Abstract
Cadmium (Cd) poisoning in humans and fish represents a significant global problem. Bacillus cereus (B. cereus) is a widely used probiotic in aquaculture. The objective of this study was to evaluate the potential of B. cereus in ameliorating Cd-induced toxicity in mirror carp. The biosorption rate of Zn for the B. cereus in 85.99% was significantly more than five strains. All fishes were exposed for 30 days to dietary ZnCl2 (30mg/kg), waterborne Cd (1 mg/L), and/or dietary Zn-enriched B. cereus (Zn 30mg/kg and 107cfu/g B. cereus). At 15 and 30 days, the fishes were sampled, and bioaccumulation, antioxidant activity, and intestinal microbiota were measured. Waterborne Cd exposure caused marked alterations in the composition of the microbiota. Dietary supplementation with Zn-enriched B. cereus can reduce the changes in the composition of intestinal microbiota in Cd exposure and decrease the pathogenic bacteria of Flavobacterium and Pseudomonas in Zn-enriched B. cereus groups. The results obtained indicate that Zn-enriched B. cereus can provide a significant protective effect on the toxicity of cadmium by inhibiting alterations in the levels of bioaccumulation and antioxidant enzyme including superoxide dismutase (SOD), catalase (CAT), total antioxidant (T-AOC), and malonaldehyde (MDA). Our results suggest that administration of Zn-enriched B. cereus has the potential to combat Cd toxicity in mirror carp.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Heavy metals with the unknown biological role persist in the natural environment and have the capacity to exert toxic effects at relatively low levels[1]. Cadmium (Cd) is utilized worldwide to facilitate many industrial applications. In addition, Cd, a toxic metal, has been recognized as one of the most deleterious heavy metal pollutants that severely threaten the health of the human and other organisms, particularly aquatic animals because it is not only readily assimilated at late in fish and in mammals along the food chain but it is highly toxic with a long biological half-time of 10–30 years [2]. As a nonessential biological element, Cd is widely toxic, affecting the intestinal microbiota and causing oxidative stress, even leading to death (at high concentrations) [3].
Cd can accumulate at relatively high levels in animals; it can also accumulate in fish upon occurrence of high levels of either waterborne or foodborne Cd [4]. The accumulation of Cd can induce overproduction of reactive oxygen species (ROS) in various organisms, and this overproduction results in oxidative stress [5]. Oxidative stress can also increase the hosts’ susceptibility to damage by promoting the reactivity of ROS with proteins, lipids, and DNA [6]. In addition, gut microbiota plays a crucial role in remaining stable and beneficial on the host health, as they are involved in numerous important physiological, nutritional, immunologic, and metabolic processes [7, 8]. Many authors suggested that taking the adverse effect of heavy metals on the intestines into consideration as well, the gut microbiota, which lives in symbiosis with intestinal epithelial cells, could be affected by oral exposure to toxic metals [9, 10]. In previous reports, Cd exposure has been found to alter the composition and the microbial diversity of gut community [11].
Up to now, for mammals to humans, of the various available dietary supplements (e.g., essential metal, vitamins, edible plants, phytochemicals, and probiotics) [12,13,14,15], Zn and probiotics may potentially reduce the heavy metal toxicity. Zinc (Zn) is the second most abundant essential trace element for animals and humans, and it plays a role in numerous biological processes, such as cell proliferation and differentiation, signal transduction, neurodevelopment, and learning and memory [16]. Zn has been shown to reduce the oxidative damage brought about by free radicals. A few studies reported that Zn alleviated Cd toxicity in fish [17, 18]. Khan et al. reported that Zn-stimulated mucus secretion into the rainbow trout intestine inhibits Cd accumulation and Cd-induced lipid peroxidation [18]. In addition, Zn enrichment can influence the uptake and oxidative damage that is usually attributed to dietary Cd exposure [19]. Probiotics are living microorganisms that are beneficial to an individual’s health, which can protect hosts against a variety of diseases including both bacterial and viral diarrhea [20]. Many reported that probiotics show a potential for alleviating heavy metal toxicity [20]. A few reported that Lactobacillus plantarum (L. plantarum) could reverse the alterations in the intestinal microbiota composition caused by heavy metal, alleviate oxidative stress in tissues, and relieve the changes in hematological parameters [12, 21]. Compared with L. plantarum, Bacillus subtilis is a kind of probiotic and can survive in low pH environments and wider temperatures, which were widely used in aquaculture [22]. Many authors suggested that Bacillus, including Bacillus subtilis or Bacillus cereus, can reduce Cd and Pb accumulation in organs, modulate intestinal microbiota, and change antioxidant activity following Cd or Pb exposure in Carassius auratus gibelio [11, 23, 24].
