Abstract
Migraine is one of the most common neurological disorders associated with recurrent attacks of moderate to severe headache. Oxidative stress may play an important role in migraine pathogenesis. This study aimed to measure and compare the serum levels of Selenium, total antioxidant capacity (TAC), and malondialdehyde) MDA (in migraine patients and healthy individuals. This case–control study was performed on 31 migraine patients and 30 age and gender-matched healthy controls. The severity of headache was assessed with a standard questionnaire, and the serum levels of Selenium (Se), MDA, and TAC were measured via biochemical methods. The odds of migraine were calculated across quartile of Se and oxidative stress biomarkers via binary logistic regression. Migraine patients had a significant lower Se levels (81.06 ± 8.66 vs. 88.94 ± 10.23 μg/L, P = 0.002) and a significant higher MDA levels (3.04 ± 1.74 vs. 2.06 ± 0.59 nmol/ml, P = 0.005) compared to healthy participants. Although serum TAC levels (1.34 ± 0.34 vs.1.37 ± 0.33 mmol/L, P = 0.755) were not significantly different between migraine patients rather than healthy subjects. Individuals in the lowest quartile of Se levels were about eleven times more likely to have migraine than those in the highest quartile (OR: 11.2; 95%CI: 1.57 to 80.2; P-trend: 0.016). Besides, being in the highest quartile of the serum MDA level, the odds of having migraine increases 15.4 times compared to the lowest quartile (OR = 15.4, 95%CI: 1.1 to 221, P = 0.044). No significant association was found between TAC and migraine. The lower Se and MDA levels in migraine patients gives rise to the probability which oxidant status may play an underlying role in migraine pathophysiology.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Migraine is a primary headache triggered by increased central nervous system irritability [10, 42] and is one of the most common chronic and debilitating diseases affecting 10 to 20% of world population [15, 34]. Migraine also increases the risk of stroke and cardiovascular disease [19]. Lack of specific biological markers is a major obstacle to the early diagnosis of migraine and its causative factors [29]. Preventive treatment for migraine is sometimes limited by the side effects of medications [11, 20]. The molecular mechanisms of migraine attacks are not fully understood. Oxidative stress and impaired mitochondrial metabolism have largely contributed to the reduced threshold of headache. Single free electrons damage various macromolecules such as lipids, proteins, carbohydrates, deoxyribonucleic acid (DNA), and eventually the whole cellular integrity. Oxidative stress can play an important role in migraine pathogenesis by altering cerebral blood flow [4, 17, 21, 26]. Selenium (Se) involves in many nervous system functions and neuronal pathways [12]. The total amount of Se in the body is 10–20 mg, which varies depending on living place, food sources, and health. The brain contains 2.3% of the total body’s Se, and in Se deficiency, the brain has a priority to get it. Se as a trace element is incorporated into selenoproteins which are present in the brain [9]. Selenoproteins such as glutathione peroxidases (GPx), thioredoxin reductases (TrxRs), or selenoprotein P (SelP have antioxidant activity; play an important role to maintain the physiological function of neurons and glial cells [43]; hence, dysfunction or destruction of these enzymes can lead to brain damage [14]. In animal models of headache inflicted by glyceryl trinitrate, Se showed protective roles as an antioxidant [32]. Considering the global burden of migraine and the limited or contradictory evidences regarding the role of oxidant status in this patients [40], the present study was carried out to investigate the serum levels of Se, malondialdehyde (MDA) as a marker of lipid peroxidation, and total antioxidant capacity (TAC) in migraine patients and healthy individuals to uncover any possible association of these parameters with migraine characteristics and finally for better management of these patients.
Materials and Methods
Study Design and Participants
Among the patients with headache referred to the neurology clinic of Valiasr Hospital, Arak, Iran, 31 migraine patients aged 20–50 years were selected according to International Headache Society criteria (ICHD) [22] via a neurologist (F.F) and enrolled in the present case–control study during the year 2019. Thirty age- and gender-matched healthy volunteers were also included in this study. The study was approved by the ethics committee of Science and Research Branch, Islamic Azad University, Tehran, Iran (IR.IAU.SRB.REC.1397.167). All subjects were informed about the study, and written consent was obtained.
