Abstract
Zinc takes part in several of cellular signaling pathways, containing defense against free radicals, apoptosis, and inflammation. However, interaction between zinc and prostate cancer progression is poorly understood. Therefore, zinc treatment in DU-145 human prostate cancer cells was investigated. First, zinc sulfate (ZnSO4) concentrations with antiproliferative effect were determined using MTT assay. Then, ZnSO4-induced oxidative damage was evaluated by malondialdehyde (MDA) levels, glutathione (GSH) levels, total oxidant status (TOS) levels, and total antioxidant status (TAS) levels. Apoptotic effects of ZnSO4 were determined by measuring biochemical and immunohistochemical parameters including caspase 3 (CASP3), cytochrome C (CYC), Bcl-2-associated X protein (Bax), and B cell CLL/lymphoma 2 (Bcl-2) levels. Inflammatory effects of ZnSO4 were investigated by measuring interleukin-6 (IL-6) levels and tumor necrosis factor-alpha (TNF-α) levels. Finally, morphological analysis was performed using hematoxylin-eosin staining. We found that ZnSO4 caused a concentration-dependent increase in oxidative stress, apoptosis, and inflammation pathways. Moreover, there were a number of morphological alterations in treated cells depending on the ZnSO4 concentration. Consequently, our data showed that zinc acts as a regulator of increased oxidative damage and apoptosis through the upregulation of TNF-α and IL-6.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Prostate cancer is globally among the first five cancer cases in terms of incidence and mortality rate [1]. Prostate cancer is usually the most common malignancy that affects 1 in 5 men, and each year ~1.5 million men are diagnosed with and over 300,000 men die due to prostate cancer [2]. Despite improvements in early diagnosis, ~ 50% of men recrudesce after prostatectomy [3]. Therefore, prostate cancer is an important part of health problems for men. Recently, the correlation between eating habits and cancer threat has increased the interest of researchers day by day. Normal prostate cells have the highest zinc concentration, and the alteration of zinc levels in the prostate tissue is related to the progress of prostate cancer [4]. It was suggested that decreased levels of zinc in cancer patients could be a probable correlation between zinc and cancer development [5]. Furthermore, studies have demonstrated that is a relationship between dietary compounds and reduced menace for prostate cancer [6, 7].
Zinc is a significant trace element required for many signaling pathways in the human body by acting as a cofactor of more than 300 enzymes. These enzymes are related to the proliferation, metabolism, and functions of cells [8]. Zinc concentrations throughout the prostate can be prominently about 10 times more than typically found in other tissues. Notably, malignant prostate cells lose the talent of zinc gathering, which considerably diminishes zinc concentrations in the cells. It is supposed that this reduction contributes to the fate of prostate carcinogenesis, since androgen-dependent and androgen-independent prostate cancer cells have generally been declared to own a fairly poor zinc concentration [9]. A previous study showed that zinc treatment inhibited cell proliferation and increased apoptosis by inducing Bcl-2-associated X protein (Bax) and B cell CLL/lymphoma 2 (Bcl-2) expressions in prostate cancer cells [10]. Additionally, it was reported that zinc supplementation in nude mice grafted with PC3 cells correlated with decreased prostate tumor growth [11]. Furthermore, high concentrations of zinc are toxic to cells, and also, it induces a number of intracellular pathways provoking reactive oxygen species (ROS) generation [12, 13]. However, connections among zinc, apoptosis, and oxidative mechanisms in prostate cancer are not well understood.
Tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6), which have pleiotropic effects on proliferation and differentiation of tumor cells, are multifunctional inflammatory markers that can be generated by cancer cells [14, 15]. TNF-α and IL-6 induce a number of cellular pathways, which regulate invasion and metastasis of cancer cells [16]. A previous study showed that DU-145 and PC3 androgen-independent prostate cancer cell lines can secrete IL-6 and TNF-α in vitro. This cytokines normally have a role in cancer growth at low levels (0.05–0.5 U/mL), but enhanced levels (5–50 U/mL) dose-dependently prevent the cell growth [17].
