Abstract
Although migraine is a neurological disorder known since long, its physiopathology remains unclear. Recent studies suggest that migraine is associated with oxidative stress; however, they report divergent results. The aim of the present study was to evaluate total antioxidant status (TAS), total oxidant status (TOS), oxidative stress index (OSI), and serum thiol level in migraine patients with or without aura. The study group consisted of 141 migraine patients. The control group included 70 healthy subjects. TAS, TOS, OSI were evaluated using a method developed by Erel. Serum thiol level was measured using the Hu method. No difference was found in TAS, TOS, OSI between the patients and controls. The level of thiol was significantly lower in patients than in controls. Negative correlations were detected between thiol level and Migraine Disability Assessment score in patients. Although TAS, TOS, and OSI were similar to those of the control group, serum thiol level, an important marker of antioxidant capacity, was significantly lower in migraines compared with controls, and caused more serious disability. Novel treatment approaches may be developed based on these data, and compounds containing thiol, such as alpha lipoic acid and N-acetyl cysteine, may be used in prophylaxis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Migraine is a common, chronic, disabling neurovascular disorder that is characterized by recurrent and severe headache attacks with neurological, gastrointestinal, and autonomic symptoms. Although the pathophysiology of migraine remains unclear, several vascular, neuroinflammatory, and neurological mechanisms have been suggested. Oxidant–antioxidant balance disorders lie under several acute and chronic diseases of the central nervous system (CNS). It is believed that oxidative stress plays a role in the pathogenesis of migraine [1, 2].
The oxidant–antioxidant balance is an important mechanism for homeostasis in an organism. Reactive oxygen species (ROS), such as superoxide radical anions, hydroxyl radicals, and hydrogen peroxide, are formed as a result of various metabolic and physiological processes, and harmful oxidative reactions may occur. Oxidative stress is described to occur when an “imbalance between oxidant and antioxidant is shifting toward oxidants [3]”. The term “thiol” refers to compounds containing sulfur. Sulfur is an essential element for biological environments because of its incorporation into amino acids, proteins, and other biomolecules [4]. Thiol groups are important members of the antioxidant cascade because they destroy ROS and other free radicals by enzymatic as well as nonenzymatic mechanisms [5]. It was reported that the measurement of total thiol levels can be used to evaluate excess free radical generation, both in physiological and pathological conditions [6].
The hypothesis of the involvement of oxidative stress in migraine has been considered in the last few years. Data on oxidative stress were reported in studies on migraine patients [7]. However, the results are controversial [1]. We measured plasma total antioxidant status (TAS), total oxidative status (TOS), and oxidative index (OSI) using the method developed by Erel [8, 9] in migraine patients with and without aura. In addition, we measured serum total thiol levels. We hypothesize that thiols consumed to the greater extent in case of migraine is accompanied by excess free radical generation. To determine this we compared TAS, TOS and thiol levels of migraine patients with those of a control group of healthy individuals, and evaluated the relationship between their levels and the functional loss in migraine patients.
Materials and methods
Subjects
One hundred and fifty-one patients (122 females and 29 males, mean age 33.96 ± 9.40 years) who presented at the Neurology Outpatient Clinic of the Ankara Ataturk Education and Research Hospital were prospectively included in the study. Moreover, 70 age- and sex-matched healthy control subjects (53 females and 17 males, mean age 32.99 ± 8.51 years) were also enrolled, for comparison.
Patients diagnosed as having migraine according to the criteria of the International Headache Society (2004) were classified into two groups: those with migraine with aura (MWA) and those with migraine without aura (MWOA) [10]. The frequency of episodes, pain localization, autonomic findings, severity of pain, and functional loss of the patients were evaluated. The frequency of episodes was evaluated in two groups, one with less than four episodes per month and the other with more than four episodes per month. The severity of the pain was evaluated using the Visual Analogue Scale (VAS), and loss of function was evaluated using the Migraine Disability Assessment (MIDAS) [11]. Patient exclusion criteria included the presence of a systemic disease, a history of malignancy, smoking, pregnancy, lactation, and intake of medications in the last two weeks.
Written informed consents were received from the patients and controls and the study was approved by Local Ethics Committee of Ankara Atatürk Education and Research Hospital (2011/143). All investigators confirm to ethical standards as described in the Declaration of Helsinki.
Blood samples
Blood samples were obtained after an overnight fast. Samples were collected from a cubital vein into blood tubes, and serum was separated from the cells by centrifugation at 3,000 rpm for 10 min. Samples were stored at −80 °C until the time of analysis.