Inclusion and Exclusion Criteria
Eligible participants were women between 20 and 50 years of age with migraine, those who were willing to participate in the trial included. The patients with any gastrointestinal, renal, hepatic, lung or heart disease, diabetes mellitus, depression, any pathological lesion in brain MRI or CT scan, using anti-migraine medications, hormonal therapy, and antioxidant or vitamin supplements in the past 6 months were excluded from the study.
Migraine Disability Assessment
Migraine Disability Assessment Score (MIDAS) standard questionnaire was used to assess the disability of migraine [2]. MIDAS questionnaire was used to assess the degree of disability of patients caused by headaches during the last 3 months. The final score of this questionnaire is calculated as the total score of 5 separate questions. The scores are classified based on the following scale: 0–5, little or no disability (grade 1); 6–10, mild disability (grade 2); 11–20, moderate disability (grade 3); 21 + , severe disability (grade 4).
Biochemical Assessment
Five milliliters (5 ml) of fasting blood sample were taken from each participant, and after centrifugation at 3000 rpm for 15 min, the serums were isolated and frozen at – 70 °C. The serum Se, TAC, and MDA levels were determined by the atomic absorption, ferric reducing ability of plasma (FRAP), and the thiobarbituric acid (TBA) method, respectively [5, 39].
Statistical Analysis
Kolmogorov–Smirnov test confirmed the normality of the data. The means of variables were compared by independent samples t test. Analysis of variance (ANOVA) was also applied to analyze the differences of means among groups. The Pearson test measured the statistical relationship or association between variables. Binary logistic regression was performed to predict the odds (OR) of having migraine with a 95% confidence interval (CI). All tests were considered statistically significant at P < 0.05. Data were presented as means ± standard deviations. SPSS software version 21 was used for statistical analysis.
Results
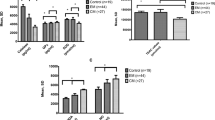
The age range of the migraine patients (male: 4, female: 27) was 27.8 ± 7.3 year and in the control group (male: 8, female: 22) was 25.7 ± 3.9 year. Among the patients, 18 cases (58%) had a family history of migraine. The frequent symptoms were photophobia (54.8%), phonophobia (51.6%), unilateral headache (51.6%), and throbbing headache (41.9%). The frequency of migraine with aura (MWA) was 19.3%. The results of MIDAS questionnaire showed that the grade of disability due to headache was little (grade 1) in 5 patients (16.1%), mild (grade 2) in 10 patients (32.3%), moderate (grade 3) in 9 patients (29%), and severe (grade 4) in 7 patients (22.6%). Mean serum Se values were significantly lower in the patient group than in the control group (P = 0.002). Mean serum MDA values were significantly higher in the migraine group (P = 0.005). However, mean serum level of TAC was not significantly different between the two groups (Table 1).
The serum level of Se (76.95 ± 5.56 vs. 82.04 ± 9.06 μg/L), MDA (3.23 ± 1.65 vs. 2.99 ± 1.79 nmol/ml), and TAC (1.21 ± 0.22 vs. 1.37 ± 0.36 mmol/l) were not significantly different between migraine with aura and migraine without aura subgroups, respectively. About 11 subjects (35.5%) of migraine patients were in the lowest quartile (Q1) of serum Se, whereas 4 subjects (13.3%) of the healthy controls were in the same quartile, which is significantly higher (P = 0.011). About 14 subjects (45.2%) of migraine patients were in the highest quartile (Q4) of serum MDA, whereas 2 subjects (6.7%) of the healthy controls were in the same quartile, which is significantly higher (P = 0.001). However, there was no significant difference between two groups according to the quartiles of serum levels of TAC (Table 2). A binary logistic regression model that adjusted for gender and age revealed an increase in migraine risk across quartiles of Se (OR = 11.24, 95% CI = 1.57–80.2, first quartile: Q1) and [(OR = 8.16, 95%CI = 1.28–51.8, second quartile: Q2), (P for trend = 0.007)]. The risk of having migraine in the first and second quartiles compared to the fourth quartile has increased by roughly 11 and 8 times, respectively. The risk of having migraine in the highest quartile of serum MDA (Q4) was higher than the lowest quartile (Q1), approximately 5 times by unadjusted regression [(OR = 5.4, 95% CI = 0.92–32.3), P = 0.062] and about 15 times by adjusted regression [(OR = 15.4, 95%CI = 1.1–221), P = 0.044]. There was no significant difference across the quartiles of serum levels of TAC (Table 3).
