Abstract
The aim of this study was to evaluate the comparative effects of CGs on heart physiology. Twenty-eight Wistar rats were distributed into four groups (n = 7), control group received NaCl 0.9% every 24 h for 21 days; treated groups received respectively 50 μg/kg of digoxin (DIG), ouabain (OUA) and oleandrin (OLE) every 24 h for 21 days. Serial ECGs were performed, as well as serum levels of creatinine kinase (CK), its MB fraction, troponin I (cTnI), calcium (Ca2+) and lactic dehydrogenase (LDH). Heart tissue was processed for histology, scanning electron microscopy and Western blot analysis for cTnI, brain natriuretic peptide (BNP), sodium potassium pump alpha-1 and alpha-2. Ventricle samples were also analyzed for thiobarbituric acid reactive substances and antioxidant enzymes (SOD, GPX, and CAT). ECGs showed decrease in QT and progressive shortening of QRS. No arrhythmias were observed. No significant differences were associated with CGs treatment and serum levels of CK, CK-MB, and cTnI. Only oleandrin increased LDH levels. Histological analysis showed degenerative changes and only oleandrin promoted moderate focal necrosis of cardiomyocytes. Scanning microscopy also confirmed the greatest effect of oleandrin, with rupture and shortening of cardiac fibers. The expression of troponin I and alpha-1 isoform were not altered, however, the protein levels of BNP and alpha-2 were higher in the groups that received oleandrin and ouabain in relation to the digoxin group. All GCs affected the production of ROS, without causing lipid peroxidation, through the activation of different antioxidant pathways. It is concluded that the administration of digoxin, ouabain, and oleandrin at 50 µg/kg for 21 days caused cardiovascular damage that represent an important limitation into its future use in heart failure and antineoplastic therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cardiac glycosides (CGs) are well-known substances frequently used in the treatment of heart failure (HF) [1, 3]. The proposed mechanism of action of CGs is the inhibition of the sodium and potassium ATPase (NKA) current, leading to an increase in intracellular sodium (Na+). The immediate consequence of such rise is the escalation of cardiomyocyte intracellular calcium (Ca2+) levels due to the action of the sodium calcium exchanger (NCX). Increases of intracellular Ca2+ promote positive inotropism and can be beneficial for patients with HF [24].
Digoxin and digitoxin are one of the few CGs available commercially for the treatment of HF, but several others have been studied over the years, including ouabain, oleandrin, bufalin, and marinobufagenin [6, 12, 17, 21, 32]. Although the main mechanism of action is similar, little is known about the possible differences in cardioactive potential amongst these substances. Previous researches indicate there are species specificity and possible modulation differences in NKA isoforms expression and activity [13].
Recent studies have described digoxin, ouabain, and oleandrin as potential anticancer drugs. There is an apparent in vitro cytotoxicity towards cancer cells from lungs, prostate, and breasts [7, 28]. However, the known toxicity of these substances may represent a limitation to its clinical use and yet no consensus or standard therapy has been proposed [5]. The most important therapeutic limitation is the dose-dependent cardiotoxicity [11]. This effect is a direct result from the CGs mechanism of action. Increased and prolonged inhibition of NKA may disrupt cardiomyocyte homeostasis and cause severe damage. Relevant clinical implications have been associated with digoxin treatment, increasing arrhythmias, hospitalization time, poor outcome, and death [11, 17]. Oleandrin intake is also associated with a variety of arrhythmias, neurological disturbances, and death in intoxication case reports [23, 36] and experimental models [6]. Ouabain, however, is poorly studied, lacking information regarding its potential therapeutic action and toxicity [32].
Despite possible toxicity, low doses of CGs are currently associated with beneficial effects in heart failure and cancer therapies. However, most of these studies lack toxicity estimation. The aim of this study was to evaluate the comparative effects of low doses of digoxin, ouabain, and oleandrin on cardiac electrochemical remodeling, aiming to elucidate differences in cardioactive potential and possible restrictions in their clinical use.
Material and Methods
All experimental procedures in this study were in accordance with the guidelines stated by the Ethical Principles in Animal Research adopted by the Brazilian College of Animal Experimentation (COBEA) and were approved by the Ethics Committee in Animals Use of Universidade Federal de Minas Gerais (Protocol # 74/2017). Our research was strictly carried out in accordance with these approved guidelines.