Measurement of the TAS of the serum
Serum TAS level was measured using a novel automated colorimetric measurement method developed by Erel [8]. The principle of this measurement method is based on the oxidation of the 2.2′-azino-bis (3-ethylbenzthiazoline-6-sulphonic acid) (ABTS) molecule to the ABTS+ molecule in the presence of hydrogen peroxide. The rate of the reaction is calibrated with the standard method of Trolox which is a vitamin E analog, and its unit is mmol Trolox Equivalent/L.
Measurement of the TOS of the serum
The total oxidant level was measured using a fully automated colorimetric method developed by Erel [9]. The principle of this method is based on the oxidation of ferrous ion–o-dianisidine complex to ferric ion by the oxidants present in the sample. The density of the color, which is correlated with the amount of oxidants in the sample, was measured by spectrophotometry. The assay calibrated with hydrogen peroxide and the results are expressed in terms of micromolar hydrogen peroxide equivalent per liter (µmol H2O2 Equiv/L).
Oxidative stress index
The percent ratio of the TOS to the TAS gives the oxidative stress index (OSI).To perform the calculation, the result unit of TAS, mmol Trolox Equivalent/L, was changed to μmol Trolox Equivalent/L, and the OSI value was calculated as follows: OSI = [(TOS, μmol/L)/(TAS, μmol Trolox equivalent/L)/100].
Measurement of the total thiol level of the serum
Total serum thiol concentration or sulfhydryl groups (thiol) were measured using the methods described originally by Ellman [12] and modified by Hu [13]. After manuel spectrophotometric optimization processes the method was applied to an automated analyzer. The format of the test is shown below:
-
Sample volume: 10 μL
-
Reagent 1 volume: 110 μL (TRIS buffer 100 mM pH: 8.2 and EDTA 10 mM)
-
Reagent 2 volume: 10 μL, (10 mM DTNB in metanol).
-
Calibration Type: Linear (Reduced glutathion is used as calibrators for the assay)
Sample and reagent 1 were mixed. After 90 s, reagent 2 was added. Here, thiols interact with 5,5′-dithiobis-(2-nitrobenzoic acid) (DTNB), forming a highly colored anion (5-thio-2-nitrobenzoic acid) with a maximum peak at 412 nm (ε412 = 13,600 M−1 cm−1). The measurements were performed on an automated biochemistry analyzer (Roche Cobas C 501). and the result was expressed in mmol/L.
Statistical analysis
All statistical analyses were performed using the Statistical Package for Social Sciences (SPSS), version 15.0 for Windows (SPSS; Chicago, IL, USA). Data were expressed as the mean ± standard deviation.
The Chi-squared test was used for dual and multiple comparisons of independent categorical variables, when the conditions of Chi-squared were met; otherwise, Fisher’s exact test was used. Student’s t test was used for dual comparisons of independent numerical variables showing a normal distribution, whereas analysis of variance (ANOVA) was used for multiple comparisons. The Mann–Whitney U test was used for dual comparisons, and the Kruskal–Wallis test was used for multiple comparisons of variables that did not show a normal distribution. The Mann–Whitney U test was used for the comparison of subgroups, with Bonferroni correction. Relationships between variables were analyzed by Pearson’s or Spearman’s correlation analysis according to the distribution type of the parameters. P < 0.05 was considered statistically significant.
Results
The demographic and clinical data of the subjects are summarized in Table 1. There were no significant differences in sex and age between the patient and control groups.
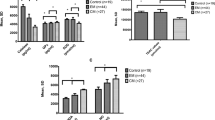
No significant difference was found regarding TAS, TOS, and OSI between the patient and control groups (P = 0.45, P = 0.15, and P = 0.41, respectively). The level of thiol was significantly lower in migraine patients than it was in healthy controls (P < 0.001) (Table 2).
We divided migraine patients into MWA and MWOA subgroups and compared them. We did not observe any significant differences in TAS, TOS, and OSI between these groups. However, the level of thiol was lower in both groups compared with the control group, but there were no significant differences in thiol level between the two groups (Table 3). More MWA patients had a history of migraine compared with MWOA patients (P = 0.026) (Table 1).
A statistically significant negative correlation was found between thiol levels and MIDAS scores (r = −0.263; P = 0.001) in migraine patients (Fig. 1). Similarly, a negative correlation was detected between MIDAS scores and thiol levels in MWA patients (r = −0.323; P = 0.004). Moreover, a negative correlation was found between the total thiol level and age in the group of migraine patients (r = −0.250; P = 0.004), MWA patients (r = −0.234; P = 0.041), and in the control group (r = −0.415; P < 0.001).