No significant relationship between MIDAS score and the serum levels of Se, MDA, and TAC in the patient group was found (P value > 0.05) (Table 4). There was no association among serum Se, MDA, and TAC levels, and headache characteristics.
Discussion
Migraine is the main cause of disability in people under 50 years of age. The pathophysiology of migraine is not fully understood. Different theories were suggested, including vascular impairment, neuroinflammation, and trigeminovascular activity [31]. In recent years, the importance of oxidative stress and its biomarkers in migraine was considered. Oxidative stress is one of the molecular changes underlying the pathogenesis of migraine and its role in the migraine is important because it stimulates the transient receptor potential subfamily A member1 (TRPA1) ion channel on pain receptors of the meninges and induces neuroinflammation [7, 23, 30].
The role of Se in migraine, as an important factor in the antioxidant defense system, is still unclear. Se deficiency has a detrimental effect on the normal activity of cells in the nervous system and even leads to cell death (22).The current study is the first report that showed significantly lower serum Se level in migraine patients (mean = 81.06 μg/L) than healthy group (mean = 88.94 μg/L), although serum Se levels in both groups were approximately within the normal range (< 90 μg/L) [37]. According to in vitro and in vivo studies, Se plays an important role to regulate intracellular molecular and oxidation processes, inactivating reactive oxygen species, maintaining normal brain functions, and reducing oxidative stress in various neurological disorders such as stroke, epilepsy, Alzheimer’s and Parkinson’s diseases. Based on the data collected from Canadian Health Measures Survey (CHMS 2007–2011, n = 7065) and the US National Health and Nutrition Examination Survey (NHANES 2011–2012, n = 5030), an inverse correlation between serum level of Se and prevalence of stroke was obtained [25]. Furthermore, the results of a case–control study by Hu et al. on 1255 stroke patients showed that there was negative association between serum Se levels and the incidence of the first attack of stroke [24]. In the study of Fathi et al. [18], serum Se levels in MS patients were significantly lower than control subjects (60.87 ± 13 vs. 85.74 ± 12, P value < 0.0001). Katarzyna Socha et al. in a study on 101 MS patients showed that serum Se levels were significantly lower than in healthy individuals [36]. Similarly, Katarzyna Socha et al. showed that serum Se levels in Alzheimer’s disease were significantly lower than in healthy individuals [35].
Se may play a role in the pathophysiology and increasing the risk of migraine; thus, for using Se as a supplement, more clinical trial studies needed to be carried out. Hence, there is no consensus on whether high levels of Se are safe for health because high Se intake exacerbates oxidative stress by increasing nitric oxide (NO) production and decreasing GSH [41]. For assessing the effects of oxidative stress on health and disease, biomarkers such as MDA are used [28]. Our study indicated that the serum level of MDA was significantly higher in the migraine group. Increasing serum MDA levels leads to approximately 15 times increased odds of having a migraine. Our results are similar to the findings of some clinical studies that indicated the serum level of MDA is significantly higher in people with migraine than in healthy individuals [8, 26, 44]. Although some other studies failed to show any significant differences [6]. Such variations might be the result of limited study populations, heterogeneity of the subjects, differences in the headache phase (ictal or interictal), presence of comorbid diseases, and selection of newly diagnosed patients without any treatment.
We found that serum TAC level in migraine patients was lower than the control group, but the difference was not significant. Besides, we did not find a significant difference in odds of having migraine across the quartiles of serum TAC levels. There are controversial results for TAC status in migraine patients. Several studies have reported decreased TAC in migraine patients compared to healthy individuals [1, 13, 27, 40], although Khosravi et al. showed that TAC levels were higher in migraine patients [38]. In a study by Eren et al., oxidative stress markers in migraine patients were not different from the control group; they suggested that such difference in the outcomes among studies may be due to the differences in patient populations and the study design [16]. It seems that patient’s age, environmental and nutritional factors, genetic characteristics, and stress are other influencers that can affect the oxidant-antioxidant balance. Another interfering factor is the duration of treatment, as shown in some studies that, the serum level of TAC also decreases as the patients with migraine undergo treatment [3]. Besides, a recent meta-analysis of 19 studies showed that the role of biomarkers related to oxidative stress in migraines is still unclear [33]. Thus, further large-population studies with more precise association analysis methods using various adjustments are needed.