Zinc has promise for cancer therapy for its potential involvement in the suppression of tumor growth. However, there are few published observations about zinc treatment on the inflammatory response, apoptotic processes, and oxidative stress processes in prostate cancer cells. The aim of the present study was to focus on further investigating the effects of zinc sulfate (ZnSO4) on DU-145 relevant to cell death pathways in vitro. Inhibitory effects of ZnSO4 on DU-145 cell line were investigated by measuring some inflammatory, apoptotic, and oxidative parameters including IL-6 levels, TNF-α levels, caspase 3 (CASP3) levels, cytochrome C (CYC) levels, malondialdehyde (MDA) levels, glutathione(GSH) levels, total oxidant status (TOS) levels, and total antioxidant status (TAS) levels. In addition, morphological changes were investigated with hematoxylin-eosin staining and Bcl-2 and Bax proteins were determined immunohistochemically to show apoptotic situations in cells. Our data showed that ZnSO4 inhibits cell proliferation, causes morphological changes, and triggers apoptotic and oxidative pathways in DU-145.
Materials and Methods
Cell Culture
Human prostate cancer cells (DU-145) were purchased from the American Type Culture Collection (ATCC, Rockville, MD, USA) and were seeded in DMEM supplemented with 10% (v/v) fetal calf serum, 100 U/mL penicillin and 100 μg/mL streptomycin at 37 °C in a humidified atmosphere containing 95% air and 5% CO2. The cells were cultured in 75 cm2 cell culture flasks. Sufficiently confluent (80–90%) cells were laved with phosphate-buffered saline (PBS; pH 7.2), rewashed with EDTA, and budged with 0.25% trypsin-1 mM EDTA solution.
MTT Viability Assay
DU-145 cells were grown at a density of 5 × 103 cells per well in 96-well plates for MTT assay. Firstly, cells were allowed to adhere to flasks for 1 day before experiment. The cells were then incubated with ZnSO4 concentrations (0 to 1 mM) for 24 h except the control group. The 3-(4,5-dimethylthiazol2-yl)-2,5-diphenyltetrazoliumbromide (MTT) viability/cytotoxicity analysis was realized according to the Mosmann method [18]. Following the 24-h treatment, the cell media were discarded, and 100 μL of 0.5 mg/mL MTT diluted in a medium was added to the wells. After a 4-h incubation period at 37 °C, the formazan salts were dissolved with 100 μL DMSO by shaking. Finally, absorbance values of each well were quantified at a wavelength of 570 nm in ELISA reader (800TS, BioTek Instruments, Winooski, VT, US). Viability of ZnSO4-treated cells was calculated in reference to the untreated control cells by using the following formula:
- OD:
-
Optical density
IC25, IC50, and IC75 were estimated by the graph drawn with the help of viability percentages. These values are used for all analysis except for hematoxylin-eosin staining and immunocytochemistry. IC50 and IC75 were used for hematoxylin-eosin staining. IC25 and IC50 were used for immunocytochemistry.
Cell Lysate Preparation
Du-145 cells were lysed according to the following method to determine levels of MDA, GSH, TOS, TAS, CASP3, CYC TNF-α, and IL-6. Initially, ZnSO4 treatment (337.3, 495.7, and 653.9 μM), cells were washed with cold PBS. Following, the cells allocated by trypsin were centrifuged at 300×g for 10 min at 4 °C and then washed three times in ice-cold PBS. The cells were treated with RIPA lysis buffer (sc-24948, Santa Cruz Biotechnology, CA, USA) for 30 min at room temperature and centrifuged at 16000×g for 10 min at 4 °C to remove residues. Protein contents in cell lysates were determined by the Lowry et al. [19] method. The cell lysates were utilized for aforementioned assays.
Measurements of Oxidative and Antioxidant Markers
To determine the oxidative stress-induced lipid peroxide assay in cells, MDA levels were measured according to the method reported by Ohkawa et al [20]. In short, the pink color intensity resulted from by incubation of thiobarbituric acid and MDA for 1 h at 100 °C was measured at 532 nm. The MDA amounts were displayed as nmol/mg protein.
GSH levels, one of the important antioxidant molecules for the cell, were measured according to Srivastava and Beutler method [21]. The color density resulting from GSH and 5,5′-dithiobis (2-nitrobenzoic acid) reaction with each other was measured at 412 nm. The data were expressed as μmol/mg protein.