It has recently been demonstrated that probiotics can absorb or tolerate the micro-macro elements (e.g., Ca, Cu, and Zn) or toxic elements (e.g., Cd and Pb) [12, 25]. Many studies suggested that L. plantarum or Bacillus could tolerate Cd or Pb, which is one of the important foundations against heavy metal toxicity [11, 12, 21, 23]. However, few studies have investigated the effects of Cd and Zn-enriched B. cereus on accumulation in mirror carp. In this study, we screen the six strains of Bacillus in adsorption for Zn. Compared with the other five strains, B. cereus showed better ability in Zn adsorption. In addition, we investigated the effects of Cd and Zn-enriched B. cereus on intestinal microbiota, bioaccumulation, and oxidative stress in mirror carp. The results showed that Zn-enriched B. cereus dietary reduces the concentration Cd of tissues, modulates intestinal microbiota, and changes antioxidant activity following Cd exposure in mirror carp.
Materials and Methods
Bacterial Strain
Six Bacillus strains were used in this study, all of which were obtained from the gut of mirror carp or natural water in Jilin Province (China). This isolation is achieved by reference to the method used in previous studies [26]. Prior to use, the organism was cultured in Luria-Bertani (LB) medium at 30 °C for 18 h using an orbital shaker at 150 rpm under aerobic conditions.
Biosorption of Trace Metal in the Liquid Medium
Six Bacillus strains (B. cereus, B. subtilis, B. megaterium, B. amyloliquefaciens, B. pumilus, and B. licheniformis) were cultured in LB medium containing ZnCl2 (Sinopharm Chemical Reagent Company, Shanghai, China). The design of our experiment was based on the previous study [27]. The strains were exposed to 50mg/L of ZnCl2, and biosorption was evaluated after 3 days of exposure. Briefly, after 24-h subculture at 30 °C on LB medium, cells were harvested off the ager surface (about 50-mg dried mass) and transferred to 50-mL LB medium at 180 rpm. After 12 h of cell growth, Zn was inserted from a pre-prepared stock solution. The control was prepared in the same condition without metal. After 24 h of exposure under these conditions (30°C, 180 rpm), cells were filtered on 0.22-μm membrane and gently rinsed with 50 mL of ultrapure water in order to liberate and collect the metal adsorbed on the fungal cell wall. At the end of the experiment, three new fractions were obtained: the liquid media (fraction A) and the dried mycelium (fraction B). Fraction A and fraction B were mineralized before the analysis of the trace metals. The Zn concentration in food was measured using an Atomic Absorption Spectrophotometer AA-6300 (Shimadzu, Japan). The percentage of absorbed mental was calculated as the quantity of metal absorbed inside the cells (A, fraction B) divided by the initial quantity of metal in the culture media (B): %A=A/B.
Scanning Electron Microscope (SEM) Analysis
The scanning electron microscope was analyzed according to the method described by Wang et al [11]. Briefly, Bacillus cereus was cultured in Luria-Bertani (LB) medium at 30 °C for 12 h using an orbital shaker at 150 rpm under aerobic conditions, and then the cells were transferred to coverslip in 24-well plates in LB medium with 200-mg/L Zn at 30 °C for 4 h. Afterward, the cells were 2.5% (v/v) glutaraldehyde in 0.1 M PBS (pH 7.4) for 1 h at room temperature and then washed with PBS three times. The cells were dehydrated in a graded series of ethanol (30–100%), passed in 100% tertiary butanol (Life Technologies), and then dried in a vacuum freeze dryer and coated with gold. The specimens were examined with a S-4800 Field Emission Scanning Electron Microscope (Hitachi).
Diet Preparation
Mirror carp (45.84 ± 0.68 g; 15.1 ± 0.1 cm) were obtained from a specialized aquatic fry farm (Jilin Province, China) and transported to the laboratory. Commercial feed (crude protein 37.7%, crude lipid 7.4%, and ash 10.8%) is obtained from Jinyanhong Aquarium Products Co., Hangzhou, China, and was used as the basal diet. The fish adapted to laboratory conditions for 2 weeks before the exposure trial. The fish was granted with the commercial feed twice daily at a rate of 1–2% of body weight and sustaining experimental conditions (dissolved oxygen 6.59 ± 0.07 mg/L; pH 7.2 ± 0.3; ammonia less than 0.5 mg/L; nitrites less than 0.05 mg/L; water temperature 25 ± 2 °C; light:dark = 13h:11h). The concentration of B. cereus in the feed was determined by spread plate technique (nutrient agar incubated at 30 °C for 24 h). Experiments were performed according to the research protocols approved by the Institutional Animal Care and Use Committee, Jilin Agricultural University.