Patients with aura had lower levels of Se and TAC and elevated levels of MDA than patients without aura, but our data provided no evidence for any significant difference between the two groups. Based on MIDAS score, the levels of Se and TAC were lower, and level of MDA was higher in grade 4 compared with grade 1, although the differences are not significant. Our results are partly similar to the findings of a study by Yigit et al., which indicated that no difference in biomarkers of oxidative stress was found according to MIDAS score [2]. The small number of patients in each grade may be one of the reasons for the lack of significant difference. The main limitation of our study was small sample size that was a barrier for significant differences in our results. Although, age- and gender-matched migraine patients with healthy participant remove the effect of two important confounders on our results.
Conclusion
It can be concluded that reducing Se and increment of MDA are effective in the risk of having migraine, but are ineffective on the clinical characteristics and severity of headache. So oxidant status is an important component in migraine treatment strategy in future studies.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Change history
19 April 2022
A Correction to this paper has been published: https://doi.org/10.1007/s12011-022-03235-1
References
Alp R, Selek S, Alp SI, Taşkin A, Kocyiğit A (2010) Oxidative and antioxidative balance in patients of migraine. Eur Rev Med Pharmacol Sci 14:877–882
Asawavichienjinda T, Imruetaijaroenchoke W, Phanthumchinda K (2020) Thai-version Migraine Disability Assessment (MIDAS) Questionnaire: concurrent validity, test–retest reliability, internal consistency, and factors predictive for migraine-related disability. Asian Biomed (Res Rev News) 14:139–150
Aytac B, Coşkun Ö, Alioğlu B, Durak ZE, Buber S, Tapci E, Öcal R, İnan LE, Durak I, Yoldaş TK (2014) Decreased antioxidant status in migraine patients with brain white matter hyperintensities. Neurol Sci 35:1925–1929
Bayrami M, Hashemi T, Malekirad AA, Ashayeri H, Faraji F, Abdollahi M (2012) Electroencephalogram, cognitive state, psychological disorders, clinical symptom, and oxidative stress in horticulture farmers exposed to organophosphate pesticides. Toxicol Ind Health 28:90–96
Benzie IF, Strain J (1999) [2] Ferric reducing/antioxidant power assay: direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Methods Enzymol 299:15–27
Bernecker C, Ragginer C, Fauler G, Horejsi R, Möller R, Zelzer S, Lechner A, Wallner-Blazek M, Weiss S, Fazekas F (2011) Oxidative stress is associated with migraine and migraine-related metabolic risk in females. Eur J Neurol 18:1233–1239
Borkum JM (2018) The migraine attack as a homeostatic, neuroprotective response to brain oxidative stress: preliminary evidence for a theory. Headache J Head Face Pain 58:118–135
Bulboaca A, Dogaru G, Blidaru M, Bulboaca A, Stanescu I (2018) Evaluation of oxidative stress in migraine patients with visual aura-the experience of an Rehabilitation Hospital. Balneo Res J 9:303–308
Chen J, Berry MJ (2003) Selenium and selenoproteins in the brain and brain diseases. J Neurochem 86:1–12
Chen Z, Chen X, Liu M, Ma L, Yu S (2019) Volume of hypothalamus as a diagnostic biomarker of chronic migraine. Front Neurol 10:606
Danno D, Iigaya M, Imai N, Igarashi H, Takeshima T (2019) The safety and preventive effects of a supraorbital transcutaneous stimulator in Japanese migraine patients. Sci Rep 9:1–7
Dhillon KS, Singh J, Lyall JS (2011) A new horizon into the pathobiology, etiology and treatment of migraine. Med Hypotheses 77:147–151
Dini E, Mazzucchi S, De Luca C, Cafalli M, Chico L, Lo Gerfo A, Siciliano G, Bonuccelli U, Baldacci F, Gori S (2019) Plasma levels of oxidative stress markers, before and after BoNT/A Treatment, in Chronic Migraine. Toxins 11:608