TOS and TAS levels in cells were measured spectrophotometrically (Epoch, BioTek) according to the manufacturer’s instructions (Rel Assay Diagnostics, Gaziantep, Turkey). Shortly, the measurement of TAS levels in cells was based on the reduction of 3-ethylbenzothiazoline-6-sulfonate (ABTS) by antioxidants. The reduced ABTS levels were measured at 660 nm. TOS levels are based on the measurement of color intensity at 530 nm by oxidation of ferrous iron to ferric iron. The TAS and TOS contents were shown as mmol Trolox Equiv/L and μmol H2O2 Equiv./L, respectively.
Measurement of Apoptotic Markers
Apoptosis in cells was determined biochemically by measuring CASP3 and CYC levels with a commercially available ELISA kit (Cloud-Clone Corp., USA) according to the manufacturer’s instructions. The CASP3 and CYC levels were detected at 450 nm. Measurements were performed using a microplate spectrophotometer (Epoch, BioTek) device. The results of CASP3 and CYC were indicated as ng/mL.
Measurement of Inflammatory Markers
Inflammatory cytokine levels (TNF-α and IL-6) were measured using a commercial kit (Cloud-Clone Corp., USA, cat no. SEA133Ra and SEA079Ra). The concentrations of TNF-α and IL-6 in serum samples were shown as pg/mL in comparison with the optical density of the standard curve.
Hematoxylin-Eosin Staining
Hematoxylin and eosin stains are commonly used for investigating morphological changes of cells [22]. Firstly, DU-145 cells were grown at a density of 3 × 105 in 6-well plates and subsequently treated with determined concentrations of ZnSO4 (495.7 and 653.9 μM) for 24 h. Thereafter, the cells fixed with 100% ice-cold methanol were incubated with hematoxylin and eosin stains for 5 min each, followed by dipping into 1% ammonia solution. In the end, the cells were agitated in distilled water and monitored with a light microscope.
Immunocytochemistry
Cells were seeded at a density of 2 × 105 in 6-well plates and treated with different ZnSO4 concentrations of 337.3 and 495.7 for 24 h. After, the treated cells were washed with PBS three times. After that, the cells were fastened with 100% ice-cold methanol for 10 min and embedded in 0.2% Triton X-100 for 5 min, followed by a blocking solution for 10 min. Thereafter, the cells were treated with anti-Bax and anti-Bcl-2 antibodies overnight at 4 °C with sufficient moisture maintained to keep antibodies from drying. Subsequently, biotinylated goat anti-polyvalent antibody and streptavidin peroxidase reagents were consecutively added for 10 min each. Later, the cells were sequentially stained with 3-amino-9-ethylcarbazole and hematoxylin. Eventually, the stained cells were observed under a light microscope.
Statistical Analysis
Three independent experiments each consisting of triplicates were done. All the data of our experiment followed the Gaussian distribution as a result of the Shapiro-Wilk normality test. All statistical analyses were employed by one-way analysis of variance (ANOVA) followed by Tukey’s post hoc tests using the Graphpad Prism 7.0 statistical software (San Diego, CA). p < 0.05 was considered significant. Results were indicated as mean ± SD (standard deviation).
Results
ZnSO4 Has Anti-proliferative Effects on DU-145 Cells
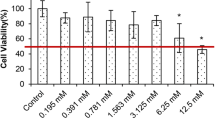
In the study, DU-145 prostate cancer cells were treated with increasing concentrations of ZnSO4 (0–1000 μM) for 24 h. It was detected that the treatments with 15.6, 31.3, 62.5, 125, and 250 μM of ZnSO4 caused an insignificant reduction in cell viability compared with the control (97.5, 97.1, 101.9, 103.6, and 88.8%, respectively) as indicated in Fig. 1.
ZnSO4 concentrations of 500 and 1000 μM showed a 49.3% and 96.9% reduction in cell viability compared with the control, respectively (P < 0.001). IC25, IC50, and IC75 concentrations in ZnSO4-treated DU-145 cells for 24 h were found as 337.3, 495.7, and 653.9 μM, respectively.