Chemicals
Digoxin, ouabain, and oleandrin were purchased as pure chemicals from Sigma Aldrich®. The purity of all analytical standard of CGs were higher than 95%. Biochemistry kits of creatine kinase, creatinine kinase MB, lactate dehydrogenase and troponin I were bought from Bioclin®.
Animals and Holding Conditions
Male Wistar rats (190-200 g) were purchased from the Central Animal House Facility of Universidade Federal de Minas Gerais, Belo Horizonte, Brazil. All were kept under standard conditions (12 h light and dark cycles) at 22 ± 2 °C, fed with laboratory rodent diet and water ad libitum.
Experimental Design
28 rats were randomly distributed into four groups, each containing seven rats. The groups were treated as follows: control group (CON) received 0.1 mL of saline; digoxin (DIG), ouabain (OUA), and oleandrin groups (OLE) received 0.1 mL of their respective cardiac glycosides, daily. Digoxin, ouabain, and oleandrin were dissolved in saline and all administrations were subcutaneous at 50 μg/kg for 21 days to evaluate potential differences in cardiac electrochemical remodeling.
Electrocardiogram
All rats were evaluated daily regarding their overall state and possible clinical alterations. Rats were anaesthetized by isoflurane (Isoflurine®, at 2.5% during induction and 1.5% for maintenance; VetCase, Brasmed®) and placed in supine position in a wooden board. A six-channel non-invasive electrocardiograph (ECG-PC version 2.07, Brazilian Electronic Technology—TEB, Belo Horizonte, MG, Brazil) was used. All procedures were performed in a quiet room to minimize stress. Readings were made in DII, at 50 mm/ s, and 2 N. Recordings were made before therapy administration (T0), and seven (T7), 14 (T14) and 21 days (T21) after.
Estimation of Cardiac Injury Serum Biomarkers
After 21 days of treatment, euthanasia was performed with isoflurane followed by intraperitoneal injection of Thiopental® (100 mg/kg). Blood was collected from abdominal aorta in tubes without anticoagulants for serum sampling. Biochemical profile was obtained through evaluation of creatine kinase (CK) and its fraction MB (CK-MB), lactate dehydrogenase (LDH), ionic calcium (Ca2+), using Veteste® commercial kits and spectrophotometry (TP Analyser®). Troponin I (cTnI) was measured with immunochromatography kit (Bioclin®).
Histopathological Evaluation
Necropsy was performed immediately after euthanasia. For microscopic analysis, myocardial samples from four animals of each group were fixed in buffered formalin solution and embedded in paraffin. Serial Sects. (4 μm) were stained with hematoxylin and eosin. Myocardial samples from the remaining three animals of each group were frozen at -80 °C for Western blot analysis and oxidative stress estimation as described below.
Western Blot
Western blotting was performed according to previous protocols [18]. A 10% tissue homogenate of each heart was prepared using ice-cold 50 mM phosphate buffer saline (pH 7.4). The homogenate was centrifuged at 2000 × g for 20 min at 4 °C and the aliquots of the supernatant were used for protein estimation. Proteins were separated on a 10% SDS-PAGE (Millipore, Darmstadt, Germany) gel electrophoresis. Proteins were transferred onto a membrane. The following primary antibodies were used: NKA alpha-1 isoform (1:5000) monoclonal (Upstated), NKA isoform alpha-2 (1:250) monoclonal (Upstated), cTnI and BNP (1:500) (ABCAM) and GADPH (Santa Cruz) monoclonal (1:1000). Secondary antibody anti-mouse monoclonal IgG were used (1:5000, Stressgen, Victoria, Canada). The numerical values above each panel of proteins represent the band intensity identified through fluorescence imaging (Luminata, Amershan, UK) and corrected with GAPDH levels.
Estimation of Oxidative Stress
We evaluated the extent of oxidative stress in the myocardial tissue across the different groups in terms of the thiobarbituric acid reactive substances (TBARS), along with the activities of catalase (CAT), glutathione peroxidase (GSH) and superoxide dismutase (SOD).
Estimation of Lipid Peroxidation
The heart homogenates were used according to previously described protocols [16, 27]. Samples of homogenates (0.05 mL) were treated with 0.5 ml of thiobarbituric acid (0.67%) and tricloroacetic acid (20%). The reaction mixture was heated at 100 °C for 20 min, cooled in ice bath and extracted with n-butanol. The n-butanol layer was separated and the absorbance of the complex estimated at 532 nm. TBARS levels are expressed as nmol de MDA/mg of protein, expressing lipid peroxidation levels.