Experimental Diets and Experimental Design
At the end of accommodation, 300 fishes were randomly divided into five groups with three replications each (fifteen fish per tank) and resided in 80-L plastic tanks. Waterborne Cd (1 mg/L) and/or dietary Zn-enriched B. cereus (including Zn 30-mg/kg and 107-cfu/g B. cereus) and/or dietary ZnCl2 (30mg/kg). The groups were distributed as follows: C0Z0B0 (control group), C0Z1B0 (Zn 30mg/kg), C0Z1B1 (Zn 30mg/kg and B. cereus 107cfu/g), C1Z0B0 (Cd 1mg/L), and C1Z1B1 (Cd 1mg/L, Zn 30mg/kg, and B. cereus 107cfu/g). During the excrement, the condition was the same as accommodation. The experiment lasted for 30 days. The control group was fed a basal diet with the same volume of sterile saline, and all food was stored at 4 °C until use. The Zn concentration in food was measured using an Atomic Absorption Spectrophotometer AA-6300 (Shimadzu, Japan). A total of 50-mL water samples were collected from fish tanks of different groups, and the Cd level was analyzed using the same method as tissues. The actual concentration of waterborne Cd and dietary-borne Zn was presented in Table 1. In addition, for steadying the Cd concentration in water, Cd was added according to the experimental concentration, and the water was substituted once a day with half a tank of water.
Sample Collection
The fishes were fasting for 24 h before collecting samples. Six fishes were randomly selected from each tank for 15 and 30 days to collect tissue samples and blood samples. The fishes were euthanized with 300 mg/L of methanesulfonate 222 (MS-222). Tissue samples were then subsequently collected as spleen and gut. The blood samples were collected using a syringe treated with heparin and then separated by centrifugation at 4000 g for 5 min at 4 °C immediately. All samples were frozen in liquid nitrogen and stored at −80 °C until the experimental assays.
Analysis of Gut Microbial Diversity
As very few gut contents could be collected from the fish in the groups, samples were pooled by tank (n = 3, each group). Genomic DNA was extracted from fecal samples using the E.Z.N.A. Soil DAN Kit (Omega Bio-tek, Norcross, GA, USA), following the standard protocol. The V3–V4 region of the bacterial 16S ribosomal RNA gene was amplified by PCR using the primers 338F 5′-ACTCCTACGGGAGGCAGCAG-3′ and 806R 5′-GGACTACHVGGGTWTCTAAT-3′, where the barcode is an eight-base sequence unique to each sample. The PCR reactions were performed in triplicate, and the mixture consisted of 10-ng template DNA, 2-μL dNTPs, 0.8-μL forward primer, 0.8-μL reverse primer, 4-μL 5 × FastPfu Buffer, 0.4-μL FastPfu Polymerase, and ddH2O in a final volume of 20 μL and the PCR amplification program according to the method described by Wang et al [28].
Amplicons were extracted from 2% agarose gels, purified using the AxyPrep DNA Gel Extraction Kit (Axygen Biosciences, CA, USA) and quantified using QuantiFluor™-ST (Promega, USA). Then, purified amplicons were pooled and paired-end sequenced (2 × 300) on an Illumina MiSeq platform (Illumina Inc., San Diego, CA, USA) according to the manufacturer’s instructions. The raw fastq files were de-multiplexed and quality-filtered using QIIME (version 1.17). Operational units (OTUs) were clustered with a 97% similarity cutoff using UPARSE. And chimeric sequences were identified and removed using UCHIME. The taxonomy of each 16S rRNA gene sequence was analyzed using the RDP Classifier against the SILVA (SSU115) 16S rRNA database using a confidence threshold of 70%.
Cd Accumulation
Five tissues (gill, kidney, gut, liver, and muscle) from each group were sampled on days 15 and 30 to observe Cd concentration. Tissues were enclosed in a digestion vessel and mixed with 10 mL of concentrated nitric acid (65% HNO3). The digestion reaction occurred from 60 °C to a finial temperature of 95 °C in the hot plate until the samples were completely dissolved. The clear liquid was diluted with demonized water to 20 mL and then assayed for concentration of Cd using an Flame Atomic Absorption Spectrophotometer AA-6300 (Shimadzu, Japan) at minimum measured concentration of 1 μg/L (0.001 mg/L). In this study, the standard reference material is 99.99 % of Cd and Zn standard. In addition, the Cd concentration in organ is determined by a third-party testing agency (Pony Testing International Group Co, Ltd, China). Quality assurance and quality control are carried out in accordance with laboratory standards (ISO/IEC 17025/ ISO/IEC 17020).
Antioxidant Response Analysis
The liver was studied for antioxidant activity on days 15 and 30 days of the feeding period. Superoxide dismutase (SOD), catalase (CAT), total antioxidant (T-AOC), and malonaldehyde (MDA) were determined using commercially available Elisa kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, Jiangsu, China) according to the method described by Dai et al [29].
Statistical Analysis
All data were submitted to analysis of variance, ANOVA, followed by Tukey’s test and using data analysis software SPSS 20.0 (SPSS, Chicago, IL, USA). The results were expressed as mean ± standard deviation (SD) and differences with P values of < 0.05 were considered to be statistically significant.