ZnSO4 Treatment Triggers Oxidative, Apoptotic, and Inflammatory Pathways in DU-145 cells
As shown in Fig. 2 a and c, the DU-145 cells treated with 337.3, 495.7, and 653.9 μM of ZnSO4 caused concentration-dependently a rise in MDA and TOS levels compared with the control. 653.9 μM ZnSO4 concentration showed two-fold increase in MDA levels and about three-fold increase in TOS levels compared with the control (P < 0.01). Additionally, 653.9 μM ZnSO4 concentration caused the highest enhancement in MDA and TOS levels (176.11 ± 10.27 nmol/mg protein and 13.85 ± 2.14 μmol H2O2 Equiv./L, respectively).
MDA, GSH, TOS, and TAS levels for determination of oxidative damage in DU-145 prostate cancer cells after 24 h of ZnSO4 treatment. a MDA levels. b GSH levels. c TOS levels. d TAS levels. *Indicates statically significant differences in comparison with control: *P < 0.05 and **P < 0.01. Data are presented as mean ± SD
Conversely, DU-145 cells demonstrated a statistically significant decrease in antioxidant defense because of the exposure to increasing concentrations. It can be seen from Fig. 2b that GSH and TAS levels displayed concentration-dependently a reduction in ZnSO4-treated DU-145 cells. 653.9 μM ZnSO4 concentration showed an important decrease in GSH levels (59.44 ± 2.07 μmol/mg protein, P < 0.01). Similar to GSH results, ZnSO4 treatment triggered a decrease in the TAS levels in DU-145 cells (Fig. 2d). 653.9 μM ZnSO4 concentration caused the highest decrease by 53% in TAS levels (1.02 ± 0.06 mmol Trolox Equiv./L) compared with the control. Conversely, 337.3 μM ZnSO4 concentration indicated an insignificant alteration in TAS levels compared with the control.
We found that treatments of ZnSO4 (337.3, 495.7, and 653.9 μM) for 24 h produced a rise in CYC and CASP3 levels by activating apoptosis in DU-145 cells treated with increasing concentrations of ZnSO4 (Fig. 3a, b). The CYC and CAPS3 levels in DU-145 cells increased approximately four-fold of the control at the 653.9 μM concentration of ZnSO4. Namely, our results demonstrated concentration-dependently a decrease in DU-145 cell viability compared with the control after a 24-h exposure with ZnSO4 concentrations.
Similarly, the results of oxidative and apoptotic markers, the TNF-α and IL-6 levels increased significantly depending on ZNSO4 concentrations (Fig. 4a, b). The highest increase in the levels of TNF-α and IL-6 were observed at the 653.9 μM ZnSO4 concentration (168.72 ± 5.29 and 203.91 ± 6.25 pg/mL, respectively).
ZnSO4 Induced Changes to Cytoplasmic, Nuclear, and Extracellular Matrices in DU-145 Cells
In Fig. 5, untreated DU-145 cells showed no morphology alterations. However, ZnSO4 treatment in DU-145 cells demonstrated numerous concentration-dependent reductions in cell number, cytoplasmic, and nuclear modifications.
Hematoxylin-eosin images of untreated and ZnSO4-treated DU-145 cells. a, b Regular-shaped DU-145 cells with their nuclei in the untreated group. c, d 495.7 μM ZnSO4-treated DU-145 cells. The abnormal cells with condensed nuclei and more eosinophilic cytoplasm (arrows). e, f 653.9 μM ZnSO4-treated DU-145 cells. In this group, abnormal cell number increases and cellular morphology becomes more severe when compared with the low dose group (arrows). Bars in a, c, and e indicate 50 μm and bars in b, d, and f indicate 20 μm
In order to explore potential signaling pathways contributing to ZnSO4-induced apoptosis, immunocytochemistry analysis was used to evaluate the Bcl-2 and Bax proteins. Concentration-dependently, the level of Bcl-2 protein decreased (Fig. 6d–f), while the level of Bax protein increased following treatment with ZnSO4 for 24 h (Fig. 6a–c).