Estimation of Reduced Glutathione
The activity of Glutathione Peroxidase was measured according to previous research [30]. Heart homogenate (0.004 ml) were treated with 0.2 ml of potassium buffer (100 mM, Ph = 7.5), with 2 mM of reduced glutathione (GSH), 0.1U/ml of glutathione reductase, 0.12 mM of NADPH, 2 mM of H2O2, and 1 mM of sodium azide. The optical density was measured at 340 nm using a microplate spectrophotometer and expressed at nmol NADPH/min/ml.
Determination of Catalase Activity
As reported by [30], the heart homogenate (0.06 ml) was added to 2 ml of phosphate buffer (50 mM, pH 7.0), followed by treatment with 0.04 ml of H202 (3 M). The reduction in optical density was monitored for 1 min at 25ºC using microplate spectrophotometer at 240 nM. The rate of optical density decrease was considered the indicator of catalase activity expressed as▲E/min/mg of protein.
Estimation of Superoxide Dismutase (SOD) Activity
The heart homogenate (20 μl) was added to a mixture of phosphate buffer saline (pH 7.2), MTT (1.25 mM) and pirogarol (100 mM), according to [10]. After five minutes, reaction was interrupted by the addition of DMSO. The optical density was measured at 570 nm in kinetics mode for 3 min at 1 min intervals. The rate increase in optical density was determined as indicator of SOD activity at U.mg of protein.
Statistical Analysis
Data are presented as means ± SD. Normality of data distribution was evaluated using the Kolmogorov–Smirnov test. Statistical significance of parametric data was determined by ANOVA, followed by Tukey's test. Non-parametric data were compared by Kruskal Wallis’ test. Significance was set at P < 0.05. Data were analyzed using GraphPad Prism v.7.0 (Systat Software Inc., USA).
Results
Arrhythmogenic Potential and Serum Biomarkers of Cardiovascular Disease
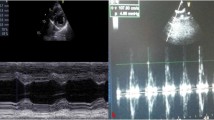
Cardiac glycosides therapy for 21 days did not cause arrhythmias in Wistar rats. However, it led to increase in QRS complex duration in all treated groups (Fig. 1) and progressive shortening of corrected QT interval. No significant differences were associated with CGs treatment and serum levels of CK, CK-MB, and cTnI. Oleandrin increased LDH levels (Fig. 1), an important biomarker for cardiovascular injury.
Cardiac Remodeling
We assessed cardiac remodeling through histopathological analysis associated with measurement of heart biomarkers of cardiovascular disease. Digoxin caused discrete cardiomyocyte focal degeneration. Ouabain and oleandrin induced focal and discrete loss of cardiac striation, discrete degeneration, mild and moderate necrosis, respectively (Fig. 2). CGs treatment did not alter heart tissue cTnI levels; however, oleandrin and ouabain increased BNP levels when compared to digoxin (Fig. 3). These results suggest that ouabain and oleandrin induce greater cardiac remodeling than digoxin.
Histological analysis of hematoxylin and eosin of Wistar rats after 21 days of oral cardenolide treatment. CON group without changes (a, b); DIG group with discrete focal intracytoplasmic vacuolization of cardiomyocytes from the papillary muscle (black arrows) (c, d); OUA group exhibited focal and discrete loss of cardiac streaks in areas of the papillary muscle, associated with discrete focal myocardial intracytoplasmic vacuolization (black arrows) and discrete in the focal area of cardiomyocyte necrosis (asterisk) (e, f). OLE group presents alterations similar to the OUA group, with focal and discrete loss of cardiac striations accompanied by discrete to moderate multifocal intracytoplasmic vaccination of cardiomyocytes (black arrows) and moderate focal cardiomyocyte necrosis (asterisks) (g, h).