Results
Biosorption Experimentation and SEM Analysis
Table 1 shows the biosorption percentages measured under the different conditions of our experiment.
The biosorption rate of Zn for the B. cereus was 85.99% (729.83 mg/kg) on the LB medium, but only Zn concentrations as low as 76.77% (651.13 mg/kg), 69.18% (586.75), 67.7% (574.21 mg/kg), 70.25% (595.83 mg/kg), and 81.56% (691.75 mg/kg) were able to absorb completely B. subtilis, B. megaterium, B. amyloliquefaciens, B. pumilus, and B. licheniformis. Compared with the other strain, B. cereus has shown the highest biosorption rate. The SEM result was indicated in Fig. 1. Compared with the control group (Fig. 1a), the cell morphology of B. cereus with Zn treatment showed a complete cell morphology and zinc adsorption particles Table 2.
Gut Microbial Diversity
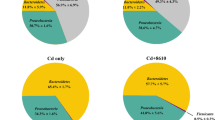
As shown in Fig. 2 and Table 3, gut microbial diversity in the gut during 30 days is presented. At a threshold of 97% sequence identity, a total of 1146 OTUs were identified in the present study. Briefly, mean of 814, 1013, 816, 925, and 963 OTUs were assigned to C0Z0B0, C0Z1B0, C0Z1B1, C1Z0B0, and C1Z1B1 groups (Fig. 2a). In the phylum (Fig. 2c) and genus (Fig. 2d) levels, both Cd exposure and B. cereus treatment altered the structure and composition of the fish gut microbiota. Compared with the control group, Photobacterium sp. was significantly decreased in other groups; Flavobacterium, uncultured_bacterium_f_Micrococcaceae, and Pseudomonas were significantly increased in C1Z0B0 and C1Z1B1 groups; Cetobacterium was significantly increased in C0Z1B0; uncultured_bacterium_f_Mycoplasmataceae was significantly decreased in C1Z0B0 and C1Z1B1; and uncultured_bacterium_f_Mycoplasmataceae was significantly increased in C0Z1B1.
Five groups of gut microbiota in OTUs (a) and Venn (b); community bar-plot analysis shows relative abundance of gut microbiota in each group at the phylum level (c) and genera level (d). C0Z0B0 (control group), C0Z1B0 (Zn 30mg/kg), C0Z1B1 (Zn 30mg/kg and B. cereus 107cfu/g), C1Z0B0 (Cd 1mg/L), C1Z1B1 (Cd 1mg/L, Zn 30mg/kg and B. cereus 107cfu/g)
Cd Accumulation
Zn-enriched B. cereus significantly reduced the levels of the concentration of Cd in tissues (Fig. 3). Compared with the control group, accumulation of Cd in the tissues increased significantly following exposure to waterborne cadmium with a positive correlation to the concentration. In addition, compared with the Cd-only administration group, Cd concentration in all studied tissues was significantly decreased (P < 0.05) in the Zn-enriched B. cereus group.
Antioxidant Responses
The antioxidant responses to the liver of common carp was evaluated by measuring SOD, CAT, MDA, and T-AOC activity following exposure to waterborne Cd and/or dietary Zn-enriched B. cereus (Fig. 4).
Compared with the control group, SOD and CAT were significantly increased in the 30-mg/kg Zn and Zn-enriched B. cereus group, but significantly decreased in the Cd exposure group (1-mg/L Cd and Zn-enriched B. cereus plus Cd groups); however, MDA and T-AOC were significantly decreased in the 30 mg/kg Zn and Zn-enriched B. cereus group, but significantly increased in the Cd exposure group (1mg/L Cd). Compared with the only 1 mg/L Cd group, SOD and CAT were significantly increased in the Zn-enriched B. cereus plus Cd group; however, MDA and T-AOC were significantly decreased in the Zn-enriched B. cereus plus Cd group.
Discussion
Fish is rich in multiple essential nutrients and proteins, which can also be easily digested and absorbed by human. However, as industrial development progresses, the general public is facing serious Cd pollution problems [30]. Due to the bioaccumulation, Cd was accumulated to higher levels in fish with a risk for consumers [31]. In addition, Cd accumulation causes intestinal flora disorders and oxidative stress [10, 32]. Therefore, it is of importance to develop innovative dietary therapeutic strategies for the prevention and treatment of Cd toxicity. The aim of this study consisted of Bacillus strains with potential properties against Cd toxicity in common carp.