Dominiak A, Wilkaniec A, Adamczyk A (2016) Selenium in the therapy of neurological diseases. Where is it going? Curr Neuropharmacol 14:282–299
Ebrahimi-Monfared M, Sharafkhah M, Abdolrazaghnejad A, Mohammadbeigi A, Faraji F (2017) Use of melatonin versus valproic acid in prophylaxis of migraine patients: A double-blind randomized clinical trial. Restor Neurol Neurosci 35:385–393
Eren Y, Dirik E, Neşelioğlu S, Erel Ö (2015) Oxidative stress and decreased thiol level in patients with migraine: cross-sectional study. Acta Neurol Belg 115:643–649
Faraji F, Ranjbar A, Eshrati B, Talaei A, Shafiei N, Pirasteh S (2008) Comparing the oxidative stress indexes of CVA patients with control group. J Arak Uni Med Sci 11:109-116
Fathi F, Mehrpour M, Akbari ME, Sohrabzadeh K, Fathi S, Joghataie M-T, Nejad MR (2013) A concentration of serum selenium in multiple sclerosis patients compare to healthy subject in Tehran. Arch Adv Biosci 4
Folsom AR, Lutsey PL, Misialek JR, Cushman M (2019) A prospective study of migraine history and venous thromboembolism in older adults. Res Pract Thromb Haemost 3:357–363
Ghavami A, Khorvash F, Heidari Z, Khalesi S, Askari G (2021a) Effect of synbiotic supplementation on migraine characteristics and inflammatory biomarkers in women with migraine: Results of a randomized controlled trial. Pharmacol Res 169:105668
Ghavami A, Khorvash F, Khalesi S, Heidari Z, Askari G (2021b) The effects of synbiotic supplementation on oxidative stress and clinical symptoms in women with migraine: A double-blind, placebo-controlled, randomized trial. J Funct Foods 86:104738
Göbel CH, Karstedt SC, Munte TF, Göbel H, Wolfrum S, Lebedeva ER, Olesen J, Royl G (2020) ICHD-3 is significantly more specific than ICHD-3 beta for diagnosis of migraine with aura and with typical aura. J Headache Pain 21:1–6
Gumusyayla S, Vural G, Bektas H, Neselioglu S, Deniz O, Erel O (2016) A novel oxidative stress marker in migraine patients: dynamic thiol–disulphide homeostasis. Neurol Sci 37:1311–1317
Hu H, Bi C, Lin T, Liu L, Song Y, Wang B, Wang P, Zhou Z, Fang C, Ma H (2021) Sex difference in the association between plasma selenium and first stroke: a community-based nested case-control study. Biol Sex Differ 12:1–15
Hu XF, Stranges S, Chan LH (2019) Circulating selenium concentration is inversely associated with the prevalence of stroke: results from the Canadian Health Measures Survey and the National Health and Nutrition Examination Survey. J Am Heart Assoc 8:e012290
Khosravi A, Nakhaee A, Ghoreishi A, Arefpoor Z, Sadeghi M (2019) Impaired oxidative-antioxidative balance during migraine attack. Biomed Res Ther 6:2996–3002
Lucchesi C, Baldacci F, Cafalli M, Chico L, Lo Gerfo A, Bonuccelli U, Siciliano G, Gori S (2015) Evidences of reduced antioxidant activity in patients with chronic migraine and medication-overuse headache. Headache J Head Face Pain 55:984–991
Marrocco I, Altieri F, Peluso I (2017) Measurement and clinical significance of biomarkers of oxidative stress in humans. Oxidative medicine and cellular longevity, 2017
Mehrifar Y, Pirami H, Farhang Dehghan S (2018) The relationship between exposure to manganese in welding fumes and incidence of migraine headache symptoms. Tehran University Medical Journal TUMS Publications 76:135–141
Mohammadi H, Talebi S, Ghavami A, Rafiei M, Sharifi S, Faghihimani Z, Ranjbar G, Miraghajani M, Askari G (2021) Effects of zinc supplementation on inflammatory biomarkers and oxidative stress in adults: a systematic review and meta-analysis of randomized controlled trials. J Trace Elem Med Biol 126857
Nattagh-Eshtivani E, Sani MA, Dahri M, Ghalichi F, Ghavami A, Arjang P, Tarighat-Esfanjani A (2018) The role of nutrients in the pathogenesis and treatment of migraine headaches. Biomed Pharmacother 102:317–325