Discussion
Migraine is a neurovascular disease that is characterized by cortical depression, neurogenic inflammation, and vasodilation. Although migraine is an illness that has been known since long, its etiology and pathophysiology remain unclear. At present, a genetic change in brain excitability, intracranial arterial vasodilation, and sensitization in the trigeminovascular pathway are implicated in the pathophysiology of migraine [14]. Migraine patients are genetically predisposed to these neurovascular reactions, which are induced by various endogenous and exogenous factors [15]. The balance between inhibition and stimulation of the nervous system determines the degree of stimulation of this individual threshold. Factors such as dysfunction of ion channels, neuropeptides, disorders of magnesium metabolism, and oxidative phosphorylation in mitochondria may trigger migraine attacks by increasing neuronal excitability [16]. Moreover, oxygen free radicals and antioxidant molecules may play a role in the pathophysiology of migraine by affecting all these processes [1].
It is well known that harmful free radicals occur as a result of metabolic and physiological processes in the organism. These are removed by the antioxidant system via enzymatic or nonenzymatic pathways. The oxidative–antioxidative balance shifts toward the oxidative status if the oxidant material increases or the antioxidant mechanisms become insufficient [8].
The hypothesis of oxidative stress in migraine was recently proposed. Oxidative stress in migraine was reported to be associated with increased nitric oxide (NO) [1]. NO plays a role in trigeminovascular inflammation, which occurs during migraine attacks and precipitates bouts of migraine by dilating cerebral vessels [16]. An increase in NO and its metabolites was detected in the plasma, urine, and platelets of migraine patients [1, 17, 18]. Yilmaz et al. [18] reported that NO and its metabolites are good markers of oxidative stress in migraine patients. Malonyl aldehyde (MDA) is a major indicator of lipid peroxidation and determines the activities of antioxidant enzymes, such as superoxide dismutase (SOD), glutathione peroxidase (Glut-px), glutathione reductase (GSSG-R), and catalase (CAT). Studies have shown the lipid peroxidation and levels of these antioxidant enzymes in migraine patients [19–23]. Conversely, studies of this type are few and their results are controversial. Bockwski et al. [1] found lower MDA concentrations in plasma and erythrocytes. A prominent increase in GSSG-R activity was detected; however, the increase in Glut-Px activity and decrease in SOD activity were not statistically significant [1]. Bolayir et al. [23] compared patients with tension-type headache with a control group and found that SOD and Glut-Px activities were prominently higher in the erythrocytes of migraine patients. Tuncel et al. [22] found higher MDA plasma levels and similar SOD and CAT activities in patients migraine compared with the control group. In contrast, Shukla et al. [24] found no significance differences in the activities of CAT, SO, and Glut-Px in neutrophils, or in platelet SOD in migraine patients compared with the control group.
The cause of the different results from investigations of oxidative stress in migraine is the evaluation of antioxidant enzyme activities in serum, erythrocytes, platelets, and neutrophils because the activities of these enzymes vary among these structures. There are few data about lipid peroxidation processes in more components. Lipid peroxidation levels may be observed based on serum TOS measurements using the method developed recently by Erel [9]. On the other hand, TAS is a useful method to determine antioxidant activity in the biological environment [8]. OSI is calculated from the ratio of TOS to TAS. OSI includes antioxidants that may be evaluated, as well as those that are not yet defined or measured in the serum. We did not find a difference between migraine patients and the control group in terms of TAS, TOS, and OSI. Yilmaz et al. [7] found increased levels of TOS and OSI in migraine patients; however TAS levels were not different from the control group. Alp et al. [25] reported increased TOS and OSI and decreased TAS in MWOA patients. The differences observed regarding the results of these studies may be due to differences in patient selection and study design. Patient age, dose of medications and duration of treatment, environmental factors, nutritional preferences, stress, and genetic characteristics may have an effect on the antioxidant–oxidant balance.