Probiotics, including Bacillus and lactic acid bacteria, are living microorganisms that are beneficial to an individual’s health. It has been reported that lactic acid bacteria are effective in alleviating oxidative stress induced by several diseases. In addition, Zhai et al (2014) demonstrated that lactic acid bacteria have a strong cadmium tolerance and adsorption [25]. Apart from Cd tolerance and adsorption, lactic acid bacteria also have a strong lead, gastrointestinal properties, and perfluorooctanoate tolerance and adsorption [12, 25, 33]. Bacillus strains were widely used as a probiotic in aquaculture because it is able to tolerate a wide range of temperatures and low pH. In previous study, our data suggest that Bacillus can tolerate the higher concentration of Cd [32]. In addition, the minimal inhibitory concentration (MIC) of Cd for B. cereus was 300 mg/L on the LB medium (previous study). In this study, we screened six Bacillus for zinc adsorption and transformation ability. Interestingly, B. cereus also shows the strongest for Zn biosorbed in vitro. Meanwhile, the SEM result shows the same as adsorption result. The cadmium tolerance and zinc adsorption capacity of B. cereus in vitro provide a strong theoretical basis for reducing cadmium toxicity in vivo.
Gut microbiota play a role in remaining stable and beneficial on the host’s health, as they are involved in numerous important physiological, nutritional, immunologic, and metabolic processes. Mu et al. suggested that Cd can induce a significant change in the intestinal microbiota composition of Rana chensinensis tadpoles [34]. Similar results were also observed in Carassius auratus gibelio and Nile tilapia [11, 35]. Previous reports have shown that there are four phyla of dominant bacteria in the intestinal tract of common carp (Cyprinus carpio L.): Fusobacteria, Proteobacteria, Firmicutes, and Bacteroidetes [36]. In this study, the dominant bacteria at the phylum level of mirror carp was Proteobacteria, Firmicutes, Actinobacteria, and Bacteroidetes. In our previous study, Cd or B. cereus treatment was found to have significantly decreased the population of the Proteobacteria and considerably increased the Firmicutes at the phylum level in the gut of the Carassius auratus gibelio [11, 32]. Our results are consistent with previous studies at the phylum level in the gut of the common carp. Interestingly, Photobacterium sp. is the dominant bacteria of common carp intestines. In this study, this phenomenon is distinct from other studies [10, 34]. This may be related to the environment in mirror carp. In addition, in this study, our data suggested that Photobacterium sp. was significantly decreased in all experimental groups compared with the control group. Currently, we have isolated Photobacterium sp. in the common carp intestine (data unpublished), and then the function of Photobacterium sp. will be studied next. A few reports suggested that the pathogenic bacteria in fish were significantly increased in cadmium exposure [5, 10, 11]. A few authors suggested that Flavobacterium and Pseudomonas have been mainly isolated from fish, suggesting that they could be saprophytic or commensal organisms that may act as opportunistic fish pathogens [37, 38]. In this study, our data suggested that Flavobacterium and Pseudomonas were significantly increased in 1-mg/L Cd group. However, Flavobacterium and Pseudomonas were significantly increased in Zn-enriched B. cereus. Many reports suggested that reduced proportion of pathogenic bacteria can effectively alleviate intestinal inflammation [39, 40]. Cetobacterium is the most dominant microbiota in the intestine of fish, and this bacterium has been proven to produce vitamin B12 [41, 42]. Our results demonstrated that the abundance of Cetobacterium was reduced in 1-mg/L Cd groups. In previous studies, we observed the same result. Therefore, the increased abundance of these bacteria may enhance cadmium isolation in the gut due to cellular accumulation and biological clearance mechanisms.
It is reported that the concentration of Cd can be increased in the tissues of aquatic animals through accumulation [4]. The bioaccumulation pattern of waterborne Cd in different tissues of Mugil cephalus is liver > gill > muscle > alimentary canal > heart [43]. Wang et al. demonstrated that the order of distribution of Cd in the organs was intestine > kidney > liver > gill > muscle [32]. Uptake routes (whether waterborne or diet-borne) of toxic substances affect tissue accumulation of toxicants in aquatic animals [44]. Liu et al. (2015) suggested that the profile of Cd accumulation in the organs of juvenile cobia was kidneys > liver > gut > gills [45]. In this study, the bioaccumulation pattern of waterborne Cd in different tissues was kidney > gut > liver > muscle > gill. In previous study, we reported that the B. cereus can reduce the Cd accumulation in Carassius auratus gibelio [32]. In addition, a few studies reported that dietary Zn can reduce the Cd accumulation in fathead minnow (Pimephales promelas) and rainbow trout (Oncorhynchus mykiss) [17, 18]. Our results suggested that B. cereus shows the strongest for Zn biosorbed in vitro. In addition, we suggested that the Zn-enriched B. cereus can reduce the Cd accumulation in the organ of mirror carp. Many reports suggested that the accumulation of Cd can induce overproduction of reactive oxygen species (ROS) in many organisms, and this overproduction results in oxidative stress [34]. Wu et al. (2019) demonstrated that oxidative stress was significantly increased following waterborne Cd (299.6 μg/L) in female rare minnow (Gobiocypris rarus) [46]. Antioxidant enzymes, including SOD, CAT, and T-AOC, are the primary line of defense against ROS. Wang et al. (2020) reported that Cd can decrease SOD and CAT activity in human gastric epithelial cells [47]. A parallel study in grass carp kidney cells [48]. MDA, a product of lipid peroxidation, is a major contributor to the loss of cell function under oxidative stress. A few studies indicated that increased lipid peroxide levels (MDA levels) after Cd administration were previously demonstrated in rats [49]. In addition, Zhai et al (2014) reported that Lactobacillus plantarum CCFM8610 alleviates Cd toxicity in mice by oxidative stress (enhance SOD and CAT activity) and tissue damage [21]. In this study, our data suggested that SOD and CAT were significantly decreased in 1-mg/L Cd group and MDA and T-AOC were significantly increased in 1 m-/L Cd. In addition, dietary Zn and dietary Zn-enriched B. cereus were significantly alleviated the oxidative stress in mirror carp. This finding is supported by the observation that Zn or B. cereus can enhance animal antioxidant activity. The accumulation of Cd in the organ of fish fed the Zn-nonenriched and Zn-enriched B. cereus was the likely causation of the localized oxidative stress. The antioxidative ability might be one of the main mechanisms for Zn-enriched B. cereus to alleviate Cd toxicity which needs to be further confirmed.