Mean values and standard deviation of cardiac tissue biomarkers: brain natriuretic peptide (BNP) (a) and troponin I (cTnI) (b) obtained from control Wistar rats (CON) and treated for 21 days with digoxin (DIG), ouabain (OUA) and oleandrin (OLE). Parametric data were evaluated by ANOVA and post Tukey test (*p < 0.05)
Modulation of NKA Isoforms Expression
We analyzed NKA isoform alpha-1 and 2 expression in the left ventricle tissue of rats treated with digoxin, ouabain, and oleandrin. NKA alpha-1 expression was far greater than alpha-2. However, comparative analysis of the treatments indicates that NKA alpha-2, in detriment of alpha-1, was modulated by the treatments. In fact, digoxin did not cause any significant changes, whilst ouabain and oleandrin only triggered increase in NKA isoform alfph-2 expression (Fig. 4).
Expression of the alpha-1 and alpha-2 isoforms of the sodium and potassium ATpase (NKA) using Western blot analysis of heart samples from control Wistar rats (CON) and treated for 21 days with GCs with digoxin (DIG), ouabain (OUA) (c) and oleandrin (OLE). The treatments did not alter the levels of the alpha-1 (a) and alpha-2 (b) isoform. The expression of the alpha-1 isoform was significantly higher in all groups studied compared to alpha-2 (c). The relative evaluation between the alpha-1/alpha-2 isoforms also did not reveal significant differences between treatments (d). Parametric data were evaluated by ANOVA and post Tukey test (*p < 0.05)
Oxidative Potential
We evaluated oxidative potential of cardiac glycosides through identification of TBARs levels, representing possible role in lipid peroxidation, and activity of the main antioxidant enzymes: SOD, CAT, and GPx in the heart tissue. CGs did not change GPx activity; however, oleandrin treatment induced higher levels of SOD, while ouabain caused major increase in CAT and digoxin that increased both antioxidant enzymes (Fig. 5). Results suggest that oleandrin caused greater production of superoxide, ouabain of hydrogen peroxide and digoxin, both.
Analysis of oxidative stress (levels of lipid peroxidation—TBARs) and activity of the main antioxidant enzymes—SOD, CAT and GPx—in the cardiac tissue of Wistar control (CON) rats and treated for 21 days with GCs with digoxin (DIG), ouabain (OUA) (c) and oleandrin (OLE). Treatments did not alter the levels of TBARs (a). SOD showed a significant increase in its enzymatic activity in the DIG and OLE groups, in relation to the control and OUA (b). The enzymatic activity of CAT is increased in all treatments, with a significant difference in the DIG and OUA groups in relation to the control (c). The treatments did not alter the enzymatic activity of GPx (d). Parametric data were evaluated by ANOVA and post Tukey test (*p < 0.05; **p < 0.01).*Significant difference between groups
Discussion
In this study, we determined that digoxin, ouabain, and oleandrin cause different alterations regarding cardiac remodeling as seen through cardiomyocyte production of reactive oxygen species (ROS), cellular damage, and modulation of NKA isoforms that precede clinical alterations and represent an important limitation on possible treatments involving CGs. Amongst the studied CGs, oleandrin caused the most severe effects on cardiac remodeling.
CGs inhibit the sodium and potassium ATP [24], causing electrochemical imbalance that may damage the cardiovascular system even at low doses. They are considered potent cardioactive substances used in heart failure therapy, but exert dose-dependent cardiotoxicity [33]. Currently available drugs include digoxin and digitoxin, however, even these substances have their limitations especially regarding cardiovascular side effects [11]. Hence, other CGs, such as ouabain and oleandrin, have been the target of new researches. These well-known substances have recently attracted attention due to important anticancer potential. In vitro studies using cell or animal models over the last decades suggest anticancer potential over several neoplastic cells [7]. However, in vivo effects of low prolonged doses of CGs on the cardiovascular system could represent a determinant limitation of such therapy [5].
Treatment using 50 μg/kg for 21 days of digoxin, ouabain, and oleandrin did not cause clinical signs of intoxication or severe cardiac arrhythmias. Expected clinical signs in acute and chronic intoxication with cardenolides and bufodienolides may include emesis, salivation, abdominal pain, tremors, diarrhea, weak pulse, heart palpitations, apathy, depression, and incoordination [2, 14, 23, 25, 29, 31]. Several heart arrhythmias are then described, including ventricular extrasystoles, atrioventricular blockage, atrial fibrillation, and ventricular tachycardia [8, 10, 14, 23, 25, 31, 36]. The absence of such results indicate this dosage could be used for clinical experimentation in the treatment of heart failure.