Nazıroğlu M, Çelik Ö, Uğuz AC, Butun A (2015) Protective effects of riboflavin and selenium on brain microsomal Ca 2+-ATPase and oxidative damage caused by glyceryl trinitrate in a rat headache model. Biol Trace Elem Res 164:72–79
Neri M, Frustaci A, Milic M, Valdiglesias V, Fini M, Bonassi S, Barbanti P (2015) A meta-analysis of biomarkers related to oxidative stress and nitric oxide pathway in migraine. Cephalalgia 35:931–937
Rai NK, Bitswa R, Singh R, Pakhre AP, Parauha DS (2019) Factors associated with delayed diagnosis of migraine: A hospital-based cross-sectional study. J Fam Med Prim Care 8:1925
Socha K, Klimiuk K, Naliwajko SK, Soroczyńska J, Puścion-Jakubik A, Markiewicz-Żukowska R, Kochanowicz J (2021) Dietary habits, selenium, copper, zinc and total antioxidant status in serum in relation to cognitive functions of patients with Alzheimer’s disease. Nutrients 13:287
Socha K, Kochanowicz J, Karpińska E, Soroczyńska J, Jakoniuk M, Mariak Z, Borawska MH (2014) Dietary habits and selenium, glutathione peroxidase and total antioxidant status in the serum of patients with relapsing-remitting multiple sclerosis. Nutr J 13:1–6
Stoffaneller R, Morse NL (2015) A review of dietary selenium intake and selenium status in Europe and the Middle East. Nutrients 7:1494–1537
Togha M, Jahromi SR, Ghorbani Z, Ghaemi A, Rafiee P (2019) An investigation of oxidant/antioxidant balance in patients with migraine: a case-control study. BMC Neurol 19:1–10
Togha M, RazeghiJahromi S, Ghorbani Z, Martami F, Seifishahpar M (2019) Serum Vitamin B12 and Methylmalonic Acid Status in Migraineurs: A Case-Control Study. Headache J Head Face Pain 59:1492–1503
Tripathi GM, Kalita J, Misra UK (2018) A study of oxidative stress in migraine with special reference to prophylactic therapy. Int J Neurosci 128:318–324
Weldy CS, Luttrell IP, White CC, Morgan-Stevenson V, Bammler TK, Beyer RP, Afsharinejad Z, Kim F, Chitaley K, Kavanagh TJ (2012) Glutathione (GSH) and the GSH synthesis gene Gclm modulate vascular reactivity in mice. Free Radical Biol Med 53:1264–1278
Wenwen X, Jing Y, Yingchao S, Qinglu W (2019) The effect of magnesium deficiency on neurological disorders: a narrative review article. Iran J Public Health 48:379
Xiong S, Markesbery WR, Shao C, Lovell MA (2007) Seleno-L-methionine protects against β-amyloid and iron/hydrogen peroxide-mediated neuron death. Antioxid Redox Signal 9:457–467
Yigit M, Sogut O, Tataroglu Ö, Yamanoglu A, Yigit E, Guler EM, Ozer OF, Kocyigit A (2018) Oxidative/antioxidative status, lymphocyte DNA damage, and urotensin-2 receptor level in patients with migraine attacks. Neuropsychiatr Dis Treat 14:367
Acknowledgements
We especially thank Dr. Ghasem Mosayebi and Dr. Amir Almasi-Hashiani. We also appreciate the migraine patients, healthy volunteers and all executive agents of this project.
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics Approval
This study was approved by the ethics committee of Science and Research Branch, Islamic Azad University, Tehran, Iran (Code: IR.IAU.SRB.REC.1397.167).
Informed Consent
Informed consent was obtained from all participants included in the study.
Conflict of Interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original version of this article was revised due to the incorrect author name. The name of Afsoon Talaie is now corrected in the author group.
Rights and permissions
About this article
Cite this article
Talaie, A., Jafary, H., Faraji, F. et al. The Serum Oxidative Stress Biomarkers and Selenium Levels in a Group of Migraine Patients Compared with Healthy Controls: a Case–Control Study. Biol Trace Elem Res 200, 4250–4255 (2022). https://doi.org/10.1007/s12011-021-03024-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-021-03024-2