Thiol groups are sulfur-containing compounds that are present in all cells of the body [4, 26]. Proteins are the main antioxidant component in the serum, and the sulfur groups present in their structure are responsible for this antioxidant effect. Thiol protein groups are responsible for 52.9 % of the total serum antioxidant capacity, according to a study of healthy individuals [8]. Although plasma thiols are strong antioxidants, they also exert a pro-oxidant effect according to the physiological conditions. Serum thiols are physiological free-radical scavengers and modulate antioxidant enzymes related to glutathione. Cysteine, methionine, taurine, glutathione, lipoic acid, and N-acetyl cysteine are some of the sulfur-containing antioxidant compounds [4]. Free radicals were reported to cause damage in DNA, proteins, and lipids in studies conducted by various investigators [27]. On the other hand, the in vivo DNA and protein damage due to oxidative stress appears to be more important than the damage to lipids [28]. In vivo oxidative changes in proteins affect the various cellular functions that they modulate. Cellular events in which receptors, signal conduction mechanisms, structural proteins, transport systems, and enzymes play a role are affected by oxidative protein damage [29, 30]. All these cellular events play a role involved in the pathophysiology of migraine which are cortical hyperexcitability, neurogenic inflammation, activation of the trigeminovascular system, cortical spreading depression, and pain. Oxidative protein damage is characterized by an increase in protein carbonyl levels [28, 31] and a decrease in protein thiol levels [13]. The determination of plasma thiol levels is important because it reflects the extent of oxygen-radical-mediated oxidation applied to proteins [13].
We found lower levels of serum total thiol in migraine patients compared with the control group in this study. Thiol levels were not different between migraine patients with and without aura. A history of migraine was more prevalent in patients with aura compared with patients without aura. Moreover, a negative correlation was found between serum total thiol levels and MIDAS scores in MWA patients. Alp et al. [25] found lower levels of total thiol in MWOA patients, and they detected a negative correlation between the duration of headache and thiol levels. In a study of healthy individuals, thiol protein groups were reported to account for 52.9 % of serum total antioxidant capacity [8]. Our results showed that, although serum total thiol levels were lower in migraine patients, TAS levels were similar to that of the control group. This finding may reflect oxidative protein damage and thiols which are major component of the antioxidant system used more active in migraine patients. As we mentioned previously the thiols are physiological free radical scavengers. Probably in migraine patients, to remove excess free radicals generated, greater extent thiol consumption occurs. Moreover, it may be associated with the fact that it may cause a more serious loss of function. In addition, a negative correlation was observed in the migraine and control groups between age and total thiol levels. This result suggests that individuals are affected more profoundly by thiol levels as they age, regardless of the presence of migraine. Patients with aura were reported to be more prone to oxidative stress. We found a family history of migraine more frequently in MWA patients compared with those without aura, and detected a negative relationship between thiol levels and MIDAS. Genetic heritance may affect these parameters.
Decreasing the severity, duration, and frequency of attacks is the aim of preventive treatment in migraine patients. Flunarizine, which is used in preventive treatment, decreases the oxidative stress via its free-radical scavenger properties [32]. Antioxidant vitamin complexes, such as vitamin C and E, were used in migraine patients, and encouraging results were obtained [1]. According to our data, antioxidants containing sulfur, such as alpha lipoic acid and N-acetyl cysteine, may be used in the prophylaxis of migraine.
In conclusion, we found similar levels of TAS, TOS, and OSI in migraine patients and in the control group in this pilot study. Conversely, a decrease in serum thiol levels and an increase in MIDAS scores were observed. These findings may be associated with an insufficiency of the antioxidant system in migraine patients, which may cause a more serious loss of function. These data may be a cause, as well as a result, of the disease. Complex studies analyzing the dynamic changes in the oxidant–antioxidant balance during and after migraine attacks are required.