In accordance, the CGs studied also did not alter serum biomarkers such as CK, CK-MB, and cTnI. Previous reports have shown significant increases in biomarkers in intoxication cases, in both human [11, 33] and animals [2], as spontaneous [36] or experimental intake [6]. However, most studies involved significantly higher doses of CGs as purified substances, plants or venoms that contain CGs. These differences are probably associated with the dose-dependent mechanism of action related to such substances.
Nonetheless, detailed analysis of ECG tracings showed increase in QRS segment in all CGs treated groups. Such segment alteration is often overlooked, but is considered an early predictor of ventricular arrhythmias [4] and should be carefully evaluated in further studies. These discrete alterations were similarly found in histopathological analysis. Digoxin caused minor cardiac degeneration while ouabain and oleandrin mild to moderate necrosis, suggesting that ouabain and oleandrin provoke greater cardiac remodeling and damage than digoxin. Although results were discrete, they remain relevant as a clear representation of CGs’ cardiotoxicity even at low doses, preceding clinical signs and heart arrhythmias. Such results are similar to previous findings [2, 6, 32] that often describe degeneration and necrosis of cardiomyocytes due to intracellular ion imbalance.
In order to further investigate cardiotoxicity, we evaluated heart levels of both BNP and cTnI. Digoxin did not cause alterations in such proteins, while OUA and OLE provoked significant increase in BNP. These results express the same level of cardiotoxicity as observed in histopathological findings in the left ventricle. BNP is an important biomarker of cardiac remodeling and is also a predictor of arrhythmias [35], which leads to the conclusion that both OUA and OLE induced greater cardiac remodeling and possible damage than DIG. Our results show that cardiac glycosides therapeutical potential for both heart failure and cancer treatment, especially OUA and OLE, even at low dosages may represent a risk for cardiotoxicity.
Cardiomyocyte ion equilibrium is partially maintained by the action of the NKA. Considering the importance of such pump and the known selective mechanism of CGs, we analyzed isoforms alpha-1 and alpha-2 from the left ventricle of all treated groups. Results showed that NKA alpha-1 was far greater expressed in all rat heart cells, but only alpha-2 was positively modulated by the CGs treatment, especially by OUA and OLE. Similar findings were reported regarding rat expression of NKA alpha-1 [9], but this is the first research regarding comparative expression of NKA with chronic administration of CGs. The impact of such differences remains to be discovered, but NKA alpha-2 is mostly expressed in cardiomyocyte T-tubules [9], causing local electrochemical alterations that directly impact cell contraction. The fact that OUA and OLE modulate the expression of this isoform suggests that their mechanism of action is slightly different and possibly more cytotoxic than digoxin.
Regarding such cytotoxicity, we evaluated the oxidative potential of cardiac glycosides through lipid peroxidation assay and production of ROS. DIG, OUA, and OLE did not increase TBARs levels, showing lipid peroxidation was not installed, but caused increase in different antioxidant enzymes. These results suggest that OLE caused greater production of superoxide, OUA of hydrogen peroxide and DIG, both. Oxidative stress following toxic doses of CGs is expected [15], however, low doses of CGs are associated with beneficial effects [22, 34], and the present work is one of the few that revealed such cytotoxicity even at low dose. This is an important finding as it represents a limitation regarding prolonged treatments with CGs. Researches involving CGs and cancer treatment have increased over the last decades, with promising results [19, 20], as CGs induce oxidative stress in cancer cells resulting in apoptosis [26]. Conversely, ROS increase in the heart caused by the three CGs in the present study may limit its beneficial effects. Patients using CGs could present cardiac damage, without clinical alterations perceived by the medical staff, even at low doses. Our work provides evidences that oleandrin, ouabain, and digoxin at 50 µg/kg for 21 days cause early stages of electromechanical remodeling of the heart.
Conclusion
We conclude that the administration of digoxin, ouabain, and oleandrin at 50 µg/kg for 21 days caused cardiovascular damage that represent an important limitation into its future use in heart failure and antineoplastic therapy. CGs cause cardiovascular damage even at low doses, with important differences in NKA isoform modulations, associated with ROS production and cardiac remodeling, preceding clinical alterations, including those of serum biomarkers of heart failure and arrhythmias. Amongst the studied substances, oleandrin presented the highest cardiotoxic potential.
