. G., et al. (2012). A Study on the toxic effects of doxorubicin on the histology of certain organs. Toxicology International, 19(3), 241–244.
Bourdois, P. S., Dancla, J. L., Faccini, J. M., et al. (1982). The sub-acute toxicology of digoxin in dogs; clinical chemistry and histopathology of heart and kidneys. Archives of Toxicology, 51, 273–283.
Botelho, A. F. M., Santos-Miranda, A., Joca, H. C., et al. (2017). Hydroalcoholic extract from Nerium oleandar L. (Apocynaceae) elicits arrhythmogenic activity. Journal of Ethnopharmacology, 206, 170–177.
Wu, J., Li, D., Du, L., et al. (2015). Ouabain prevents pathological cardiac hypertrophy and heart failure through activation of phosphoinositide 3-kinase α in mouse. Cell & Bioscience, 64, 1–15.
Funding
Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG).
Author information
Authors and Affiliations
Contributions
RSF performed the clinical procedures and data analysis and interpretation and drafted the manuscript. PBUF performed the clinical procedures and laboratory analysis. JPOC performed the clinical procedures and laboratory analysis. FLAS performed the clinical procedures and laboratory analysis. MRL performed the clinical procedures and pharmacokinetic analysis and contributed to statistical analysis. GNC performed the clinical procedures. JCCV contributed to scientific discussions and helped laboratory analysis; participated in the study design; and revised the manuscript. MMM contributed to scientific discussions and statistical analysis. AFMB was responsible for the study design and academic direction and contributed to revising, editing, and writing of manuscript. MMM was responsible for the study design and academic direction and contributed to revising, editing, and writing of manuscript. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Vera Costa.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
da Silva Ferreira, R., Fernandes, P.B.U., da Cruz, J.P.O. et al. Comparative Therapeutic Potential of Cardioactive Glycosides in Doxorubicin Model of Heart Failure. Cardiovasc Toxicol 22, 78–87 (2022). https://doi.org/10.1007/s12012-021-09702-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12012-021-09702-w