Bax and Bcl-2 staining of untreated and ZnSO4-treated DU-145 cells. a The nuclei of regular-shaped DU-145 cells stains sparsely (arrows). b Bax staining is conspicuously higher (arrows) than the untreated group in 337.3 μM (IC25) of the ZnSO4-treated group. c Higher Bax staining persists (arrows) although many cells shrank in 495.7 μM (IC50) of the ZnSO4-treated group. d The nuclei of regular-shaped DU145 cells stain intensely (arrows). e The intensity of Bcl-2 staining decreases (arrows) in 337.3 μM (IC25) of the ZnSO4-treated group. f Bcl-2 staining decreases (arrows) even more in 495.7 μM (IC50) of the ZnSO4-treated group. All bars indicate 50 μm
Discussion
Zinc is one of the important trace elements that play a role in many biological functions related to cellular differentiation and proliferation, protein synthesis, DNA synthesis, ROS clearance, and apoptosis [23]. Zinc has conflicting reports that may exhibit inducing or inhibitory effects according to the cell type and its concentration. For example, a current study has reported that 75–600 μM zinc concentrations hindered cell proliferation of mouse thymocytes but 7.5–15 μM zinc concentrations enhanced it [24]. Conversely, Schrantz et al. [25] found that zinc at 10–50 μM low concentrations inhibited the growth of Ramos cells but at 50–100 μM high concentrations promoted it. Also, it was reported that zinc induces apoptosis in prostate cancer cell lines unlike normal prostate cells [26]. Although, in the current study, zinc treatments were greater than plasma levels which are normally 10 to 20 μM, the zinc concentrations in the prostate tissue that increase 1.5 mM levels [27]. Also, we demonstrated that ZnSO4 treatment (more than 500 μM) was cytotoxic to DU-145 prostate cancer cells.
The increase of redox imbalance of cancer cells triggers ROS production [28]. ROS, produced either by endogenous or by exogenous sources, plays a vital role in the regulation of biochemical processes (oxidative stress, apoptosis, inflammation, etc.) [29]. ROS generation has been associated with oxidative damage or apoptosis, which is traditionally the inhibition of cellular proliferation or differentiation [30]. Actually, proliferation, apoptosis, and inflammation-related processes are the response of signaling pathways to changes in intracellular ROS levels [31]. Therefore, ROS production or a deficiency of the antioxidant defense system can affect the fate of many cancer types [32]. Zinc also acts as a pro-oxidant and leads to oxidative stress when its concentration is in excess since this case promotes the ROS formation and decreases antioxidant defense [33]. A previous study showed that zinc treatment in Hep-2 cells promoted the generation of ROS and this situation was accompanied by gradual depletion of GSH [34]. Our data verify these evidences and additionally show that zinc induces oxidative damage by a concentration-dependent manner in the DU-145 cells. Moreover, we found that the increase in MDA and TOS correlated in ZnSO4-treated cells with decreasing levels of GSH and TAS. Noteworthy, this study indicated that ZnSO4 concentrations higher than 500 μM on prostate cancer cells are correlated with increased oxidative stress.
Zinc takes an active part in regulating apoptosis. Apoptosis, called programmed cell death, is defined by several morphological and biochemical conditions containing cell contraction, crescent-shaped nuclei, diminished Bcl-2 protein levels, increased Bax, CASP3, and CYC levels [35]. CASP3 is known as a significant enzyme for the regulation of apoptosis [36]. CASP3 is closely related to the reorganization of Bcl-2 and Bax proteins. The anti-apoptotic Bcl-2 protein controls apoptosis by regulating the migration of CYC from the mitochondria to the cytoplasm, whereas the Bax protein forms a heterodimer with Bcl-2 and activates Bcl-2-suppressed apoptosis by promoting CYC release [37]. It was reported that the apoptotic effects of zinc directly stemmed from an increase in Bax protein expression, and the release of cytochrome C from mitochondria to cytosol that causes the activation of caspase [38]. Our results are in agreement with a previous study where the apoptosis in zinc-treated DU-145 cells was triggered through the reduction of Bcl-2 and the increase of Bax related to the caspase-3 activation [10]. In our experiment, the amount of Bcl-2 and Bax proteins correlated with increased CAPS3 and CYC levels. Reduced Bcl-2 protein levels and increased Bax protein, CASP3, and CYC levels in DU-145 cells treated with ZnSO4 in this study (Fig. 6) indicate that zinc-induced apoptosis is pertinent to the mitochondrial death-signal pathway in DU-145 cells. Additionally, we used hematoxylin-eosin staining to the morphological changes in the DU-145 cells following ZnSO4 exposure for 24 h. The ZnSO4-treated DU-145 cells showed a concentration-dependent decrease in adherence to culture dish and an increase in morphological alterations such as flattened and low-volume cells.