References
Atkins, C., Bonagura, J., Ettinger, S., et al. (2009). Guidelines for the diagnosis and treatment of canine chronic valvular disease. JVIM, 23, 1142–1150.
Barbosa, R. R., Fonetele-Neto, J. D., & Soto-Blanco, B. (2008). Toxicity in goats caused by oleander (Nerium oleander). Research in Veterinary Science, 85, 279–281.
Bocchi, E. A., Braga, F. G. M., Bacal, F., et al. (2012). III Diretriz Brasileira de Insuficiência Cardíaca Crônica. Arquivos Brasileiros de Cardiologia, 98, 1–33.
Borleffs, C. J., Shcerptong, R. W., Man, S. C., et al. (2009). Predicting ventricular arrhythmias in patients with ischemic heart disease: clinical application of the ECG-derived QRS-T angle. Circulation Arrhythmia and Electrophysiology, 2, 548–554.
Botelho, A. F. M., Pierezan, F. P., Soto-Blanco, B., & Melo, M. M. (2019). A review of cardiac glycosides: structure, toxicokinetics, clinical signs, diagnosis and antineoplastic potential. Toxicon, 158, 63–68.
Botelho, A. F. M., Santos-Miranda, A., JOCA, H. C., et al. (2017). Hydroalcoholic extract from Nerium oleander L (Apocynaceae) elicits arrhythmogenic activity. Journal of Ethnopharmacology, 206, 170–177.
Calderón-Montaño, J. M., Burdos-Móron, E., Ortal, M. L., et al. (2013). A hydroalcoholic extract from the leaves of Nerium oleander inhibits glycolysis and induces selective killing of lung cancer cells. Planta Medica, 79, 1017–1023. https://doi.org/10.1055/s-0032-1328715.
Camphausen, C., Hass, N. A., & Mattke, A. C. (2005). Successful treatment of oleander intoxication (cardiac glycosides) with digoxin-specific Fab antibody fragments in a 7-year-old child. Zeitschrift fur Kardiologie, 94, 817–823. https://doi.org/10.1007/s00392-005-0293-3.
Despa, S., & Bers, D. M. (2007). Functional analysis of Na+/K+-ATPase isoform distribution in rat ventricular myocytes. American Journal of Physiology, 293, 321–327.
Dieterich, S., Bieligk, U., Beulich, K., Hasenfuss, G., & Prestle, J. (2000). Gene expression of antioxidative enzymes in the human heart. Increased expression of catalase in the end-stage failing heart. Circulation, 101, 33–39.
Trial, D., & The Digitalis Investigation Group. (1997). The Effect of Digoxin on Mortality and Morbidity in Patients with Heart Failure. The New England Journal of Medicine., 336, 525–533. https://doi.org/10.1056/NEJM199702203360801.
Gadelha, I. C. N., Lima, J. M., Batista, J. S., et al. (2014). Toxicity effects of toad (Rhinella jimi Stevaux, 2002) venom in chicken (Gallus gallus domesticus). The Scientific World Journal, 2014, 1–6.
Gupta, R. S., Chopra, A., & Stetsko, D. K. (1986). Cellular basis for the species differences in sensitivity to cardiac glycosides (digitalis). Journal of Cellular Physiology, 127, 197–206.
Haynes, B. E., Bessen, H. A., & Wightamn, W. D. (1985). Oleander tea: herbal draught of death. Annals of Emergency Medicine, 14, 350–353.
Ho, H., Stevens, S. C. W., Terentyeva, R., et al. (2011). Arrhythmogenic adverse effects of cardiac glycosides are mediated by redox modification of ryanodine receptors. Journal of Physiology, 589, 4697–4708.
Janero, D. (1990). Malondialdehyde and thiobarbituric acid-reactivity as diagnostic indices of lipid peroxidation and peroxidative tissue injury. Free Radical Biology and Medicine., 9, 515–540.
Joost, A., Kurowki, V., & Kaiser, A. (2010). Intoxication by Digitalis purpurea in suicidal intention – a case report. Medicina Interna, 4, 77–81.
Joviane-Santos, J. V., Santos-Miranda, A., Botelho, A. F. M., et al. (2019). Increased oxidative stress and CaMKII activity contribute to electro-mechanical defects in cardiomyocytes from a murine model of Huntington’s disease. The FEBS Journal, 286, 110–123. https://doi.org/10.1111/FEBS.14706.