References
Bockwski L, Sobaniec W, Kulak W et al (2008) Serum and intraerythrocyte antioxidant enzymes and lipid peroxides in children with migraine. Pharmacol Rep 60:542–548
Gruber HJ, Bernecker C, Lechner A et al (2010) Increased nitric oxide stress is associated with migraine. Cephalalgia 30:486–492
Stocker R, Keaney JF Jr (2004) Role of oxidative modifications in atherosclerosis. Physiol Rev 84:1381–1478
Atmaca G (2004) Antioxidant effects of sulfur-containing amino acids. Yonsei Med J 45:776–788
Cadenas E (1989) Biochemistry of oxygen toxicıty. Annu Rev Biochem 58:79–110
Pasaoglu H, Sancak B, Bukan N (2004) Lipid peroxidation and resistance to oxidation in patients with type 2 diabetes mellitus. Tohoku J Exp Med 203:211–218
Yilmaz N, Aydin O, Yegin A et al (2011) Increased levels of total oxidant status and decreased activity of arylesterase in migraineurs. Clin Biochem 44:832–837
Erel O (2004) A novel automated direct measurement method fortotal antioxidant capacity using a new generation, morestable ABTS radical cation. Clin Biochem 37:277–285
Erel O (2005) A new automated colorimetric method for measuring total oxidant status. Clin Biochem 38:1103–1111
Headache Classification Subcommittee of the International Headache Society (2004) The International Classification of Headache Disorders: 2nd edition. Cephalalgia 4:9–160
Stewart WF, Lipton RB, Dowson AJ et al (2001) Development and testing of the Migraine Disability Assessment (MIDAS) Questionnaire to assess headache-related disability. Neurology 56:S20–S28
Ellman GL (1959) Tissue sulphydryl groups. Arch Biochem Biophys 82:70–77
Hu ML (1994) Measurement of protein thiol groups and glutathione in plasma. Methods Enzymol 233:380–385
Noseda R, Burstein R (2013) Migraine pathophysiology: anatomy of the trigeminovascular pathway and associated neurological symptoms, CSD, sensitization and modulation of pain. Pain. doi:10.1016/j.pain.2013.07.021
Glaubic-Łatka M, Łatka D, Bury W et al (2004) Current opinions on migraine pathophysiology. Neurol Neurochir Pol 38:307–315
Lance JW (1993) Current concepts of migraine pathogenesis. Neurology 43:S11–S15
Uzar E, Evliyaoglu O, Toprak G et al (2011) Increased asymmetric dimethylarginine and nitric oxide levels in patients with migraine. J Headache Pain 12:239–243
Yilmaz G, Sürer H, Inan LE et al (2007) Increased nitrosative and oxidative stress in platelets of migraine patient. Tohoku J Exp Med 211:23–30
Shimomura T, Kowa H, Nakamo T et al (1994) Platelet superoxide dismutase in migraine and tension type headache. Cephalalgia 14:215–218
Tozzi-Ciancarelli MG, De Matteis G, Di Masimo C et al (1997) Oxidative stress and platelet responsiveness in migraine. Cephalalgia 17:580–584
Ciancarelli I, Tozzi-Ciancarelli MG, Di Masimo C et al (2003) Urinary nitric oxide metabolites and lipid peroxidation by-products in migraine. Cephalalgia 23:39–42
Tuncel D, Tolun FI, Gokce M et al (2008) Oxidative stress in migraine with and without aura. Biol Trace Elem Res 126:92–97
Bolayir E, Celik K, Kugu N et al (2004) İntra erythrocyte antioxidant enzyme activities in migraine and tension-type headaches. J Chin Med Assoc 67:263–267
Shukla R, Barthwal MK, Srivastava N et al (2004) Neutrophil-free radical generation and enzymatic antioxidants in migraine patients. Cephalalgia 24:37–43
Alp R, Selek S, Alp SI et al (2010) Oxidative and antioxidative balance in patients of migraine. Eur Rev Med Pharmacol Sci 14:877–882
Morıarty-Craıge SE, Jones D (2004) Extracellular thiols and thiol/disulfide redox in metabolism. Annu Rev Nutr 24:481–509
Liu J, Wang X, Shigenaga MK et al (1996) Immobilization stress causes oxidative damage to lipid, protein, and DNA in the brain of the rats. FASEB J 10:1532–1538
Reznick AZ, Packer L (1994) Oxidative damage to proteins: spectrophotometric method for carbonyl assay. Methods Enzymol 233:357–363
Deneke SM (2000) Thiol-based antioxidants. Curr Top Cell Regul 36:151–180
Wlodek L (2002) Beneficial and harmful effects of thiols. Pol J Pharmacol 54:215–223
Levine RL, Williams JA, Stadtman ER et al (1994) Carbonyl assays for determination of oxidatively modified proteins. Methods Enzymol 233:347–357
Ciancarelli I, Tozzi-Ciancarelli MG, Di Masimo C et al (2004) Flunarizine effects on oxidative stress in migraine patients. Cephalalgia 24:528–532
Acknowledgments
This research received no specific grant from any funding agency in public, commercial, or not-for-profit sectors.
Conflict of interest
The authors declare that there is no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Eren, Y., Dirik, E., Neşelioğlu, S. et al. Oxidative stress and decreased thiol level in patients with migraine: cross-sectional study. Acta Neurol Belg 115, 643–649 (2015). https://doi.org/10.1007/s13760-015-0427-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13760-015-0427-y