TNF-α and IL-6 are produced by cancer cells and affect various physiologic processes including cancer progression, migration, and differentiation [39]. IL-6 and TNF- α are responsible for a number of signaling mechanisms, which cause either apoptosis or proliferation based on the IL-6 and TNF-α levels of a cell [40]. TNF-α and IL-6 are multifunctional cytokines that regulate proliferation and differentiation depending on their intracellular concentration [17]. Few studies investigated the relation between IL-6 and proliferation of prostate cancer cells. Increased IL-6 level inhibited growth in androgen-independent cell lines (DU-145 and PC3), but no effect on androgen-sensitive cell line (LNCaP) [41]. Similarly, it was reported that TNF-α at concentrations higher than 100 ng/mL inhibited the proliferation of PC3 prostate cancer cells; TNF-α at concentrations lower than 50 ng/mL induced the growth of PC3 [42]. Besides, Lu et al. [43] suggested that 10 ng/mL TNF-α in MDA-MB-231 breast cancer cells induced apoptosis by upregulation of caspases. Our study is the first to demonstrate that zinc treatment leads to a concentration-dependent enhancement of TNF-α and IL-6 levels in the DU-145 cells. Additionally, by deteriorating the equilibrium between redox mechanisms that are liable for inducing apoptosis, zinc may contribute to activation of the inflammatory response. It can be supposed that this imbalance promotes the vulnerability of prostate cancer cells to the oxidative and apoptotic effects of TNF-α and IL-6, finally causing DU-145 cell death.
Conclusions
To summarize, we here have shown that ZnSO4 can control proliferation in the DU-145 cells via inducing oxidative damage, apoptosis, inflammation, and morphological changes. Interestingly, it has been shown that zinc treatment not only enhances anti-proliferative effects associated with activation of oxidative damage and apoptosis but also elevates the expression of inflammatory cytokines and provokes chronic inflammation. In addition, zinc treatment in DU-145 prostate cancer cell line negatively affects the proliferation of cells by decreasing the antioxidant defense system. However, for clinical application of zinc in the prevention and treatment of prostate cancer, further studies are needed reflecting the metabolism of zinc and its compounds in other prostate cancer cell lines and experimental animal models.
References
Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F (2015) Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 136:359–386. https://doi.org/10.1002/ijc.29210
Pernar CH, Ebot EM, Wilson KM, Mucci LA (2018) The epidemiology of prostate cancer. Cold Spring Harb Perspect Med 8:a030361. https://doi.org/10.1101/cshperspect.a030361
Moul JW (2003) Variables in predicting survival based on treating “PSA-only” relapse. Urol Oncol 21:292–304. https://doi.org/10.1016/S1078-1439(03)00103-0
Costell LC, Franklin RB (1998) Novel role of zinc in the regulation of prostate citrate metabolism and its implications in prostate cancer. Prostate 35:285–296. https://doi.org/10.1002/(SICI)1097-0045(19980601)35:4<285::AID-PROS8>3.0.CO;2-F
Federico A, Iodice P, Federico P, Del Rio A, Mellone MC, Catalano G (2001) Effects of selenium and zinc supplementation on nutritional status in patients with cancer of digestive tract. Eur J Clin Nutr 55:293–297. https://doi.org/10.1038/sj.ejcn.1601157
Rohrmann S, Giovannucci E, Willett WC, Platz EA (2007) Fruit and vegetable consumption, intake of micronutrients, and benign prostatic hyperplasia in US men. Am J Clin Nutr 85:523–529. https://doi.org/10.1093/ajcn/85.2.523
Kristal AR, Arnold KB, Schenk JM, Neuhouser ML, Goodman P, Penson DF, Thompson IM (2008) Dietary patterns, supplement use, and the risk of symptomatic benign prostatic hyperplasia: results from the prostate cancer prevention trial. Am J Epidemiol 167:925–934. https://doi.org/10.1093/aje/kwm389