Conclusion
In conclusion, our results suggested that Zn-enriched B. cereus can modulate intestinal microbiota, reduce Cd accumulation in organ, and change antioxidant activity following Cd exposure in mirror carp. These results indicated that Zn-enriched B. cereus may be useful as supplement to minimize the toxic side effects of cadmium in aquaculture.
References
Taweel A, Shuhaimi-Othman M, Ahmad AK (2013) Assessment of heavy metals in tilapia fish (Oreochromis niloticus) from the Langat River and Engineering Lake in Bangi, Malaysia, and evaluation of the health risk from tilapia consumption. Ecotoxicol Environ Saf 93:45–51
Deng G, Li M, Li H, Yin L, Li W (2014) Exposure to cadmium causes declines in growth and photosynthesis in the endangered aquatic fern (Ceratopteris pteridoides). Aquat Bot 112:23–32
Almeida JA, Diniz YS, Marques SFG, Faine LA, Ribas BO, Burneiko RC, Novelli ELB (2002) The use of the oxidative stress responses as biomarkers in Nile tilapia (Oreochromis niloticus) exposed to in vivo cadmium contamination. Environ Int 27:673–679
Heydarnejad MS, Khosravian-Hemamai M, Nematollahi A (2013) Effects of cadmium at sub-lethal concentration on growth and biochemical parameters in rainbow trout (Oncorhynchus mykiss). Ir Vet J 66
Lang Q, Guo Z, Feili Y, Cong Y, Ting Z (2019) Single and combined exposures of waterborne Cu and Cd induced oxidative stress responses and tissue injury in female rare minnow (Gobiocypris rarus). Comp Biochem Physiol Toxicol Pharmacol
Farombi EO, Adelowo OA, Ajimoko YR (2007) Biomarkers of oxidative stress and heavy metal levels as indicators of environmental pollution in African cat fish (Clarias gariepinus) from Nigeria Ogun River. Int J Environ Res Public Health 4:158–165
Thaiss CA, Zmora N, Levy M, Elinav E (2016) The microbiome and innate immunity. Nature 535:65–74
Gómez GD, Balcázar JL (2010) A review on the interactions between gut microbiota and innate immunity of fish. FEMS Immunol Med Microbiol 52:145–154
Larsen AM, Mohammed HH, Arias CR (2014) Characterization of the gut microbiota of three commercially valuable warmwater fish species. J Appl Microbiol 116
Chang X, Li H, Feng J, Chen Y, Nie G, Zhang J (2019) Effects of cadmium exposure on the composition and diversity of the intestinal microbial community of common carp (Cyprinus carpio L.). Ecotoxicol Environ Saf 171:92–98
Wang N, Zhengyao G, Yilin Z, Peijun Z, Jia L, Yi C, Lei Z, Yuehong L (2020) Effect on intestinal microbiota, bioaccumulation, and oxidative stress of Carassius auratus gibelio under waterborne cadmium exposure. Fish Physiol Biochem:1–8
Tian F, Zhai Q, Zhao J, Liu X, Wang G, Zhang H, Zhang H, Chen W (2012) Lactobacillus plantarum CCFM8661 alleviates lead toxicity in mice. Biol Trace Elem Res 150:264–271
Luchese C, Brandão R, Oliveira RD, Nogueira CW, Santos FW (2007) Efficacy of diphenyl diselenide against cerebral and pulmonary damage induced by cadmium in mice. Toxicol Lett 173:181–190
El-Sokkary GH, Awadalla EA (2011) The protective role of vitamin C against cerebral and pulmonary damage induced by cadmium chloride in male adult albino rat. Open Neuroendocrinol J 411:1–8
Pérez Díaz MFF, Acosta M, Mohamed FH, Ferramola ML, Oliveros LB, Gimenez MS (2013) Protective effect of soybeans as protein source in the diet against cadmium-aorta redox and morphological alteration. Toxicol Appl Pharmacol 272:806–815
Chung MJ, Walker PA, Brown RW, Hogstrand C (2005) ZINC-mediated gene expression offers protection against H2O2-induced cytotoxicity. Toxicol Appl Pharmacol 205:225–236
Driessnack MK, Jamwal A, Niyogi S (2017) Effects of chronic waterborne cadmium and zinc interactions on tissue-specific metal accumulation and reproduction in fathead minnow (Pimephales promelas). Ecotoxicol Environ Saf 140:65–75