Kaushik, V., Azad, N., Yakisich, J. S., & Iyer, A. K. V. (2017). Antitumor effects of naturally ocurring cardiac glycosides convallotoxin and peruvoside on human ER+ and triple negative breast cancers. Cell Death Discov, 3, 17009.
Kepp, O., Menger, L., Vacchelli, E., et al. (2012). Anticancer activity of cardiac glycosides At the frontier between cell-autonomous and immunological effects. Oncoimmunology, 1, 1640–1642.
Khan, I., Kant, C., Sanwaria, A., et al. (2010). Acute cardiac toxicity of Nerium Oleander/Indicum poisoning (Kaner) Poisoning. Heart Views, 11, 115–116.
Ko, Y. S., Rugira, T., Jin, H., et al. (2018). Oleandrin and its derivative odoroside A, both cardiac glycosides, exhibit anticancer effects by inhibiting invasion via suppressing the STAT-3 signaling pathway. International Journal of Molecular Sciences, 19, 3350.
Kuçudkurmaz, Z., Karapinar, H., Gul, İ., et al. (2012). Complete atrioventricular blok after self-ingestion of Nerium oleander for relief of hemorrhoidal complaints. Arch. Turk. Soc. Cardiol., 40, 168–170.
Langford, S. D., & Boor, P. J. (1996). Oleander toxicity: examination of human and animal toxic exposures. Toxicology, 109, 1–13.
le Couter, D. G. L., & Fisher, A. A. (2002). Chronic and criminal administration of Nerium oleander. Clinical Toxicology, 40, 523–424.
Ma, Y., Zhu, B., Yong, L., et al. (2016). The apoptosis induction of oleandrin on osteosarcoma cells through regulations mitochondrial- and death receptor-dependent apoptotic pathways in vitro. International Journal of Molecular Sciences, 17, 1950.
Ohkawa, H., Ohishi, N., & Yagi, K. (1979). Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Analytical Biochemistry, 95, 351–358.
Osman, M. H., Farrag, E., Selim, M., et al. (2017). Cardiac glycosides use and the risk and mortality of cancer; systematic review and meta-analysis of observational studies. PLoSOne, 7, e0178611.
Page, C., & Murtaugh, R. J. (2015). Hypoglycemia associated with oleander toxicity in a dog. J. Med. Toxicol., 11(1), 141–143.
Paglia, D. E. (1967). Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. Journal of Laboratory and Clinical Medicine, 70, 158–169.
Peymani, P., Zamiri, N., & Tahmasbi, J. (2011). A case of non-fatal oleander poisoning. Iran Red. Crescent Med. J., 13, 219–220.
Ramirez-Ortega, M., Zarco, G., Maldonado, V., et al. (2007). Is digitalis compound-induced cardiotoxicity, mediated through guinea-pig cardiomyocytes apoptosis? European Journal of Pharmacology, 566, 34–42.
Roberts, D. M., Gallapathy, G., Dunuwille, A., & Chan, B. S. (2016). Pharmacological treatment of cardiac glycoside poisoning. British Journal of Clinical Pharmacology, 81, 488–495.
Schneider, N. F. Z., Cerella, C., Siões, C. M. O., & Diederich, M. (2017). Anticancer and immunogenic properties of cardiac glycosides. Molecules, 8, E1932.
Scott, P. A., Barry, J., Roberts, P. R., & Morgan, J. M. (2009). Brain natriuretic peptide for the prediction of sudden cardiac death and ventricular arrhythmias: a meta-analysis. European Journal of Heart Failure, 11, 958–966.
Senthilkumaran, S., Meenakshisundaram, R., Michaels, A. D., et al. (2011). Electrocardiographic changes during inhalational oleander toxicity. Journal of Electrocardiology, 44, 470–472.
Acknowledgements
The authors thank the funding provided by CAPES, CNPq (grant number 424241/2016-1) and FAPEMIG (grant number APQ-03578-16).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interests regarding the publication of this article.
Additional information
Handling Editor: Gen Suzuki .
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Botelho, A.F.M., Miranda, A.L.S., Freitas, T.G. et al. Comparative Cardiotoxicity of Low Doses of Digoxin, Ouabain, and Oleandrin. Cardiovasc Toxicol 20, 539–547 (2020). https://doi.org/10.1007/s12012-020-09579-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12012-020-09579-1