Costello LC, Franklin RB (2016) A comprehensive review of the role of zinc in normal prostate function and metabolism; and its implications in prostate cancer. Arch Biochem Biophys 611:100–112. https://doi.org/10.1016/j.abb.2016.04.014
Costello LC, Franklin RB, Feng P, Tan M, Bagasra O (2005) Zinc and prostate cancer: a critical scientific, medical, and public interest issue (United States). Cancer Causes Control 16:901–915. https://doi.org/10.1007/s10552-005-2367-y
Hong SH, Choi YS, Cho HJ, Lee JY, Kim JC, Hwang TK, Kim SW (2012) Antiproliferative effects of zinc-citrate compound on hormone refractory prostate cancer. Chin J Cancer Res 24:124–129. https://doi.org/10.1007/s11670-012-0124-9
Feng P, Li TL, Guan ZX, Franklin RB, Costello LC (2003) Effect of zinc on prostatic tumorigenicity in nude mice. Ann N Y Acad Sci 1010:316–320. https://doi.org/10.1196/annals.1299.056
Reyes JG (1996) Zinc transport in mammalian cells. Am J Phys 270:401–410. https://doi.org/10.1152/ajpcell.1996.270.2.C401
McCord MC, Aizenman E (2014) The role of intracellular zinc release in aging, oxidative stress, and Alzheimer's disease. Front Aging Neurosci 6:1–16. https://doi.org/10.3389/fnagi.2014.00077
Liu RY, Fan C, Mitchell S, Chen Q, Wu J, Zuckerman KS (1998) The role of type I and type II tumor necrosis factor (TNF) receptors in the ability of TNF-alpha to transduce a proliferative signal in the human megakaryoblastic leukemic cell line Mo7e. Cancer Res 58:2217–2223
Shintani Y, Fujiwara A, Kimura T, Kawamura T, Funaki S, Minami M, Okumura M (2016) IL-6 secreted from cancer-associated fibroblasts mediates chemoresistance in NSCLC by increasing epithelial-mesenchymal transition signaling. J Thorac Oncol 11:1482–1492. https://doi.org/10.1016/j.jtho.2016.05.025
Grivennikov SI, Greten FR, Karin M (2010) Immunity, inflammation, and cancer. Cell 140:883–899. https://doi.org/10.1016/j.cell.2010.01.025
Tse BW, Scott KF, Russell PJ (2012) Paradoxical roles of tumour necrosis factor-alpha in prostate cancer biology. Prostate Cancer 2012:1–8. https://doi.org/10.1155/2012/128965
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65:55–63. https://doi.org/10.1016/0022-1759(83)90303-4
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358. https://doi.org/10.1016/0003-2697(79)90738-3
Srivastava SK, Beutler E (1968) Accurate measurement of oxidized glutathione content of human, rabbit, and rat red blood cells and tissues. Anal Biochem 25:70–76. https://doi.org/10.1016/0003-2697(68)90082-1
Fischer AH, Jacobson KA, Rose J, Zeller R (2008) Hematoxylin and eosin staining of tissue and cell sections. CSH Protoc 2008:pdb.prot4986. https://doi.org/10.1101/pdb.prot4986
Ho E (2004) Zinc deficiency, DNA damage and cancer risk. J Nutr Biochem 15:572–578. https://doi.org/10.1016/j.jnutbio.2004.07.005
Provinciali M, Di Stefano G, Fabris N (1995) Dose-dependent opposite effect of zinc on apoptosis in mouse thymocytes. Int J Immunopharmacol 17:735–744. https://doi.org/10.1016/0192-0561(95)00063-8
Schrantz N, Auffredou MT, Bourgeade MF, Besnault L, Leca G, Vazquez A (2001) Zinc-mediated regulation of caspases activity: dose-dependent inhibition or activation of caspase-3 in the human Burkitt lymphoma B cells (Ramos). Cell Death Differ 8:152–161. https://doi.org/10.1038/sj.cdd.4400772
Wetherell D, Baldwin GS, Shulkes A, Bolton D, Ischia J, Patel O (2018) Zinc ion dyshomeostasis increases resistance of prostate cancer cells to oxidative stress via upregulation of HIF1α. Oncotarget 9:8463–8477. https://doi.org/10.18632/oncotarget.23893
Yan M, Hardin K, Ho E (2010) Differential response to zinc-induced apoptosis in benign prostate hyperplasia and prostate cancer cells. J Nutr Biochem 21:687–694. https://doi.org/10.1016/j.jnutbio.2009.04.002