Khan FR, Mcgeer JC (2013) Zn-stimulated mucus secretion in the rainbow trout (Oncorhynchus mykiss) intestine inhibits Cd accumulation and Cd-induced lipid peroxidation. Aquat Toxicol 142-143:17–25
Banni M, Chouchene L, Said K, Kerkeni A, Messaoudi I (2011) Mechanisms underlying the protective effect of zinc and selenium against cadmium-induced oxidative stress in zebrafish Danio rerio. Biometals 24:981–992
Halttunen, Teemu, Salminen, Seppo, and, Meriluoto, Jussi, Tahvonen (2008) Reversible surface binding of cadmium and lead by lactic acid and bifidobacteria. Int J Food Microbiol
Zhai Q, Wang G, Zhao J, Liu X, Narbad A, Chen YQ, Zhang H, Tian F, Chen W (2014) Protective effects of Lactobacillus plantarum CCFM8610 against chronic cadmium toxicity in mice indicate routes of protection besides intestinal sequestration. Appl Environ Microbiol 80:4063–4071
Lee S, Katya K, Park Y, Won S, Seong M, Hamidoghli A, Bai SC (2017) Comparative evaluation of dietary probiotics Bacillus subtilis WB60 and Lactobacillus plantarum KCTC3928 on the growth performance, immunological parameters, gut morphology and disease resistance in Japanese eel, Anguilla japonica. Fish Shellf Immunol
Yin Y, Zhang P, Yue X, Du X, Li W, Yin Y, Yi C, Li Y (2018) Effect of sub-chronic exposure to lead (Pb) and Bacillus subtilis on Carassius auratus gibelio: bioaccumulation, antioxidant responses and immune responses. Ecotoxicol Environ Saf 161:755–762
Yulin Y, Xinyan Y, Dongming Z, Peijun Z, Abedin A, Yuwei Y, Yanan C, Yuehong L (2019) Study of bioaccumulation, hematological parameters, and antioxidant responses of Carassius auratus gibelio exposed to dietary lead and Bacillus subtilis. Biol Trace Elem Res:1–8
Zhai Q, Yin R, Yu L, Wang G, Tian F, Yu R, Zhao J, Liu X, Chen YQ, Zhang H (2015) Screening of lactic acid bacteria with potential protective effects against cadmium toxicity. Food Control 54:23–30
Wang L, Ge C, Wang J, Dai J, Zhang P, Li Y (2017) Effects of different combinations of Bacillus on immunity and antioxidant activities in common carp. Aquac Int 25:2091–2099
Albert Q, Leleyter L, Lemoine M, Heutte N, Rioult J-P (2018) Comparison of tolerance and biosorption of three trace metals (Cd, Cu, Pb) by the soil fungus Absidia cylindrospora. Chemosphere Environ Toxicol Risk Assess
Hao Wang YJ, Yin C, Deng M, Tang T, Deng B, Ren W, Deng J, Yin Y (2018) Chengquan Tan, Differential analysis of gut microbiota correlated with oxidative stress in sows with high or low litter performance during lactation. Front Microbiol 9:1665
Dai J, Zhang L, Du X, Zhang P, Li W, Guo X, Li Y (2018) Effect of lead on antioxidant ability and immune responses of crucian carp. Biol Trace Elem Res:1–8
Minh ND, Hough RL, Thuy LT, Nyberg Y, Mai LB, Vinh NC, Khai NM, Born I (2012) Assessing dietary exposure to cadmium in a metal recycling community in Vietnam: age and gender aspects. Sci Total Environ 416:164–171
Almeida JA, Novelli ELB, Silva MDP, Júnior RA (2001) Environmental cadmium exposure and metabolic responses of the Nile tilapia, Oreochromis niloticus. Environ Pollut 114:169–175
Wang N, Jiang M, Zhang P, Shu H, Li Y (2019) Amelioration of Cd-induced bioaccumulation, oxidative stress and intestinal microbiota by Bacillus cereus in Carassius auratus gibelio. Chemosphere 245:125613
Jiali X, Fan W, Qi X, Boxing Y, Dongsheng F (2016) Screening of potential probiotic lactic acid bacteria based on gastrointestinal properties and perfluorooctanoate toxicity. Appl Microbiol Biotechnol
Mu D, Meng J, Xiaoxue M, Xiao H, Wang H (2018) The effect of cadmium exposure on diversity of intestinal microbial community of Rana chensinensis tadpoles. Ecotoxicol Environ Saf 154:6–12
Zhai Q, Yu L, Li T, Zhu J, Zhang C, Zhao J, Zhang H, Chen W (2017) Effect of dietary probiotic supplementation on intestinal microbiota and physiological conditions of Nile tilapia (Oreochromis niloticus) under waterborne cadmium exposure. Antonie Van Leeuwenhoek 110:501–513
Li T, Long M, Gatesoupe FOJL, Zhang Q, Li A, Gong X (2015) Comparative analysis of the intestinal bacterial communities in different species of carp by pyrosequencing. Microb Ecol 69:25–36
Wei C, Zeng Y, Jiao TN (2009) Comparison of bacterioplankton communities in three mariculture ponds farming different commercial animals in subtropical Chinese coast. Hydrobiologia 632:107–126
Kayansamruaj P, Dong HT, Hirono I, Kondo H, Senapin S, Rodkhum C (2017) Comparative genome analysis of fish pathogen Flavobacterium columnare reveals extensive sequence diversity within the species, Infection. Genet Evol 2017
Zhang D, Thongda W, Li C, Zhao H, Beck BH, Mohammed H, Arias CR, Peatman E (2017) More than just antibodies: protective mechanisms of a mucosal vaccine against fish pathogen Flavobacterium columnare. Fish Shellfish Immunol 71:160–170
Ling G, Cheng M, Wen X, Xian M, Xia H (2017) Molecular cloning, biological effect, and tissue distribution of interleukin-8 protein in mandarin fish (Siniperca chuasti) upon Flavobacterium columnare infection. Fish Shellfish Immunol 66:112
Jiajia N, Yuhe Y, Tanglin Z, Lei (2012) Comparison of intestinal bacterial communities in grass carp, Ctenopharyngodon idellus, from two different habitats. Chin J Oceanol Limnol
Tsuchiya C, Sakata T, Sugita H (2010) Novel ecological niche of Cetobacterium somerae, an anaerobic bacterium in the intestinal tracts of freshwater fish. Lett Appl Microbiol 46:43–48
Hilmy AM, Shabana MB, Daabees AY (1985) Bioaccumulation of cadmium: toxicity in Mugil cephalus. Comp Biochem Physiol C Comp Pharmacol 81:139–144
Kim JH, Kang JC (2014) The selenium accumulation and its effect on growth, and haematological parameters in red sea bream, Pagrus major, exposed to waterborne selenium. Ecotoxicol Environ Saf 104:96–102
Liu S, Chi K, Dong X, Tan B, Yang Q (2015) Toxic effects of two sources of dietborne cadmium on the juvenile cobia, Rachycentron canadum L. and tissue-specific accumulation of related minerals. Aquat Toxicol
Wu L, Qiuguo G, Zhang F, Yingying C, Yuan T, Zaizhao (2019) Single and combined exposures of waterborne Cu and Cd induced oxidative stress responses and tissue injury in female rare minnow (Gobiocypris rarus). Compar Biochem Physiol Toxicol Pharmacol
Wang K, Ma JY, Li M, Qin YS, Ma LQ (2020) Mechanisms of cd and cu induced toxicity in human gastric epithelial cells: oxidative stress, cell cycle arrest and apoptosis. Sci Total Environ:143951
Yin Y, Zhang P, Liu J, Wang N, Li Y (2020) Amelioration of Cd-induced oxidative stress, mt gene expression, and immune damage by vitamin C in grass carp kidney cells. Biol Trace Elem Res 194(1):552–559
Marina N, Aleksandra PA, Jelena D, Ivana M, Dina M, Anja P (2015) Toxicity of oral cadmium intake: impact on gut immunity. Toxicol Lett 237(2):89–99
Acknowledgements
The work was supported by the Natural Science Foundation of Jilin Province (Project Number: 20190201179JC), State Key Laboratory of Freshwater Fish Developmental Biology Project (Project Number. 2019KF001), and Jilin Province Industrial Technology Research and Development Special Project (Project Number: 2019C059-5).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics Approval
All experiments and handling of the animals were conducted according to the research protocols approved by the Institutional Animal Care and Use Committee, Jilin Agricultural University.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, N., Yin, Y., Xia, C. et al. Zn-Enriched Bacillus cereus Alleviates Cd Toxicity in Mirror Carp (Cyprinus carpio): Intestinal Microbiota, Bioaccumulation, and Oxidative Stress. Biol Trace Elem Res 200, 812–821 (2022). https://doi.org/10.1007/s12011-021-02657-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-021-02657-7