Barzilai A, Rotman G, Shiloh Y (2002) ATM deficiency and oxidative stress: a new dimension of defective response to DNA damage. DNA Repair 22:3–25. https://doi.org/10.1016/S1568-7864(01)00007-6
Naka K, Muraguchi T, Hoshii T, Hirao A (2008) Regulation of reactive oxygen species and genomic stability in hematopoietic stem cells. Antioxid Redox Signal 10:1883–1894. https://doi.org/10.1089/ars.2008.2114
Sauer H, Wartenberg M, Hescheler J (2001) Reactive oxygen species as intracellular messengers during cell growth and differentiation. Cell Physiol Biochem 11:173–186. https://doi.org/10.1159/000047804
Minelli A, Bellezza I, Conte C, Culig Z (2009) Oxidative stress-related aging: a role for prostate cancer? Biochim Biophys Acta 1795:83–91. https://doi.org/10.1016/j.bbcan.2008.11.001
Bouayed J, Bohn T (2010) Exogenous antioxidants—double-edged swords in cellular redox state: health beneficial effects at physiologic doses versus deleterious effects at high doses. Oxidative Med Cell Longev 3:228–237. https://doi.org/10.4161/oxim.3.4.12858
Maret W (2013) Zinc biochemistry: from a single zinc enzyme to a key element of life. Adv Nutr 4:82–91. https://doi.org/10.3945/an.112.003038
Rudolf E, Rudolf K, Cervinka M (2005) Zinc induced apoptosis in HEP-2 cancer cells: the role of oxidative stress and mitochondria. Biofactors 23:107–120. https://doi.org/10.1002/biof.5520230206
Nagata S (2018) Apoptosis and clearance of apoptotic cells. Annu Rev Immunol 36:489–517. https://doi.org/10.1146/annurev-immunol-042617-053010
Elmore S (2007) Apoptosis: A Review of Programmed Cell Death. Toxicol Pathol 35:495–516. https://doi.org/10.1080/01926230701320337
Youle RJ, Strasser A (2008) The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol 9:47–59. https://doi.org/10.1038/nrm2308
Costello LC, Feng P, Milon B, Tan M, Franklin RB (2004) Role of zinc in the pathogenesis and treatment of prostate cancer: critical issues to resolve. Prostate Cancer Prostatic Dis 7:111–117. https://doi.org/10.1038/sj.pcan.4500712
Michalaki V, Syrigos K, Charles P, Waxman J (2004) Serum levels of IL-6 and TNF-alpha correlate with clinicopathological features and patient survival in patients with prostate cancer. Br J Cancer 90:2312–2316. https://doi.org/10.1038/sj.bjc.6601814
Muenchen HJ, Lin DL, Walsh MA, Keller ET, Pienta KJ (2000) Tumor necrosis factor-alpha-induced apoptosis in prostate cancer cells through inhibition of nuclear factor-kappaB by an IkappaBalpha “super-repressor”. Clin Cancer Res 6:1969–1977
Giri D, Ozen M, Ittmann M (2001) Interleukin-6 is an autocrine growth factor in human prostate cancer. The Am J Pathol 159:2159–2165. https://doi.org/10.1016/S0002-9440(10)63067-2
Maolake A, Izumi K, Natsagdorj A, Iwamoto H, Kadomoto S, Makino T, Naito R, Shigehara K, Kadono Y, Hiratsuka K, Wufuer G, Nastiuk KL, Mizokami A (2018) Tumor necrosis factor-α induces prostate cancer cell migration in lymphatic metastasis through CCR7 upregulation. Cancer Sci 109:1524–1531. https://doi.org/10.1111/cas.13586
Lu L, Shi W, Deshmukh RR, Long J, Cheng X, Ji W, Zeng G, Chen X, Zhang Y, Dou QP (2014) Tumor necrosis factor-α sensitizes breast cancer cells to natural products with proteasome-inhibitory activity leading to apoptosis. PLoS One 9:e113783. https://doi.org/10.1371/journal.pone.0113783
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hacioglu, C., Kacar, S., Kar, F. et al. Concentration-Dependent Effects of Zinc Sulfate on DU-145 Human Prostate Cancer Cell Line: Oxidative, Apoptotic, Inflammatory, and Morphological Analyzes. Biol Trace Elem Res 195, 436–444 (2020). https://doi.org/10.1007/s12011-019-01879-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-019-01879-0